Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia
Abstract
1. Introduction
2. Materials and Methods
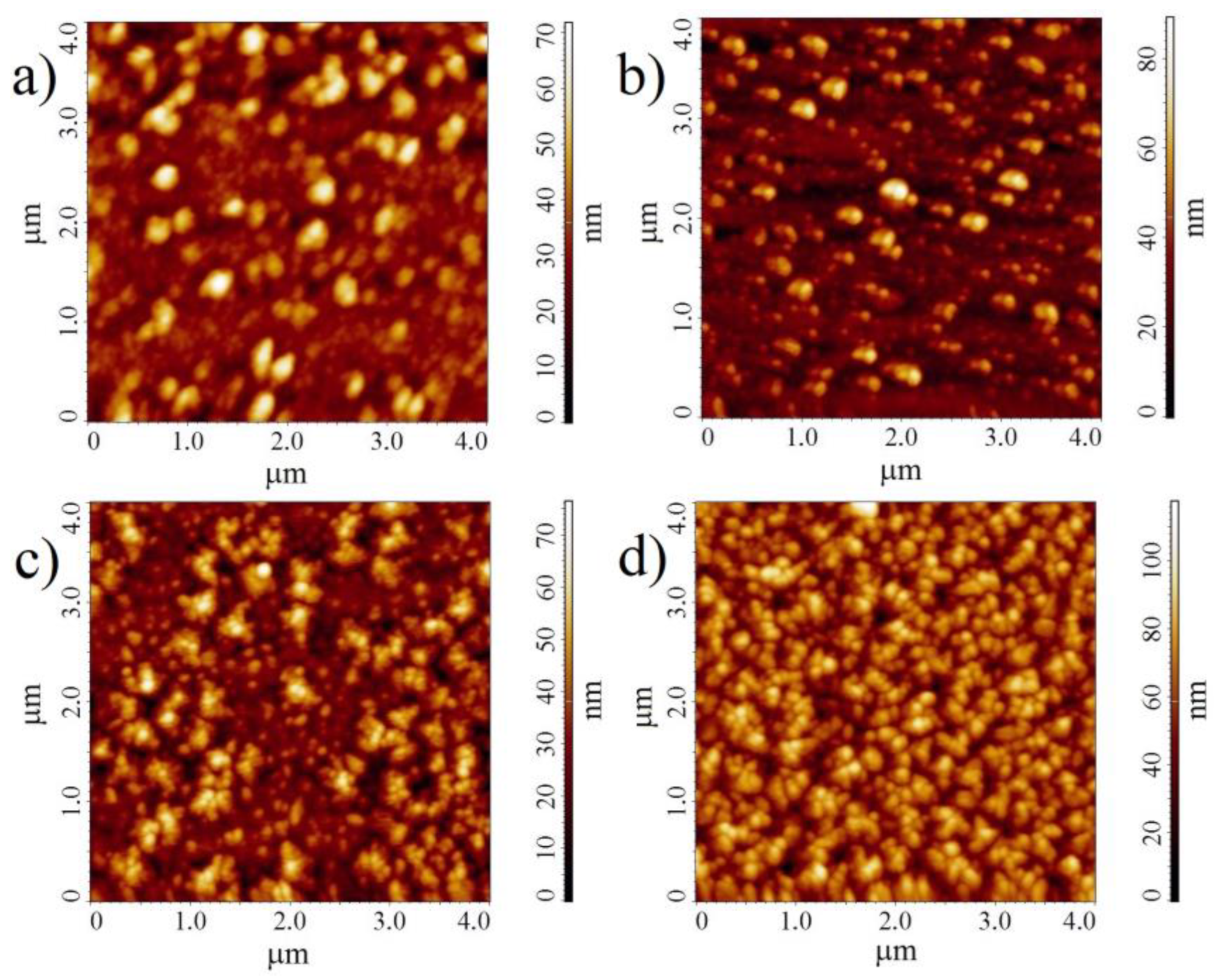
2.1. Preparation and Characterization of Thin Films
2.2. Measurements of the Sensor Response
2.3. Theoretical Calculations
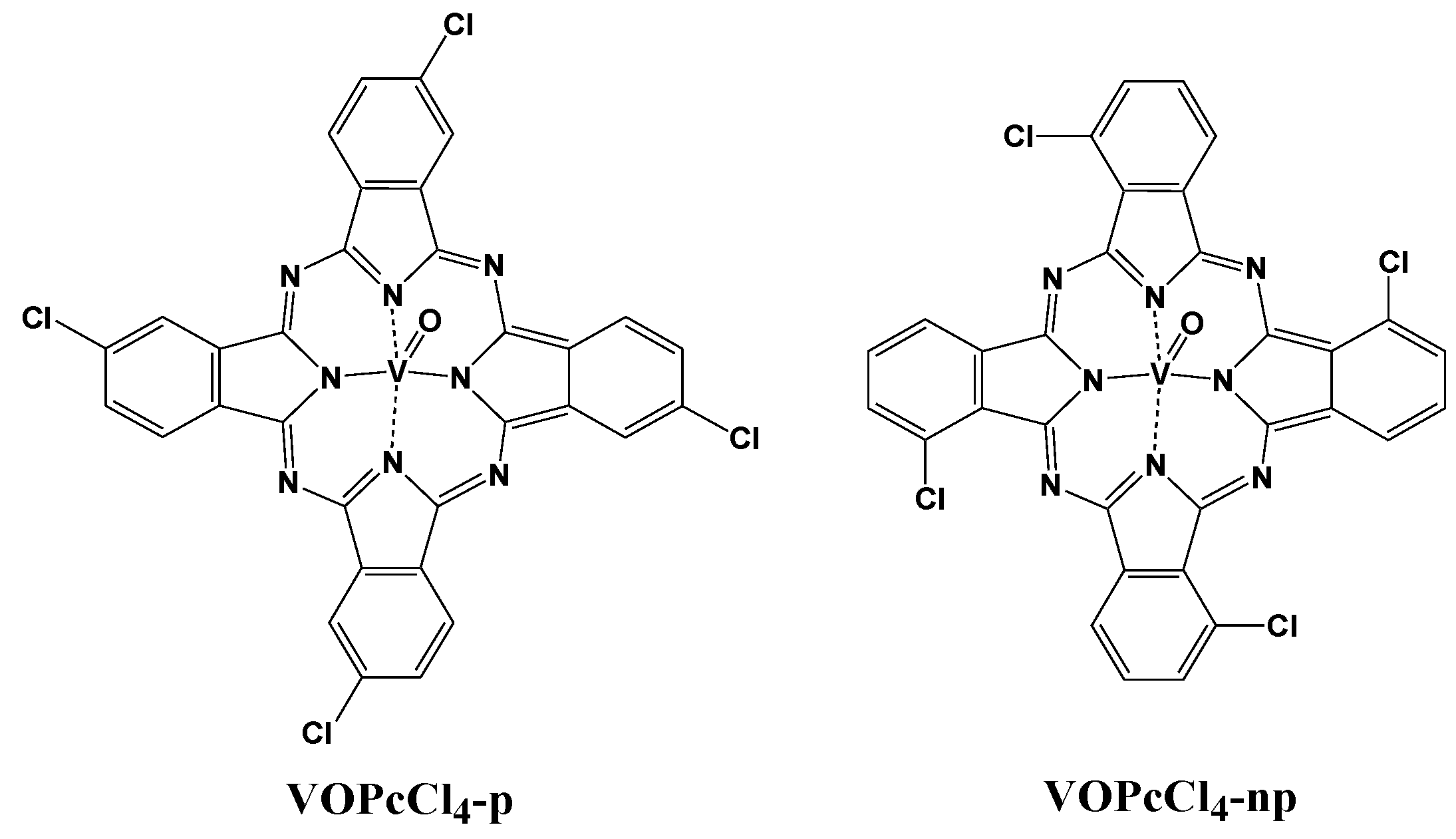
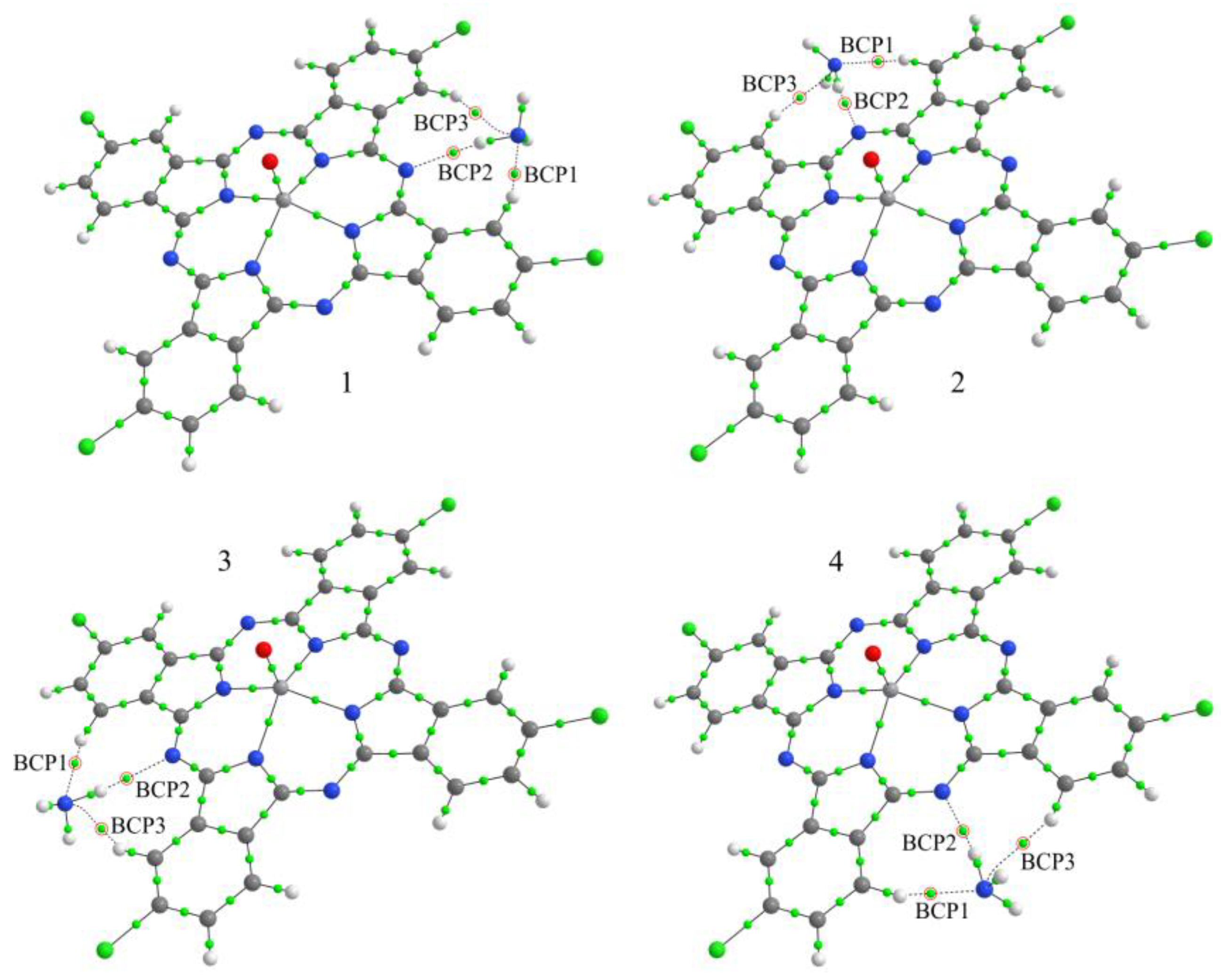
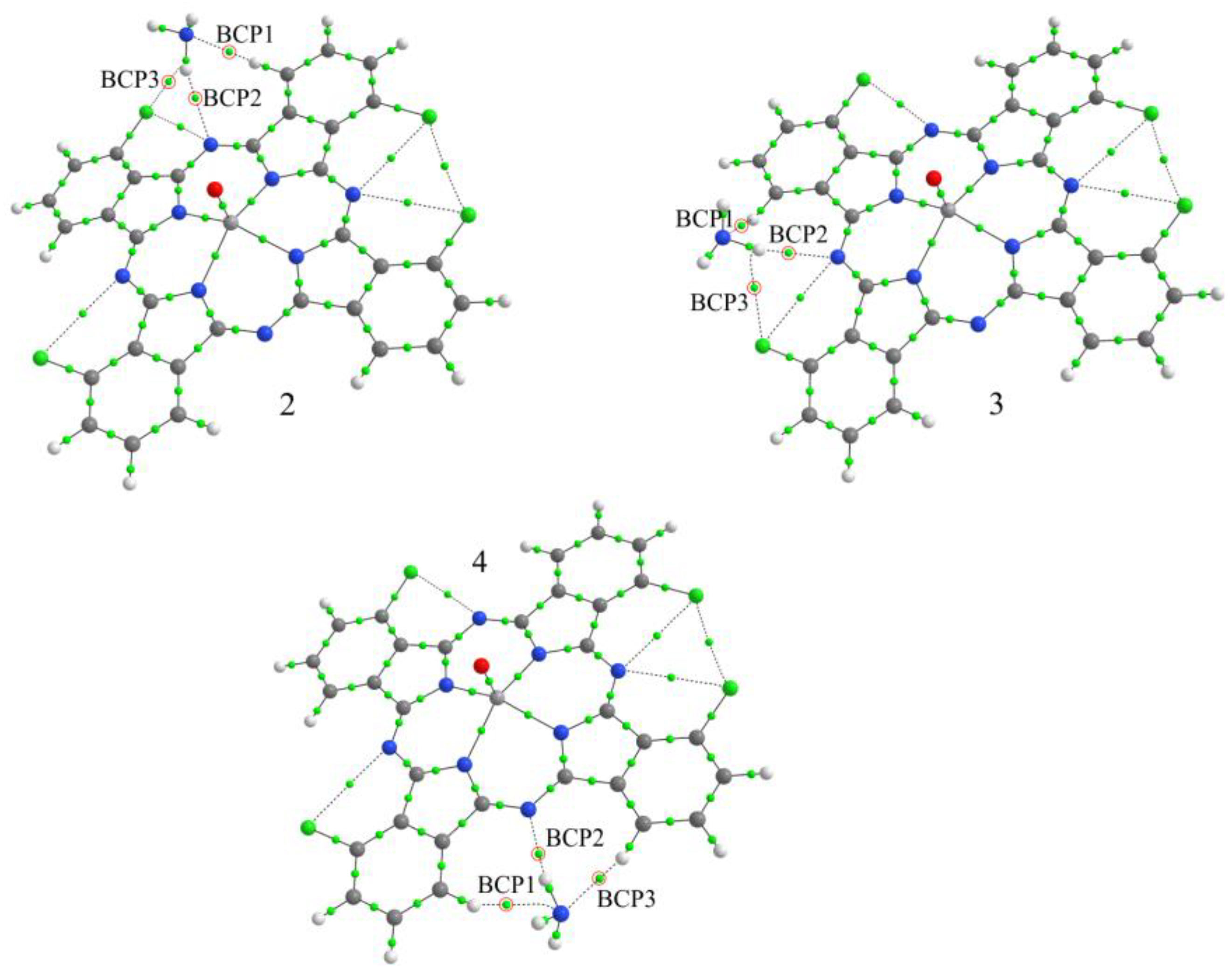
3. Results and Discussion
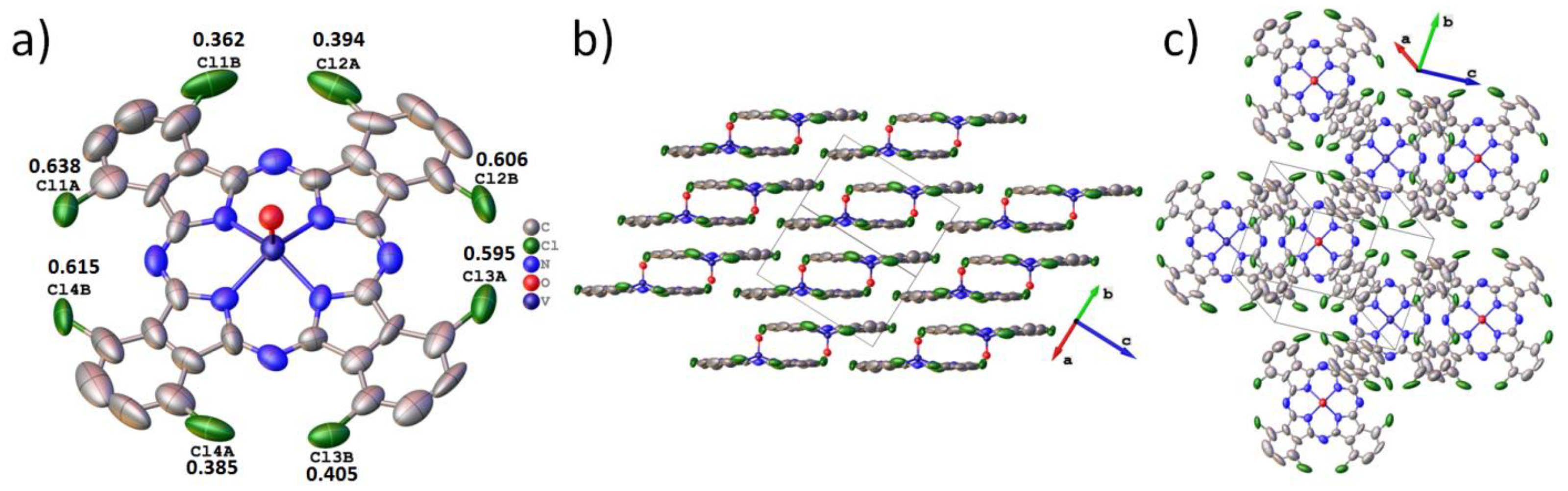
3.1. Crystal Structure of VOPcCl4-p and VOPcCl4-np
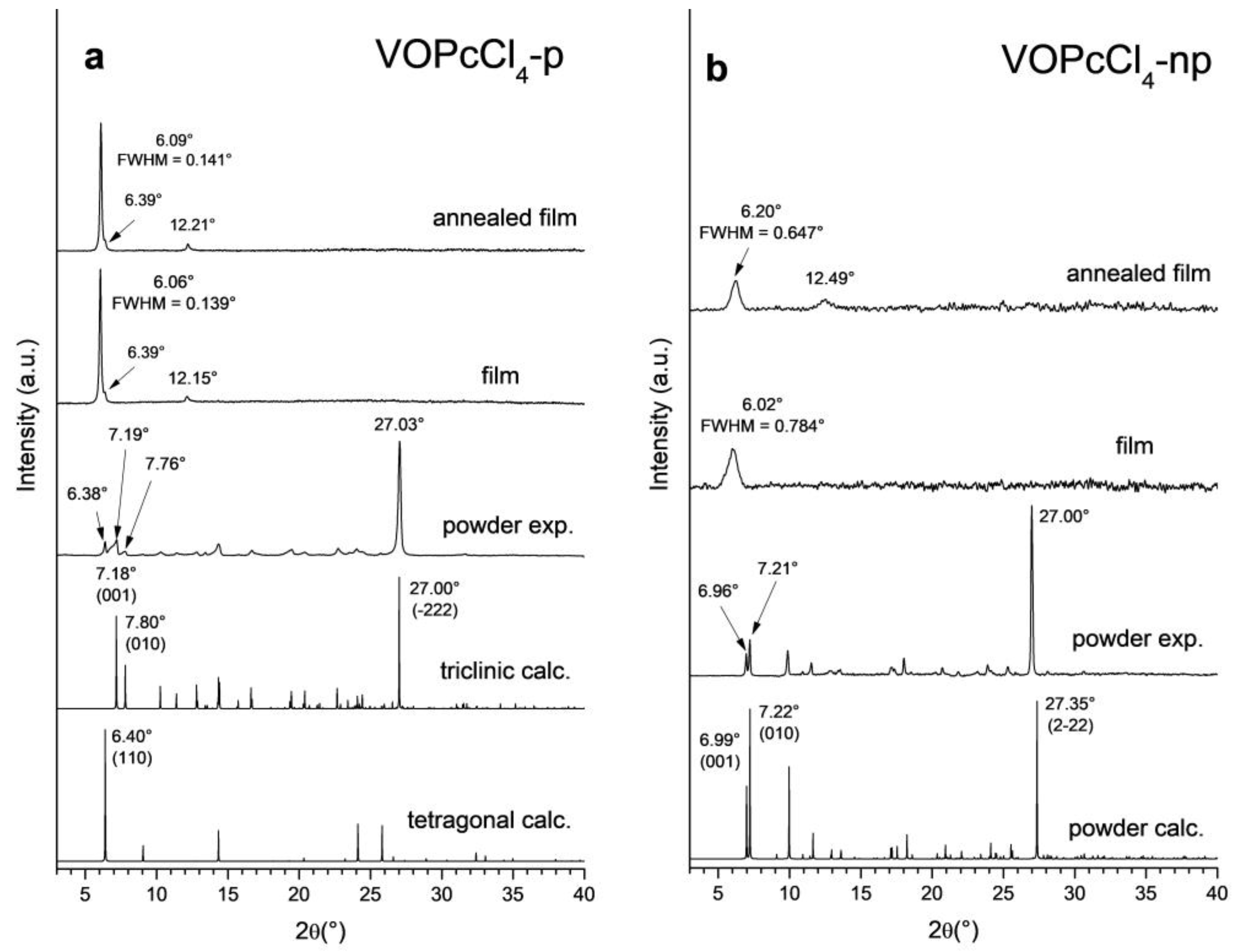
3.2. VOPcCl4-p and VOPcCl4-np Thin Films and Powder XRD Study
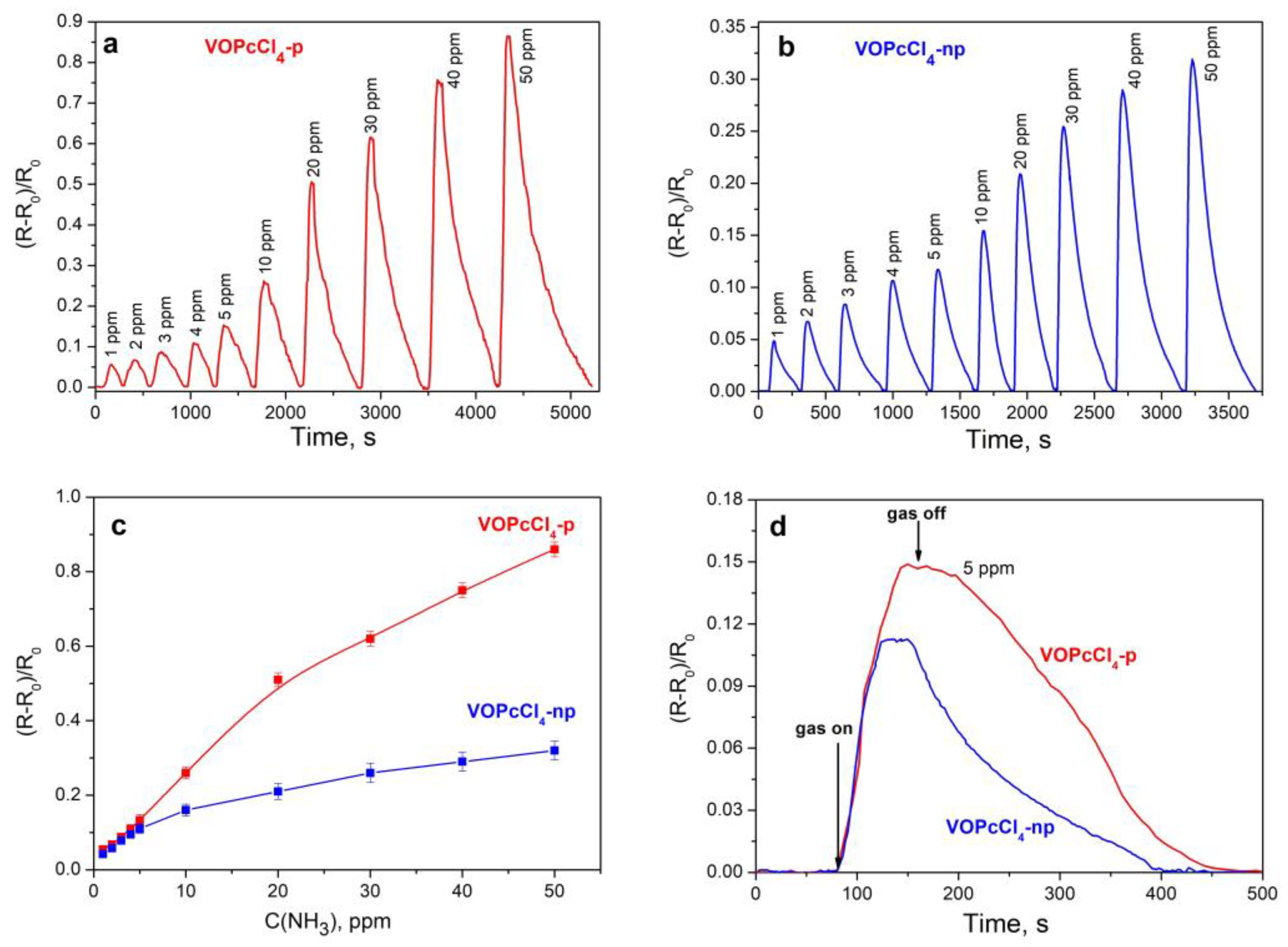
3.3. Sensor Properties of VOPcCl4-p and VOPcCl4-np Films
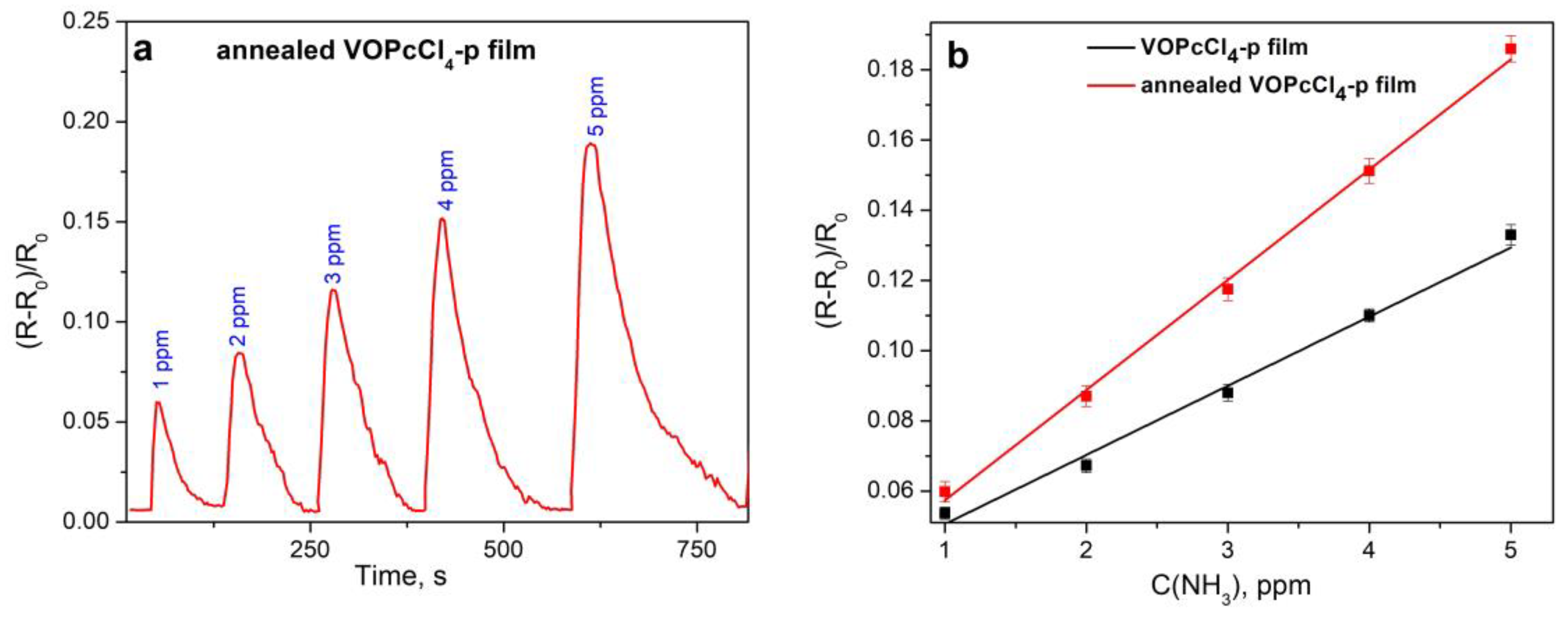
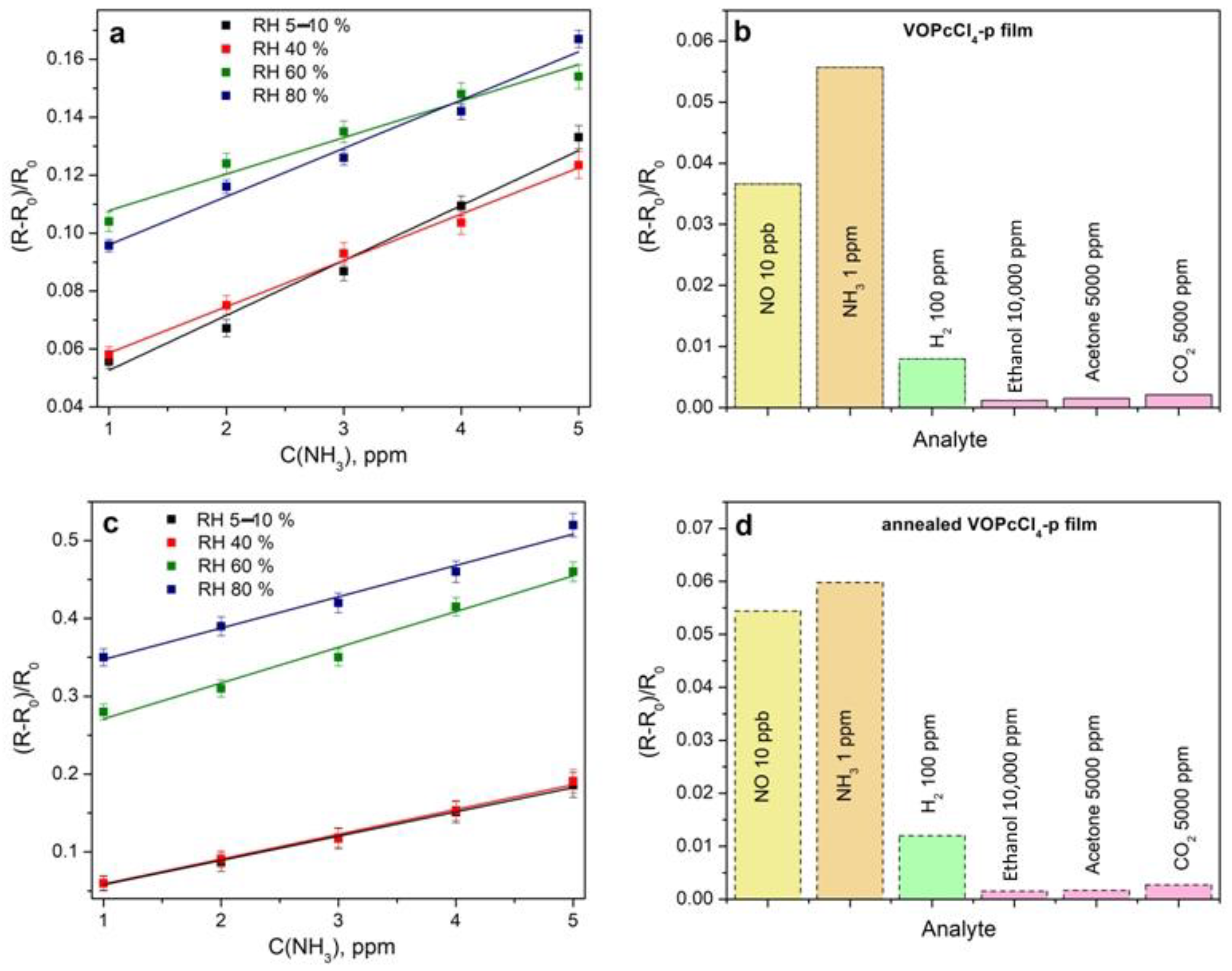
3.3.1. Comparison of the Sensor Response of VOPcCl4-p and VOPcCl4-np Films to Ammonia
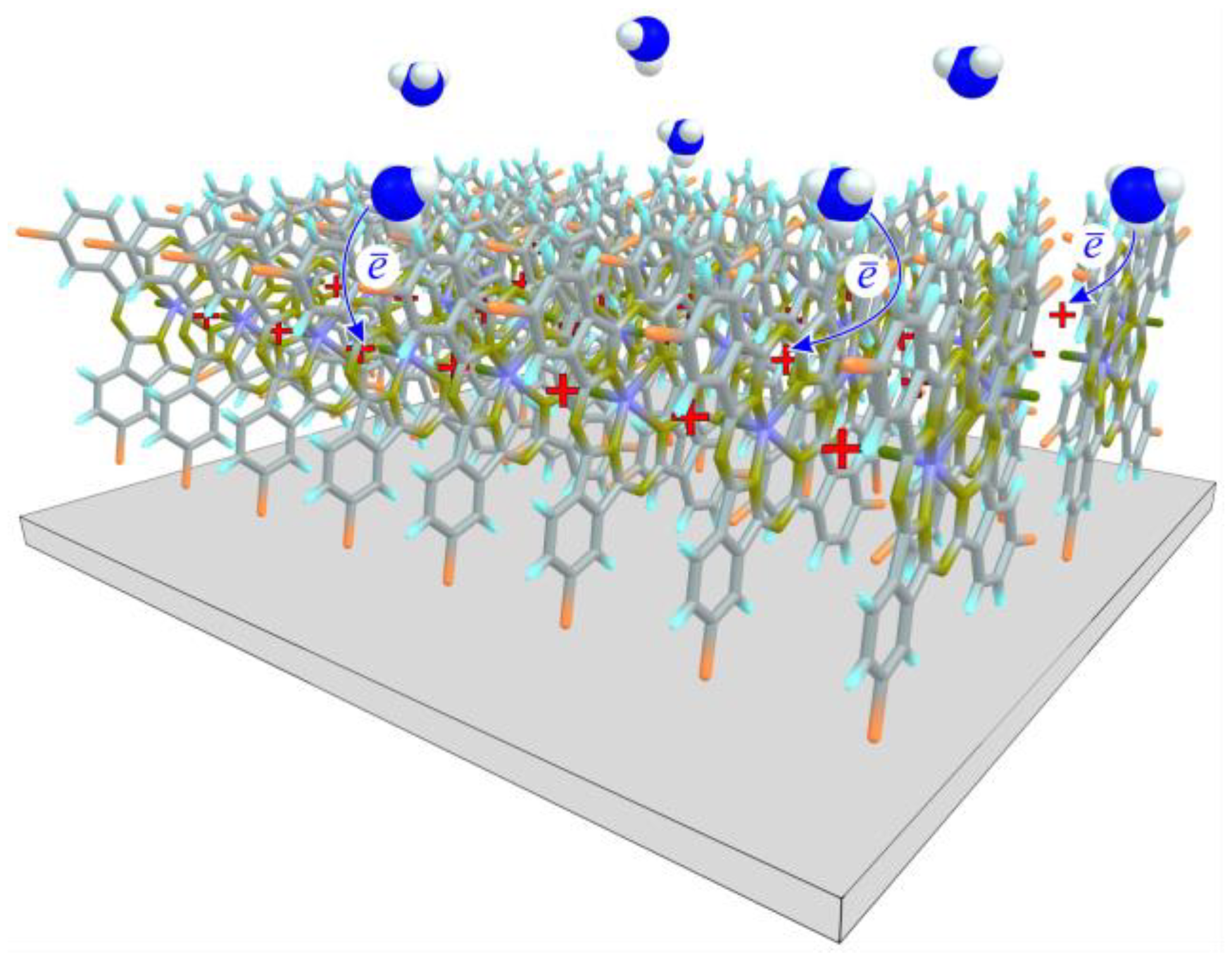
3.3.2. Study of the Nature of Interaction between NH3 and VOPcCl4 Molecules and Comparison of the Sensor Response of VOPcCl4 and VOPcF4 Films
3.3.3. Characteristics of the Sensor Based on VOPcCl4-p Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhari, U.; Jagtap, S. A panoramic view of NOx and NH3 gas sensors. Nano-Struct. Nano-Objects 2023, 35, 100995. [Google Scholar] [CrossRef]
- Close, L.G.; Catlin, F.I.; Cohn, A.M. Acute and chronic effects of ammonia burns of the respiratory tract. Arch. Otolaryngol. 1980, 106, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Freddi, S.; Marzuoli, C.; Pagliara, S.; Drera, G.; Sangaletti, L. Targeting biomarkers in the gas phase through a chemoresistive electronic nose based on graphene functionalized with metal phthalocyanines. RSC Adv. 2022, 13, 251–263. [Google Scholar] [CrossRef]
- Liu, L.; Fei, T.; Guan, X.; Zhao, H.; Zhang, T. Humidity-activated ammonia sensor with excellent selectivity for exhaled breath analysis. Sens. Actuators B Chem. 2021, 334, 129625. [Google Scholar] [CrossRef]
- Lefferts, M.J.; Castell, M.R. Ammonia breath analysis. Sens. Diagn. 2022, 1, 955–967. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J.; Kwak, H.J.; Kim, S.I.; Park, D.W.; Kim, J.W.; Kim, T.H.; Sohn, J.W.; Shin, D.H.; Park, S.S.; et al. Comparison of two exhaled nitric oxide analyzers: The NIOX MINO hand-held electrochemical analyzer and the NOA280i stationary chemiluminescence analyzer. Respirology 2012, 17, 830–834. [Google Scholar] [CrossRef]
- Pan, S.; Tian, Y.; Li, M.; Zhao, J.; Zhu, L.; Zhang, W.; Gu, H.; Wang, H.; Shi, J.; Fang, X.; et al. Quantitative detection of nitric oxide in exhaled human breath by extractive electrospray ionization mass spectrometry. Sci. Rep. 2015, 5, 8725. [Google Scholar] [CrossRef]
- Budde, W.L. Ron hites: Gas chromatography/mass spectrometry pioneer and the Great detector debate. Environ. Sci. Technol. 2015, 49, 13741–13742. [Google Scholar] [CrossRef][Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Uma, S.; Shobana, M.K. Metal oxide semiconductor gas sensors in clinical diagnosis and environmental monitoring. Sens. Actuators A Phys. 2023, 349, 114044. [Google Scholar] [CrossRef]
- Qin, R.; Shan, G.; Hu, M.; Huang, W. Two-dimensional transition metal carbides and/or nitrides (MXenes) and their applications in sensors. Mater. Today Phys. 2021, 21, 100527. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Zhu, X.; Jiang, Y.; Chang, X.; Sun, S. Two-dimensional transition metal MXene-based gas sensors: A review. Chin. Chem. Lett. 2023, 108286. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–34. [Google Scholar] [CrossRef]
- Şahin, Z.; Meunier-Prest, R.; Dumoulin, F.; Kumar, A.; Isci, Ü.; Bouvet, M. Tuning of organic heterojunction conductivity by the substituents’ electronic effects in phthalocyanines for ambipolar gas sensors. Sens. Actuators B Chem. 2021, 332, 129505. [Google Scholar] [CrossRef]
- Saini, R.; Kaur, R.; Devi, P.; Gasso, S.; Sharma, S. Tailoring the performance of phthalocyanine-based sensor: Side chain substitution and nanofabrication. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Kumar, A.; Samanta, S.; Joshi, N.; Balouria, V.; Debnath, A.K.; Prasad, R.; Salmi, Z.; Chehimi, M.M.; et al. Bending stress induced improved chemiresistive gas sensing characteristics of flexible cobalt-phthalocyanine thin films. Appl. Phys. Lett. 2013, 102, 132107. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Liu, C.J.; Peng, C.H.; Ju, Y.H.; Hsieh, J.C. Titanyl phthalocyanine gas sensor for NO2 detection. Sens. Actuators B Chem. 1998, 52, 264–269. [Google Scholar] [CrossRef]
- Elesh, E.; Mohammed, Z. Morphological, linear and nonlinear properties of gallium phthalocyanine chloride annealed thin films. Optik 2020, 219, 165176. [Google Scholar] [CrossRef]
- Ziolo, R.F.; Griffiths, C.H.; Troup, J.M. Crystal structure of vanadyl phthalocyanine, phase II. J. Chem. Soc. Dalt. Trans. 1980, 575, 2300–2302. [Google Scholar] [CrossRef]
- Handa, M.; Suzuki, A.; Shoji, S.; Kasuga, K.; Sogabe, K. Spectral and electrochemical properties of vanadyl hexadecafluorophthalocyanine. Inorganica Chim. Acta 1995, 230, 41–44. [Google Scholar] [CrossRef]
- Wang, H.; Song, D.; Yang, J.; Yu, B.; Geng, Y.; Yan, D. High mobility vanadyl-phthalocyanine polycrystalline films for organic field-effect transistors. Appl. Phys. Lett. 2007, 90, 253510. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Wang, H.; Yan, D. Room temperature nitrogen dioxide chemresistor using ultrathin vanadyl-phthalocyanine film as active layer. Sens. Actuators B Chem. 2011, 160, 115–120. [Google Scholar] [CrossRef]
- Aziz, F.; Sayyad, M.H.; Karimov, K.S.; Saleem, M.; Ahmad, Z.; Khan, S.M. Characterization of vanadyl phthalocyanine based surface-type capacitive humidity sensors. J. Semicond. 2010, 31, 114002. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abu Bakar, A.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Abdul Majid, W.H.; Supangat, A. Enhancing the performance of vanadyl phthalocyanine-based humidity sensor by varying the thickness. Sens. Actuators B Chem. 2019, 279, 148–156. [Google Scholar] [CrossRef]
- Ji, S.; Wang, H.; Wang, T.; Yan, D. A high-performance room-temperature NO2 sensor based on an ultrathin heterojunction film. Adv. Mater. 2013, 25, 1755–1760. [Google Scholar] [CrossRef]
- Schöllhorn, B.; Germain, J.P.; Pauly, A.; Maleysson, C.; Blanc, J.P. Influence of peripheral electron-withdrawing substituents on the conductivity of zinc phthalocyanine in the presence of gases. Part 1: Reducing gases. Thin Solid Film. 1998, 326, 245–250. [Google Scholar] [CrossRef]
- Bonegardt, D.; Klyamer, D.; Sukhikh, A.; Krasnov, P.; Popovetskiy, P.; Basova, T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors 2021, 9, 137. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl phthalocyanine films and their hybrid structures with Pd nanoparticles: Structure and sensing properties. Sensors 2020, 20, 1893. [Google Scholar] [CrossRef] [PubMed]
- Klyamer, D.; Bonegardt, D.; Krasnov, P.; Sukhikh, A.; Popovetskiy, P.; Basova, T. Tetrafluorosubstituted Metal Phthalocyanines: Study of the Effect of the Position of Fluorine Substituents on the Chemiresistive Sensor Response to Ammonia. Chemosensors 2022, 10, 515. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- APEX3, v.2019.1-0; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kuprikova, N.M.; Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Mrsic, I.; Basova, T.V. Fluorosubstituted lead phthalocyanines: Crystal structure, spectral and sensing properties. Dye. Pigment. 2020, 173, 107939. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Bonegardt, D.V.; Basova, T.V. Tetrachlorosubstituted Lead Phthalocyanines: Effect of the Positions of Substituents on the Structure of Single Crystals and thin Films. J. Struct. Chem. 2023, 64, 650–661. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Roy, V.A.L.; Yan, D.; Zhang, J.; Lee, C.S. Polymorphism and electronic properties of vanadyl-phthalocyanine films. Org. Electron. 2014, 15, 1586–1591. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Schlettwein, D. Energy Migratoin and Light-Induced Charge Transfer at Phthalocyanine Surfaces. In Handbook of Photochemistry and Photobiology, Vol. 3: Supramolecular Photochemistry; Nalwa, H.S., Ed.; American Scientific: Valencia, CA, USA, 2003; pp. 355–409. [Google Scholar]
- Yamashita, A.; Maruno, T.; Hayashi, T. Absorption spectra of organic-molecular-beam-deposited vanadyl- and titanylphthalocyanine. J. Phys. Chem. 2002, 97, 4567–4569. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Basova, T. Effect of non-peripheral fluorosubstitution on the structure of metal phthalocyanines and their films. Dye. Pigment. 2021, 192, 109442. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xi, Q.; Zhao, J. Fluorescent and triplet state photoactive J-type phthalocyanine nano assemblies: Controlled formation and photosensitizing properties. J. Mater. Chem. 2010, 20, 6726–6733. [Google Scholar] [CrossRef]
- Bushmarinov, I.S.; Lyssenko, K.A.; Antipin, M.Y. Atomic energy in the “Atoms in Molecules” theory and its use for solving chemical problems. Russ. Chem. Rev. 2009, 78, 283–302. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Film. 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Li, Y.; Pan, M.; Hu, Y.; Wang, Z.; Lv, W.; Peng, Y. The influence of substrate temperature on the near-infrared absorption and carrier mobility of lead phthalocyanine phototransistors. Thin Solid Film. 2021, 718, 138481. [Google Scholar] [CrossRef]
- Nénon, S.; Kanehira, D.; Yoshimoto, N.; Fages, F.; Videlot-Ackermann, C. Shelf-life time test of p- and n-channel organic thin film transistors using copper phthalocyanines. Thin Solid Film. 2010, 518, 5593–5598. [Google Scholar] [CrossRef]
- Wu, F.C.; Cheng, H.L.; Yen, C.H.; Lin, J.W.; Liu, S.J.; Chou, W.Y.; Tang, F.C. Electron transport properties in fluorinated copper-phthalocyanine films: Importance of vibrational reorganization energy and molecular microstructure. Phys. Chem. Chem. Phys. 2010, 12, 2098–2106. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Kaya, E.N.; Şenocak, A.; Klyamer, D.D.; Demirbaş, E.; Basova, T.V.; Durmuş, M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci. Mater. Electron. 2019, 8, 7543–7551. [Google Scholar] [CrossRef]
- Madhaiyan, G.; Sun, A.T.; Zan, H.W.; Meng, H.F.; Horng, S.F.; Chen, L.Y.; Hung, H.W. Solution-processed chloroaluminum phthalocyanine (Clalpc) ammonia gas sensor with vertical organic porous diodes. Sensors 2021, 21, 5783. [Google Scholar] [CrossRef]
- Ganesh Moorthy, S.; King, B.; Kumar, A.; Lesniewska, E.; Lessard, B.H.; Bouvet, M. Molecular Engineering of Silicon Phthalocyanine to Improve the Charge Transport and Ammonia Sensing Properties of Organic Heterojunction Gas Sensors. Adv. Sens. Res. 2023, 2, 2200030. [Google Scholar] [CrossRef]
- Trul, A.A.; Chekusova, V.P.; Polinskaya, M.S.; Kiselev, A.N.; Agina, E.V.; Ponomarenko, S.A. NH3 and H2S real-time detection in the humid air by two-layer Langmuir-Schaefer OFETs. Sens. Actuators B Chem. 2020, 321, 128609. [Google Scholar] [CrossRef]
- Gülmez, A.D.; Polyakov, M.S.; Volchek, V.V.; Kostakoğlu, S.T.; Esenpinar, A.A.; Basova, T.V.; Durmuş, M.; Gürek, A.G.; Ahsen, V.; Banimuslem, H.A.; et al. Tetrasubstituted copper phthalocyanines: Correlation between liquid crystalline properties, films alignment and sensing properties. Sens. Actuators B Chem. 2017, 241, 364–375. [Google Scholar] [CrossRef]
- Kumar, A.; Meunier-Prest, R.; Herbst, F.; Heintz, O.; Lesniewska, E.; Bouvet, M. Covalent grafting of aryls to modulate the electrical properties of phthalocyanine-based heterostructures: Application to ammonia sensing. Chem. Eng. J. 2022, 436, 135207. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Zhang, F.J.; Di, C.A.; Berdunov, N.; Hu, Y.; Hu, Y.; Gao, X.; Meng, Q.; Sirringhaus, H.; Zhu, D. Ultrathin film organic transistors: Precise control of semiconductor thickness via spin-coating. Adv. Mater. 2013, 25, 1401–1407. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, G.; Mohammadi, E.; Mei, J.; Diao, Y. Solution-Processed Nanoporous Organic Semiconductor Thin Films: Toward Health and Environmental Monitoring of Volatile Markers. Adv. Funct. Mater. 2017, 27, 1701117. [Google Scholar] [CrossRef]
- Li, L.; Gao, P.; Baumgarten, M.; Müllen, K.; Lu, N.; Fuchs, H.; Chi, L. High performance field-effect ammonia sensors based on a structured ultrathin organic semiconductor film. Adv. Mater. 2013, 25, 3419–3425. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Du, X.; Chu, Y.; Zhang, S.; Huang, J. Gas Sensors Based on Nano/Microstructured Organic Field-Effect Transistors. Small 2019, 15, 1805196. [Google Scholar] [CrossRef]
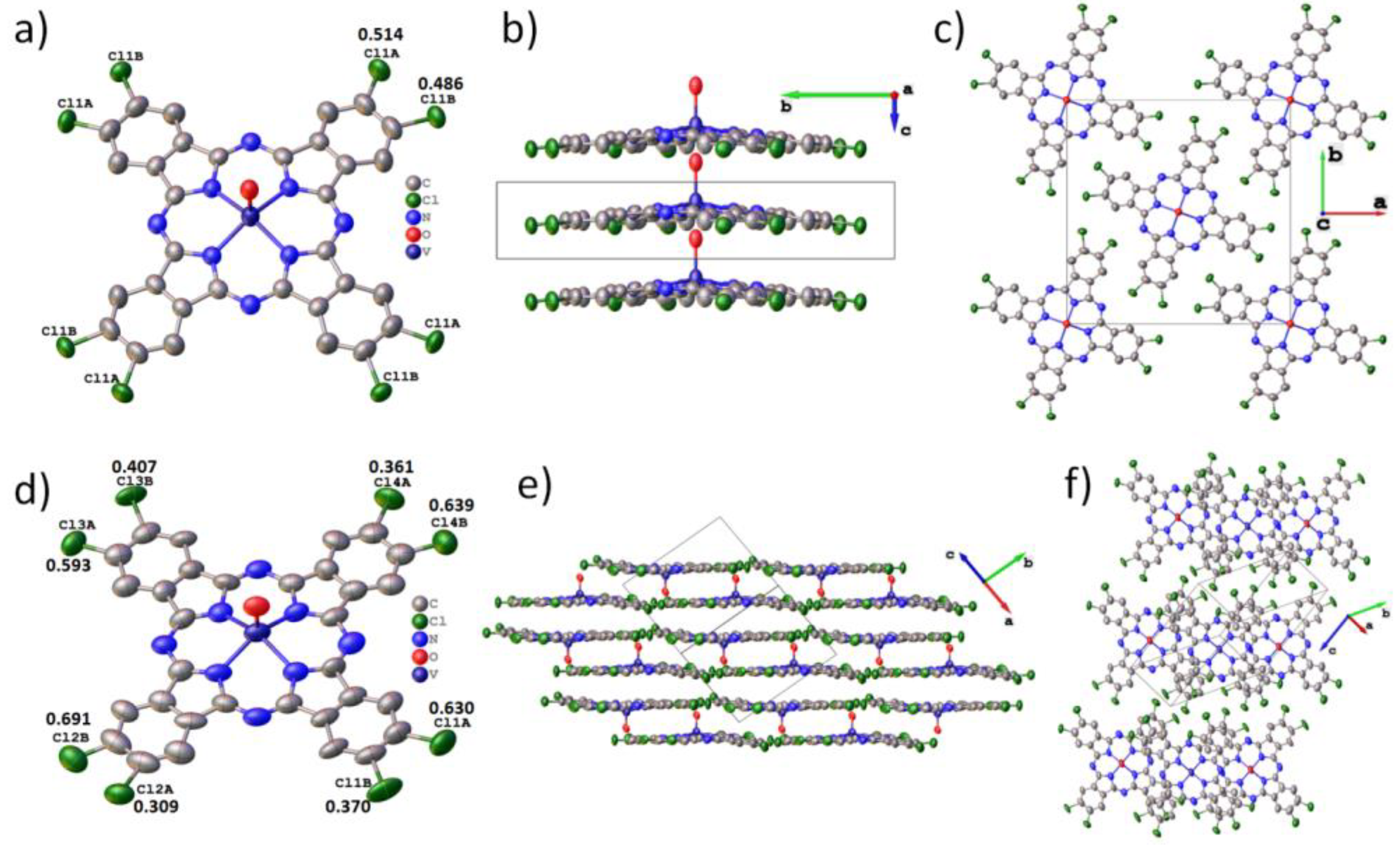

| Compound | VOPcCl4-p (Tetragonal) | VOPcCl4-p (Triclinic) | VOPcCl4-np |
|---|---|---|---|
| Empiric formula | C32H12Cl4N8OV | C32H12Cl4N8OV | C32H12Cl4N8OV |
| Formula weight | 717.24 | 717.24 | 717.24 |
| Temperature/K | 150 | 150 | 150 |
| Crystal system | Tetragonal | Triclinic | Triclinic |
| Space group | I4/m | P-1 | P-1 |
| a/Å | 19.5370(10) | 9.139(4) | 8.9851(16) |
| b/Å | 19.5370(10) | 12.716(6) | 12.505(2) |
| c/Å | 3.7577(2) | 14.291(6) | 12.937(2) |
| α/° | 90 | 114.719(10) | 99.740(5) |
| β/° | 90 | 106.252(11) | 96.323(5) |
| γ/° | 90 | 94.796(11) | 95.171(6) |
| Volume/Å3 | 1434.29(17) | 1409.5(11) | 1415.1(4) |
| Z | 2 | 2 | 2 |
| ρcalc g/cm3 | 1.661 | 1.690 | 1.683 |
| μ/mm−1 | 0.764 | 0.777 | 0.774 |
| F(000) | 718.0 | 718.0 | 718.0 |
| Crystal size/mm3 | 0.13 × 0.03 × 0.005 | 0.14 × 0.02 × 0.02 | 0.03 × 0.03 × 0.01 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.17 to 51.29 | 4.78 to 46.874 | 4.59 to 46.536 |
| Index ranges | −21 ≤ h ≤ 23, −23 ≤ k ≤ 23, −4 ≤ l ≤ 4 | −10 ≤ h ≤ 10, −13 ≤ k ≤ 14, −15 ≤ l ≤ 15 | −9 ≤ h ≤ 9, −13 ≤ k ≤ 13, −13 ≤ l ≤ 14 |
| Reflections collected | 7682 | 13,062 | 13,781 |
| Independent reflections | 791 [Rint = 0.0506, Rsigma = 0.0263] | 4063 [Rint = 0.1507, Rsigma = 0.1820] | 4054 [Rint = 0.1984, Rsigma = 0.2193] |
| Data/restraints/parameters | 791/2/107 | 4063/0/455 | 4054/12/431 |
| Goodness-of-fit on F2 | 1.055 | 0.968 | 0.954 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0600, wR2 = 0.1659 | R1 = 0.0881, wR2 = 0.2129 | R1 = 0.0918, wR2 = 0.2038 |
| Final R indexes [all data] | R1 = 0.0833, wR2 = 0.1868 | R1 = 0.2140, wR2 = 0.2839 | R1 = 0.2459, wR2 = 0.2858 |
| Largest diff. peak/hole/e Å−3 | 0.76/−0.30 | 0.53/−0.41 | 1.16/−0.33 |
| CCDC № | 2267526 | 2267527 | 2267528 |
| Compound | Eb, eV | BCP | Atoms * | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 |
|---|---|---|---|---|---|
| VOPcCl4-p/NH3-1 | 0.174 | 1 | H-N | 0.147 | 1.559 |
| 2 | N-H | 0.139 | 1.365 | ||
| 3 | H-N | 0.094 | 1.011 | ||
| VOPcCl4-p/NH3-2 | 0.180 | 1 | H-N | 0.137 | 1.489 |
| 2 | N-H | 0.134 | 1.323 | ||
| 3 | H-N | 0.094 | 1.015 | ||
| VOPcCl4-p/NH3-3 | 0.180 | 1 | H-N | 0.137 | 1.491 |
| 2 | N-H | 0.134 | 1.326 | ||
| 3 | H-N | 0.094 | 1.016 | ||
| VOPcCl4-p/NH3-4 | 0.171 | 1 | H-N | 0.139 | 1.511 |
| 2 | N-H | 0.136 | 1.337 | ||
| 3 | H-N | 0.091 | 0.974 | ||
| VOPcCl4-np/NH3-2 | 0.149 | 1 | H-N | 0.105 | 1.259 |
| 2 | N-H | 0.102 | 1.105 | ||
| 3 | Cl-H | 0.060 | 0.789 | ||
| VOPcCl4-np/NH3-3 | 0.153 | 1 | H-N | 0.105 | 1.261 |
| 2 | N-H | 0.101 | 1.104 | ||
| 3 | Cl-H | 0.060 | 0.793 | ||
| VOPcCl4-np/NH3-4 | 0.153 | 1 | H-N | 0.101 | 1.090 |
| 2 | N-H | 0.122 | 1.228 | ||
| 3 | H-N | 0.143 | 1.566 |
| Layers | Sensor Response, % * | LOD, ppb | Linear Range, ppm | Response Time, s | Recovery Time, s | Refs. |
|---|---|---|---|---|---|---|
| VOPcF4-p |
14 (5 ppm) | 40 | 1–10 | 48 (at 5 ppm) | 270 (at 5 ppm) | [32] |
| CoPcF4-p |
41 (5 ppm) | 10 | 1–10 | 55 | 215 | [32] |
| ClAlPc | 11.5 (1 ppm) | 100 | 0.1–1 | 60 (fixed) | n/a | [52] |
| Cl2SiPc/LuPc2 | 13 (10 ppm) | 100 | 10–90 | <60 (at 90 ppm) | <420 (at 90 ppm) | [53] |
| TiOTPP ** |
25 ± 2 (1 ppm) | 50 | 0–0.75 | 90 | 1200 | [54] |
| Tetrakis(n-octylthio) phthalocyaninato copper(II) |
~20 (10 ppm) | n/a | 10–50 | 50 (at 30 ppm) | 30 (at 30 ppm) | [55] |
| p-isopropylbenzene/LuPc2 |
~20 (10 ppm) | n/a | 10–90 | 60 (fixed) | 240 (fixed) | [56] |
| CoPcR8 *** | 2.8 (5 ppm) | 30 | 0.3–50 | 20 (at 5 ppm) | 40 (at 5 ppm) | [51] |
| ZnPcF4-p | 16 (5 ppm) | 10 | 0.1–50 | 45 (at 1 ppm) | 210 (at 1 ppm) | [30] |
| ZnPcCl4-p | 18 (5 ppm) | 10 | 0.1–50 | 45 (1 ppm) | 260 (1 ppm) | |
| VOPcCl4-p | 15 (5 ppm) | 70 | 1–10 | 60 (at 5 ppm) | 200 (at 5 ppm) | This work |
| Annealed VOPcCl4-p | 52 (5 ppm) | 30 | 1–10 | 50 (at 5 ppm) | 150 (at 5 ppm) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyamer, D.; Sukhikh, A.; Bonegardt, D.; Krasnov, P.; Popovetskiy, P.; Basova, T. Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia. Micromachines 2023, 14, 1773. https://doi.org/10.3390/mi14091773
Klyamer D, Sukhikh A, Bonegardt D, Krasnov P, Popovetskiy P, Basova T. Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia. Micromachines. 2023; 14(9):1773. https://doi.org/10.3390/mi14091773
Chicago/Turabian StyleKlyamer, Darya, Alexandr Sukhikh, Dmitry Bonegardt, Pavel Krasnov, Pavel Popovetskiy, and Tamara Basova. 2023. "Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia" Micromachines 14, no. 9: 1773. https://doi.org/10.3390/mi14091773
APA StyleKlyamer, D., Sukhikh, A., Bonegardt, D., Krasnov, P., Popovetskiy, P., & Basova, T. (2023). Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia. Micromachines, 14(9), 1773. https://doi.org/10.3390/mi14091773







