An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
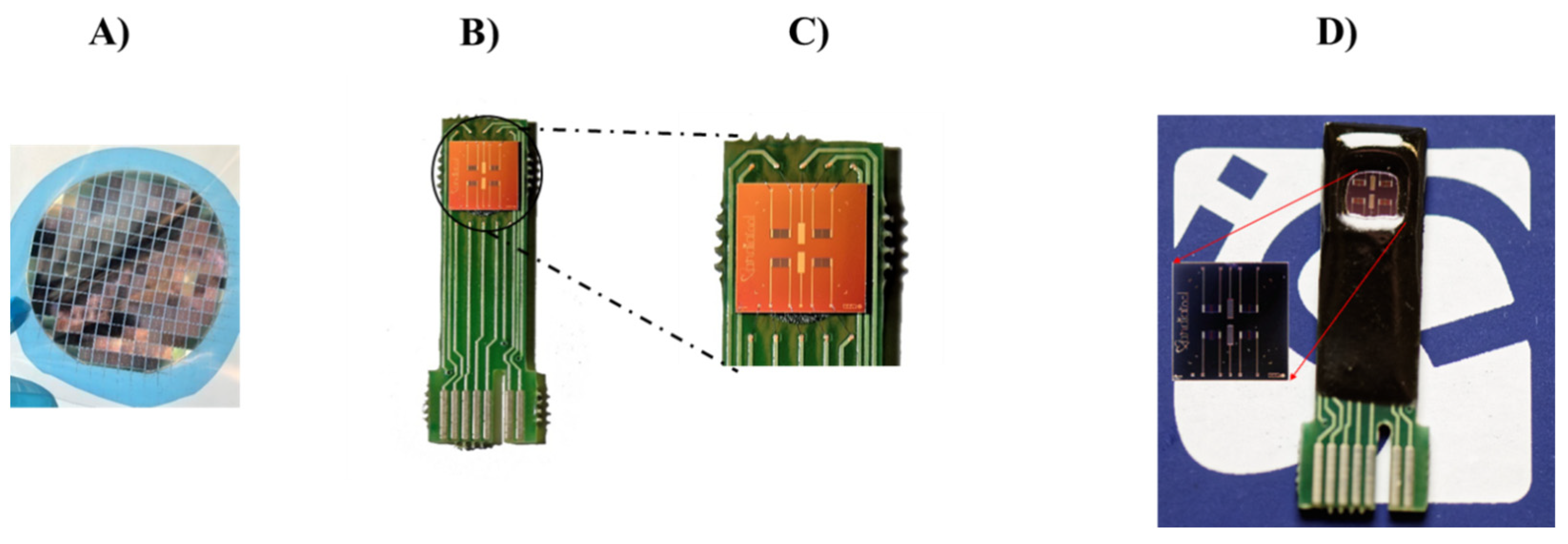
2.2. ISFET Device Fabrication
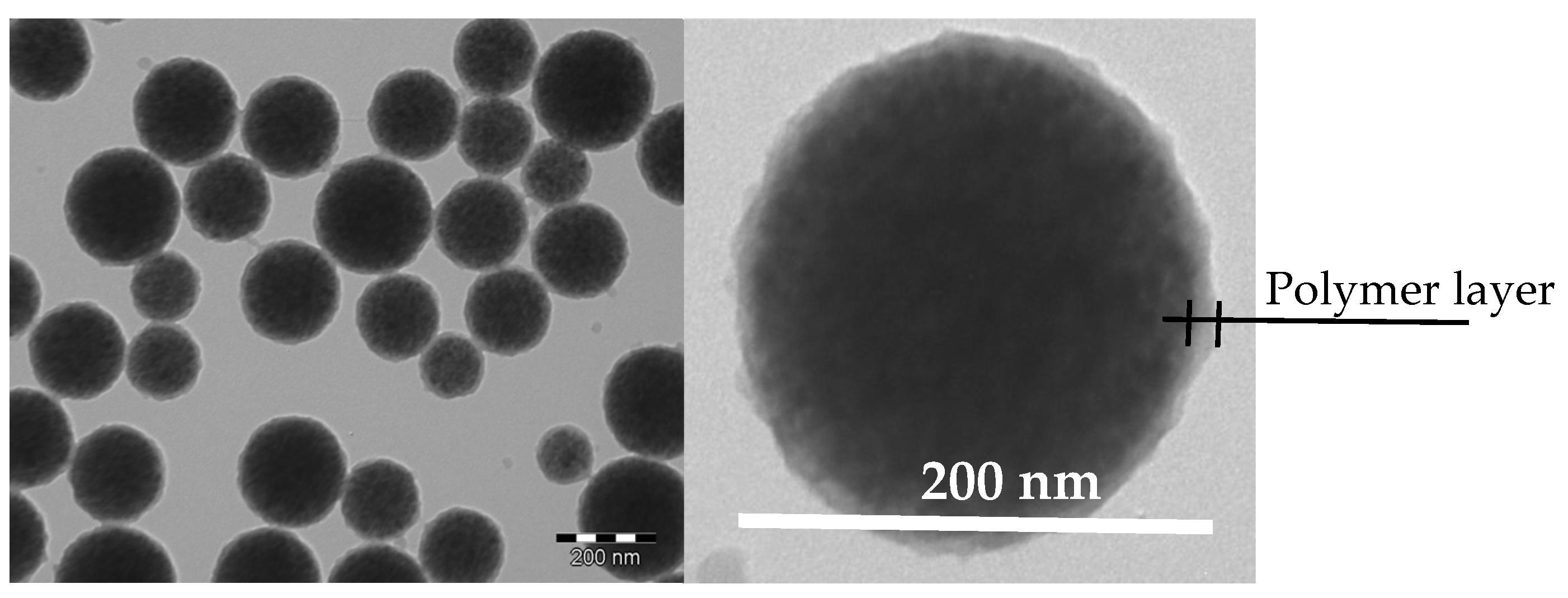
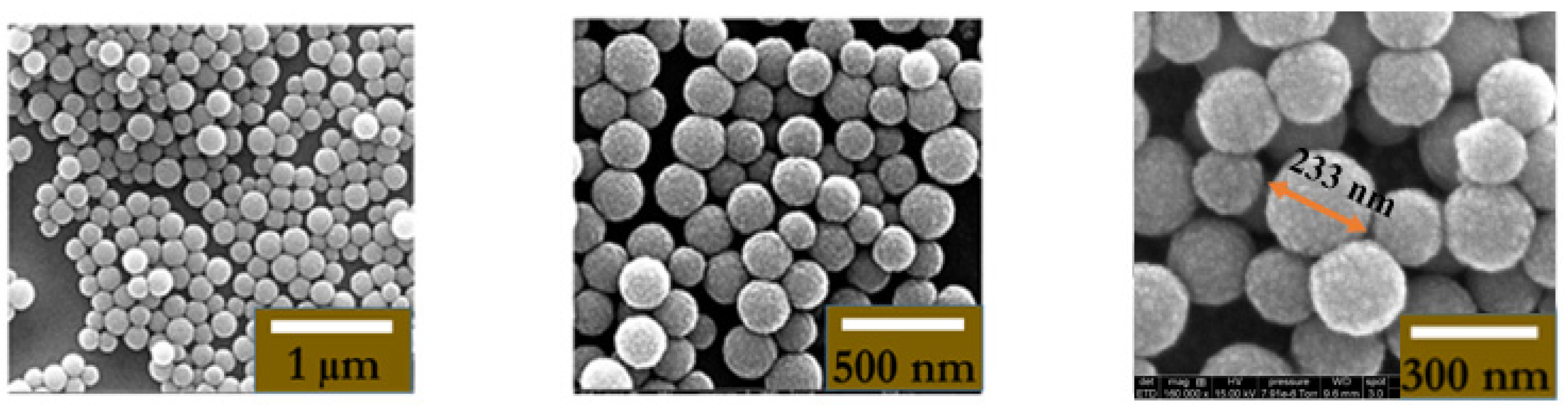
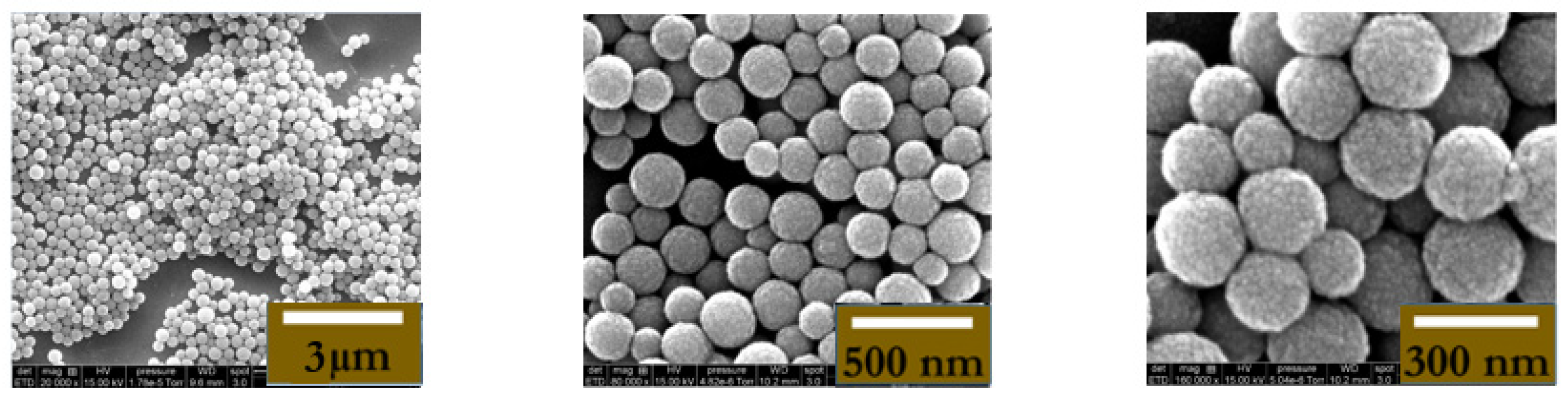
2.3. Synthesis and Characterization of MNPs
2.3.1. Seed Magnetic Latex Particle Preparation
2.3.2. Preparation of Amine-Containing Dextran Polymer
2.3.3. Adsorption of Amino Dextran onto Magnetic Latex Particles
2.3.4. Transmission Electron Microscopy Analysis
2.3.5. Hydrodynamic Particle Size
2.4. Standard Solutions
2.5. Preparation of Artificial Saliva (AS)
2.6. Sample Preparation
2.7. Biofunctionalization of MNPs
2.8. Biofunctionalization of the ImmunoFET
2.9. Electrochemical Measurements
3. Results
3.1. Coupling the Immunomagnetic Pre-concentration Process with the ImmunoFET
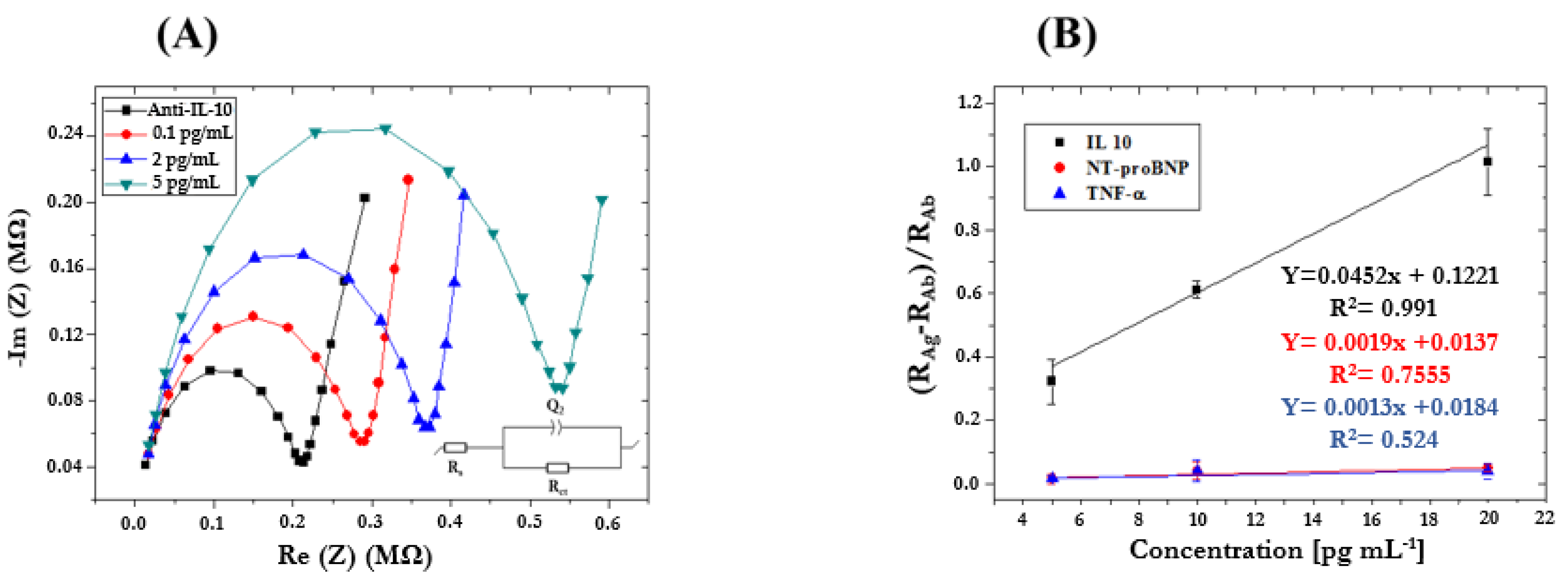
3.2. Determination of HF Biomarker in Standard Solutions by ImmunoFET
3.3. Determination of HF Biomarkers in Artificial Saliva Using the Standard Addition Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Authors/Task Force Members; Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.V.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; Van Veldhuisen, D.J.; Atar, D.; et al. ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in Collaboration with the Heart Failure Association of the ESC (HFA) and Endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2008, 29, 2388–2442. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An Endotoxin-Induced Serum Factor That Causes Necrosis of Tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Maury, C.P.J. Tumour Necrosis Factor—An Overview. Acta Medica Scand. 1986, 220, 387–394. [Google Scholar] [CrossRef]
- Le, J.; Vilcek, J. Tumor Necrosis Factor and Interleukin 1: Cytokines with Multiple Overlapping Biological Activities. Lab. Investig. 1987, 56, 234–248. [Google Scholar]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- Landry, D.W.; Oliver, J.A. The Pathogenesis of Vasodilatory Shock. N. Engl. J. Med. 2001, 345, 588–595. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva Sampling: Methods and Devices. An Overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Bolger, A.P.; Anker, S.D. Tumour Necrosis Factor in Chronic Heart Failure. Drugs 2000, 60, 1245–1257. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Redfield, M.M.; Killian, J.M.; Roger, V.L. Tumor Necrosis Factor Alpha (TNFα) and Mortality in Heart Failure: A Community Study. Circulation 2008, 118, 625–631. [Google Scholar] [CrossRef]
- Kaur, K.; Dhingra, S.; Slezak, J.; Sharma, A.K.; Bajaj, A.; Singal, P.K. Biology of TNFα and IL-10, and Their Imbalance in Heart Failure. Heart Fail. Rev. 2009, 14, 113–123. [Google Scholar] [CrossRef]
- Herrera Garza, E.H.; Herrera Garza, J.L.; Rodríguez González, H.; Treviño Treviño, A.; Ibarra Flores, M.; Torre Amione, G. Importance of Tumor Necrosis Factor-Alpha in the Pathogenesis of Heart Failure. Rev. Esp. Cardiol. 2002, 55, 61–66. [Google Scholar] [CrossRef]
- Gümüş, P.; Nizam, N.; Lappin, D.F.; Buduneli, N. Saliva and Serum Levels of B-Cell Activating Factors and Tumor Necrosis Factor-α in Patients With Periodontitis. J. Periodontol. 2014, 85, 270–280. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Arribas, A.; Leigh, J.E.; Fidel, P.L. Inhibitory Effects of Whole and Parotid Saliva on Immunomodulators. Oral. Microbiol. Immunol. 2002, 17, 100–107. [Google Scholar] [CrossRef]
- Batista, M.L.; Batista Júnior, M.L.; Lopes, R.D.; Seelaender, M.C.L.; Lopes, A.C. Anti-Inflammatory Effect of Physical Training in Heart Failure: Role of TNF-Alpha and IL-10. Arq. Bras. Cardiol. 2009, 93, 643–651, 692–700. [Google Scholar]
- Amir, O.; Rogowski, O.; David, M.; Lahat, N.; Wolff, R.; Lewis, B.S. Circulating Interleukin-10: Association with Higher Mortality in Systolic Heart Failure Patients with Elevated Tumor Necrosis Factor-Alpha. Isr. Med. Assoc. J. 2010, 12, 158–162. [Google Scholar]
- Asadullah, K.; Sterry, W.; Volk, H.D. Interleukin-10 Therapy—Review of a New Approach. Pharmacol. Rev. 2003, 55, 241–269. [Google Scholar] [CrossRef]
- Anker, S.D.; Haehling, S. von Inflammatory Mediators in Chronic Heart Failure: An Overview. Heart 2004, 90, 464–470. [Google Scholar] [CrossRef]
- Capsoni, F.; Minonzio, F.; Mariani, C.; Ongari, A.M.; Bonara, P.; Fiorelli, G. Development of Phagocytic Function of Cultured Human Monocytes Is Regulated by Cell Surface IL-10. Cell. Immunol. 1998, 189, 51–59. [Google Scholar] [CrossRef]
- Bogdan, C.; Vodovotz, Y.; Nathan, C. Macrophage Deactivation by Interleukin 10. J. Exp. Med. 1991, 174, 1549–1555. [Google Scholar] [CrossRef]
- Joyce, D.A.; Gibbons, D.P.; Green, P.; Steer, J.H.; Feldmann, M.; Brennan, F.M. Two Inhibitors of Pro-Inflammatory Cytokine Release, Interleukin-10 and Interleukin-4, Have Contrasting Effects on Release of Soluble P75 Tumor Necrosis Factor Receptor by Cultured Monocytes. Eur. J. Immunol. 1994, 24, 2699–2705. [Google Scholar] [CrossRef]
- Nozaki, N.; Yamaguchi, S.; Shirakabe, M.; Nakamura, H.; Tomoike, H. Soluble Tumor Necrosis Factor Receptors Are Elevated in Relation to Severity of Congestive Heart Failure. Jpn. Circ. J. 1997, 61, 657–664. [Google Scholar] [CrossRef]
- Nozaki, N.; Yamaguchi, S.; Yamaoka, M.; Okuyama, M.; Nakamura, H.; Tomoike, H. Enhanced Expression and Shedding of Tumor Necrosis Factor (TNF) Receptors from Mononuclear Leukocytes in Human Heart Failure. J. Mol. Cell. Cardiol. 1998, 30, 2003–2012. [Google Scholar] [CrossRef]
- Ferrari, R.; Bachetti, T.; Confortini, R.; Opasich, C.; Febo, O.; Corti, A.; Cassani, G.; Visioli, O. Tumor Necrosis Factor Soluble Receptors in Patients With Various Degrees of Congestive Heart Failure. Circulation 1995, 92, 1479–1486. [Google Scholar] [CrossRef]
- Zee, K.J.V.; Kohno, T.; Fischer, E.; Rock, C.S.; Moldawer, L.L.; Lowry, S.F. Tumor Necrosis Factor Soluble Receptors Circulate during Experimental and Clinical Inflammation and Can Protect against Excessive Tumor Necrosis Factor Alpha in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 4845–4849. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Chu, C.Q.; Brennan, F.M.; Maini, R.N.; Feldmann, M. Immunoregulatory Role of Interleukin 10 in Rheumatoid Arthritis. J. Exp. Med. 1994, 179, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, J.A.; Adabayeri, V.; Goka, B.Q.; Akanmori, B.D.; Oliver-Commey, J.O.; Nkrumah, F.K.; Behr, C.; Hviid, L. Low Plasma Concentrations of Interleukin 10 in Severe Malarial Anaemia Compared with Cerebral and Uncomplicated Malaria. Lancet 1998, 351, 1768–1772. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kribbs, S.B.; Clubb, F.J., Jr.; Michael, L.H.; Didenko, V.V.; Hornsby, P.J.; Seta, Y.; Oral, H.; Spinale, F.G.; Mann, D.L. Pathophysiologically Relevant Concentrations of Tumor Necrosis Factor-α Promote Progressive Left Ventricular Dysfunction and Remodeling in Rats. Circulation 1998, 97, 1382–1391. [Google Scholar] [CrossRef]
- Interleukin-10 and the Interleukin-10 Receptor | Annual Review of Immunology. Available online: https://www-annualreviews-org.docelec.univ-lyon1.fr/doi/abs/10.1146/annurev.immunol.19.1.683 (accessed on 13 May 2020).
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) Inhibits Cytokine Synthesis by Human Monocytes: An Autoregulatory Role of IL-10 Produced by Monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef]
- Mulligan, M.S.; Jones, M.L.; Vaporciyan, A.A.; Howard, M.C.; Ward, P.A. Protective Effects of IL-4 and IL-10 against Immune Complex-Induced Lung Injury. J. Immunol. 1993, 151, 5666–5674. [Google Scholar] [CrossRef]
- Shanley, T.P.; Schmal, H.; Friedl, H.P.; Jones, M.L.; Ward, P.A. Regulatory Effects of Intrinsic IL-10 in IgG Immune Complex-Induced Lung Injury. J. Immunol. 1995, 154, 3454–3460. [Google Scholar] [CrossRef]
- Lacraz, S.; Nicod, L.P.; Chicheportiche, R.; Welgus, H.G.; Dayer, J.M. IL-10 Inhibits Metalloproteinase and Stimulates TIMP-1 Production in Human Mononuclear Phagocytes. J. Clin. Investig. 1995, 96, 2304–2310. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.H.; Ho, A.S.; de Waal Malefyt, W.; Moore, K.W. Expression Cloning and Characterization of a Human IL-10 Receptor. J. Immunol. 1994, 152, 1821–1829. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Krause, C.D.; Izotova, L.S.; Pollack, B.P.; Wu, W.; Pestka, S. Identification and Functional Characterization of a Second Chain of the Interleukin-10 Receptor Complex. EMBO J. 1997, 16, 5894–5903. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Luo, H.; Luo, Z.; Liu, L.; Xu, W.; Zhang, T.; Yang, N.; Long, X.; Zhu, N.; et al. Ulinastatin Reduces Urinary Sepsis-related Inflammation by Upregulating IL-10 and Downregulating TNF-α Levels. Mol. Med. Rep. 2013, 8, 29–34. [Google Scholar] [CrossRef]
- Elnokeety, M.M.; Shaker, A.M.; Fayed, A.M. Urinary Interleukin-10 in Renal Transplant Recipients: Does It Predict a State of Tolerance or Rejection. Saudi J. Kidney Dis. Transplant. 2017, 28, 1196. [Google Scholar]
- Sánchez-Zauco, N.; Torres, J.; Gómez, A.; Camorlinga-Ponce, M.; Muñoz-Pérez, L.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Giono-Cerezo, S.; Maldonado-Bernal, C. Circulating Blood Levels of IL-6, IFN-γ, and IL-10 as Potential Diagnostic Biomarkers in Gastric Cancer: A Controlled Study. BMC Cancer 2017, 17, 384. [Google Scholar] [CrossRef]
- Hamzavi, M.; Tadbir, A.A.; Rezvani, G.; Ashraf, M.J.; Fattahi, M.J.; Khademi, B.; Sardari, Y.; Jeirudi, N. Tissue Expression, Serum and Salivary Levels of IL-10 in Patients with Head and Neck Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1681–1685. [Google Scholar] [CrossRef]
- Polz-Dacewicz, M.; Strycharz-Dudziak, M.; Dworzański, J.; Stec, A.; Kocot, J. Salivary and Serum IL-10, TNF-α, TGF-β, VEGF Levels in Oropharyngeal Squamous Cell Carcinoma and Correlation with HPV and EBV Infections. Infect. Agents Cancer 2016, 11, 45. [Google Scholar] [CrossRef]
- Sarris, A.H.; Kliche, K.O.; Pethambaram, P.; Preti, A.; Tucker, S.; Jackow, C.; Messina, O.; Pugh, W.; Hagemeister, F.B.; McLaughlin, P.; et al. Interleukin-10 Levels Are Often Elevated in Serum of Adults with Hodgkin’s Disease and Are Associated with Inferior Failure-Free Survival. Ann. Oncol. 1999, 10, 433–440. [Google Scholar] [CrossRef]
- Gupta, M.; Han, J.J.; Stenson, M.; Maurer, M.; Wellik, L.; Hu, G.; Ziesmer, S.; Dogan, A.; Witzig, T.E. Elevated Serum IL-10 Levels in Diffuse Large B-Cell Lymphoma: A Mechanism of Aberrant JAK2 Activation. Blood 2012, 119, 2844–2853. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Chi, P.; Wang, W.; Chen, X.; Geng, Q.; Xia, Z.; Lu, Y. High Level of Interleukin-10 in Serum Predicts Poor Prognosis in Multiple Myeloma. Br. J. Cancer 2016, 114, 463–468. [Google Scholar] [CrossRef]
- Hempel, L.; Körholz, D.; Nußbaum, P.; Bönig, H.; Burdach, S.; Zintl, F. High Interleukin-10 Serum Levels Are Associated with Fatal Outcome in Patients after Bone Marrow Transplantation. Bone Marrow Transplant. 1997, 20, 365–368. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Hangouët, M.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Zine, N.; Bausells, J.; Errachid, A. A Novel IMFET Biosensor Strategy for Interleukin-10 Quantification for Early Screening Heart Failure Disease in Saliva. Electroanalysis 2022, 35, elan.202200141. [Google Scholar] [CrossRef]
- Subirana, I.; Fitó, M.; Diaz, O.; Vila, J.; Francés, A.; Delpon, E.; Sanchis, J.; Elosua, R.; Muñoz-Aguayo, D.; Dégano, I.R.; et al. Prediction of Coronary Disease Incidence by Biomarkers of Inflammation, Oxidation, and Metabolism. Sci. Rep. 2018, 8, 3191. [Google Scholar] [CrossRef]
- Gassen, J.; Prokosch, M.L.; Eimerbrink, M.J.; Proffitt Leyva, R.P.; White, J.D.; Peterman, J.L.; Burgess, A.; Cheek, D.J.; Kreutzer, A.; Nicolas, S.C.; et al. Inflammation Predicts Decision-Making Characterized by Impulsivity, Present Focus, and an Inability to Delay Gratification. Sci. Rep. 2019, 9, 4928. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Engelhard, M.H.; Lin, Y. Sensitive Immunoassay of a Biomarker Tumor Necrosis Factor-α Based on Poly(Guanine)-Functionalized Silica Nanoparticle Label. Anal. Chem. 2006, 78, 6974–6979. [Google Scholar] [CrossRef]
- Van Der Linden, M.W.; Huizinga, T.W.J.; Stoeken, D.-J.; Sturk, A.; Westendorp, R.G.J. Determination of Tumour Necrosis Factor-α and Interleukin-10 Production in a Whole Blood Stimulation System: Assessment of Laboratory Error and Individual Variation. J. Immunol. Methods 1998, 218, 63–71. [Google Scholar] [CrossRef]
- Jones, L.J.; Singer, V.L. Fluorescence Microplate-Based Assay for Tumor Necrosis Factor Activity Using SYTOX Green Stain. Anal. Biochem. 2001, 293, 8–15. [Google Scholar] [CrossRef]
- Teppo, A.M.; Maury, C.P. Radioimmunoassay of Tumor Necrosis Factor in Serum. Clin. Chem. 1987, 33, 2024–2027. [Google Scholar] [CrossRef]
- Wang, J.; Munir, A.; Zhou, H.S. Au NPs-Aptamer Conjugates as a Powerful Competitive Reagent for Ultrasensitive Detection of Small Molecules by Surface Plasmon Resonance Spectroscopy. Talanta 2009, 79, 72–76. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Z.; Ma, L. Determination of Recombinant Human Tumor Necrosis Factor-α in Serum by Chemiluminescence Imaging. Anal. Chim. Acta 2005, 539, 277–282. [Google Scholar] [CrossRef]
- Halima, H.B.; Zine, N.; Gallardo-Gonzalez, J.; Aissari, A.E.; Sigaud, M.; Alcacer, A.; Bausells, J.; Errachid, A. A Novel Cortisol Biosensor Based on the Capacitive Structure of Hafnium Oxide: Application for Heart Failure Monitoring. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; IEEE: Berlin, Germany, 2019; pp. 1067–1070. [Google Scholar]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A Novel Biosensor Based on Hafnium Oxide: Application for Early Stage Detection of Human Interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Electrochemical Immunosensors for the Detection of Cytokine Tumor Necrosis Factor Alpha: A Review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef]
- Pérez, D.J.; Patiño, E.B.; Orozco, J. Electrochemical Nanobiosensors as Point-of-Care Testing Solution to Cytokines Measurement Limitations. Electroanalysis 2022, 34, 184–211. [Google Scholar] [CrossRef]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Amperometric Magnetoimmunoassay for the Direct Detection of Tumor Necrosis Factor Alpha Biomarker in Human Serum. Anal. Chim. Acta 2014, 838, 37–44. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.K.; Kim, M. Ion-Sensitive Field-Effect Transistor for Biological Sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef]
- Estrela, P.; Pachauri, V.; Ingebrandt, S. Biologically Sensitive Field-Effect Transistors: From ISFETs to NanoFETs. Essays Biochem. 2016, 60, 81–90. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. A Silicon Nitride ISFET Based Immunosensor for Tumor Necrosis Factor-Alpha Detection in Saliva. A Promising Tool for Heart Failure Monitoring. Anal. Chim. Acta 2021, 1161, 338468. [Google Scholar] [CrossRef]
- Vozgirdaite, D.; Ben Halima, H.; Bellagambi, F.G.; Alcacer, A.; Palacio, F.; Jaffrezic-Renault, N.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. Development of an ImmunoFET for Analysis of Tumour Necrosis Factor-α in Artificial Saliva: Application for Heart Failure Monitoring. Chemosensors 2021, 9, 26. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Brunon, F.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Errachid, A. Immuno Field-Effect Transistor (ImmunoFET) for Detection of Salivary Cortisol Using Potentiometric and Impedance Spectroscopy for Monitoring Heart Failure. Talanta 2022, 257, 123802. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Hangouët, M.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. A Novel Electrochemical Strategy for NT-proBNP Detection Using IMFET for Monitoring Heart Failure by Saliva Analysis. Talanta 2023, 251, 123759. [Google Scholar] [CrossRef]
- Ben Halima, H.; Zine, N.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis. Micromachines 2022, 13, 2235. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Yaakoubi, N.; Bausells, J.; Errachid, A. A Flexible Electrochemical Micro Lab-on-Chip: Application to the Detection of Interleukin-10. Microchim. Acta 2016, 183, 2155–2162. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Yaakoubi, N.; Trivella, M.G.; Zabala, M.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. Diazonium Modified Gold Microelectrodes onto Polyimide Substrates for Impedimetric Cytokine Detection with an Integrated Ag/AgCl Reference Electrode. Sens. Actuators B Chem. 2013, 189, 165–172. [Google Scholar] [CrossRef]
- Mouaziz, H.; Braconnot, S.; Ginot, F.; Elaissari, A. Elaboration of Hydrophilic Aminodextran Containing Submicron Magnetic Latex Particles. Colloid. Polym. Sci. 2009, 287, 287–297. [Google Scholar] [CrossRef]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A Fully Integrated Electrochemical Biosensor Platform Fabrication Process for Cytokines Detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef]
- Nessark, F.; Eissa, M.; Baraket, A.; Zine, N.; Nessark, B.; Zouaoui, A.; Bausells, J.; Errachid, A. Capacitance Polypyrrole-Based Impedimetric Immunosensor for Interleukin-10 Cytokine Detection. Electroanalysis 2020, 32, 1795–1806. [Google Scholar] [CrossRef]
- Longo, A.; Baraket, A.; Vatteroni, M.; Zine, N.; Baussells, J.; Fuoco, R.; Di Francesco, F.; Karanasiou, G.S.; Fotiadis, D.I.; Menciassi, A.; et al. Highly Sensitive Electrochemical BioMEMS for TNF-α Detection in Humansaliva: Heart Failure. Procedia Eng. 2016, 168, 97–100. [Google Scholar] [CrossRef]
- Bahri, M.; Baraket, A.; Zine, N.; Ben Ali, M.; Bausells, J.; Errachid, A. Capacitance Electrochemical Biosensor Based on Silicon Nitride Transducer for TNF-α Cytokine Detection in Artificial Human Saliva: Heart Failure (HF). Talanta 2020, 209, 120501. [Google Scholar] [CrossRef]
- Barhoumi, L.; Bellagambi, F.; Vivaldi, F.; Baraket, A.; Clément, Y.; Zine, N.; Ben Ali, M.; Elaissari, A.; Errachid, A. Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva Using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode. Sensors 2019, 19, 692. [Google Scholar] [CrossRef]





| Technique | Electrode | Immobilizing Biomolecules | Analyte | Linear Range | LOD | Reference |
|---|---|---|---|---|---|---|
| EIS | Substrate “HfO2” | mAb-IL-10 | PBS 1× | 0.1 pg/mL–50 ng/mL | - | [57] |
| EIS | Au | mAb-IL-10 | PBS 1× | 1–15 pg/mL | 0.3 pg/mL | [71] |
| EIS | Substrate “Si3N4” | mAb-IL-10 | PBS 1× | 0.1–50 pg/mL | 0.3 pg/mL | [72] |
| EIS | ISFET “Si3N4” | pAb-IL-10 | AS | 0.1–5 pg/mL | 0.03 pg/mL | This work |
| EIS | Au | mAb-TNF-α | AS | 1–15 pg/mL | 1 pg/mL | [73] |
| Mott–Schottky | Substrate “Si3N4” | mAb-TNF-α | AS | 1–30 pg/mL | 1 pg/mL | [74] |
| Amperometry | Au | mAb-TNF-α | AS | 1–15 pg/mL | 0.3 pg/mL | [75] |
| Electrical measurement | ISFET “Si3N4” | mAb-TNF-α | AS | 5–20 pg/mL | 5 pg/mL | [64] |
| EIS | ISFET “Si3N4” | pAb-TNF-α | AS | 0.1–5 pg/mL | 0.03 pg/mL | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Halima, H.; Zine, N.; Nemeir, I.A.; Pfeiffer, N.; Heuberger, A.; Bausells, J.; Elaissari, A.; Jaffrezic-Renault, N.; Errachid, A. An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers. Micromachines 2024, 15, 296. https://doi.org/10.3390/mi15030296
Ben Halima H, Zine N, Nemeir IA, Pfeiffer N, Heuberger A, Bausells J, Elaissari A, Jaffrezic-Renault N, Errachid A. An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers. Micromachines. 2024; 15(3):296. https://doi.org/10.3390/mi15030296
Chicago/Turabian StyleBen Halima, Hamdi, Nadia Zine, Imad Abrao Nemeir, Norman Pfeiffer, Albert Heuberger, Joan Bausells, Abdelhamid Elaissari, Nicole Jaffrezic-Renault, and Abdelhamid Errachid. 2024. "An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers" Micromachines 15, no. 3: 296. https://doi.org/10.3390/mi15030296
APA StyleBen Halima, H., Zine, N., Nemeir, I. A., Pfeiffer, N., Heuberger, A., Bausells, J., Elaissari, A., Jaffrezic-Renault, N., & Errachid, A. (2024). An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers. Micromachines, 15(3), 296. https://doi.org/10.3390/mi15030296









