Advancements in SARS-CoV-2 Testing: Enhancing Accessibility through Machine Learning-Enhanced Biosensors
Abstract
1. Introduction
2. The Current State of SARS-CoV-2 Testing
2.1. Overview of Current Invasive Testing Methods
2.2. Limitations of Invasive Testing Methods
2.3. Overview of Current Non-Invasive Testing Methods
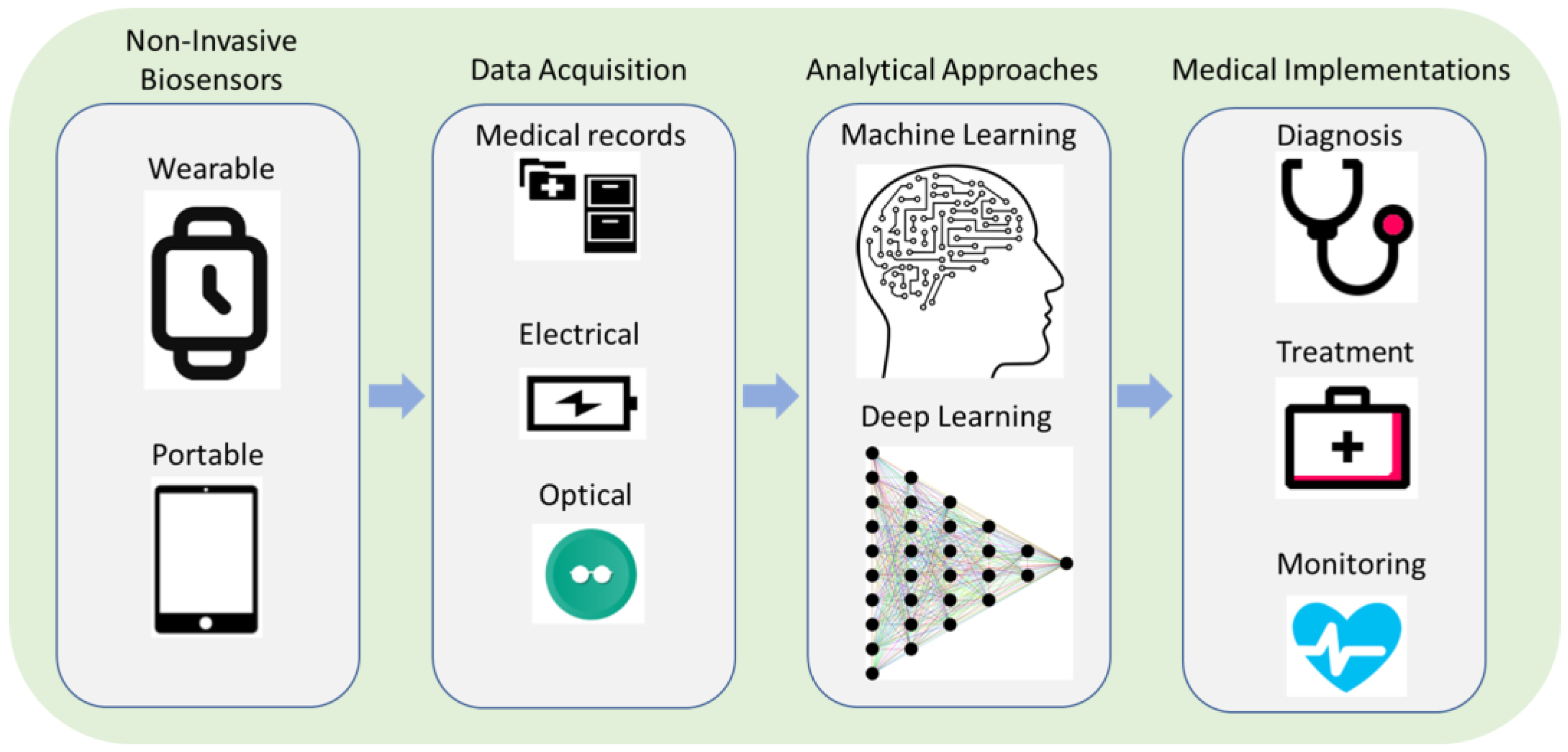
3. Machine Learning-Enhanced Biosensors for Non-Invasive Sampling
3.1. Machine Learning-Enhanced Biosensors
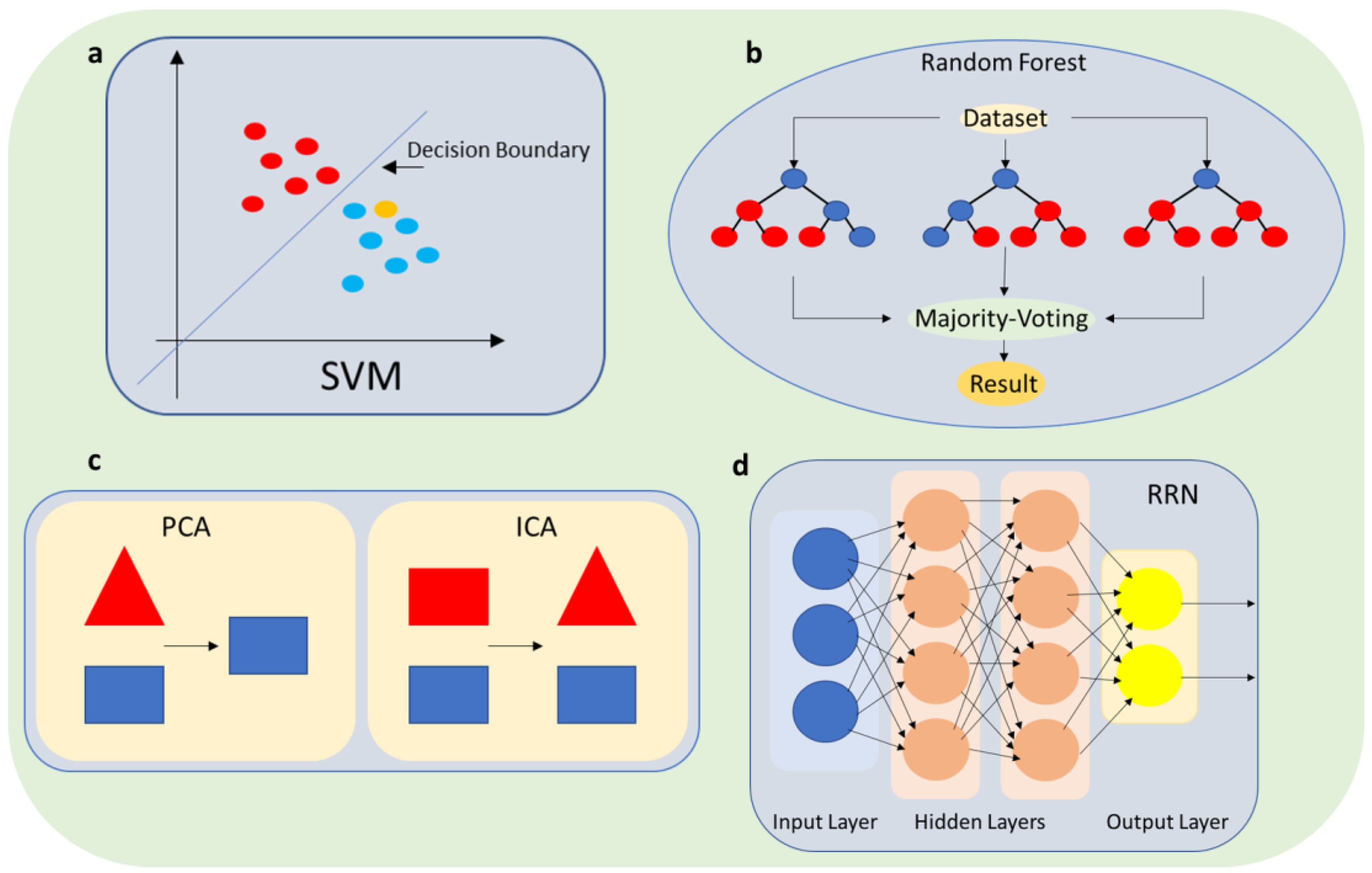
3.2. Pattern Recognition and Error Detection
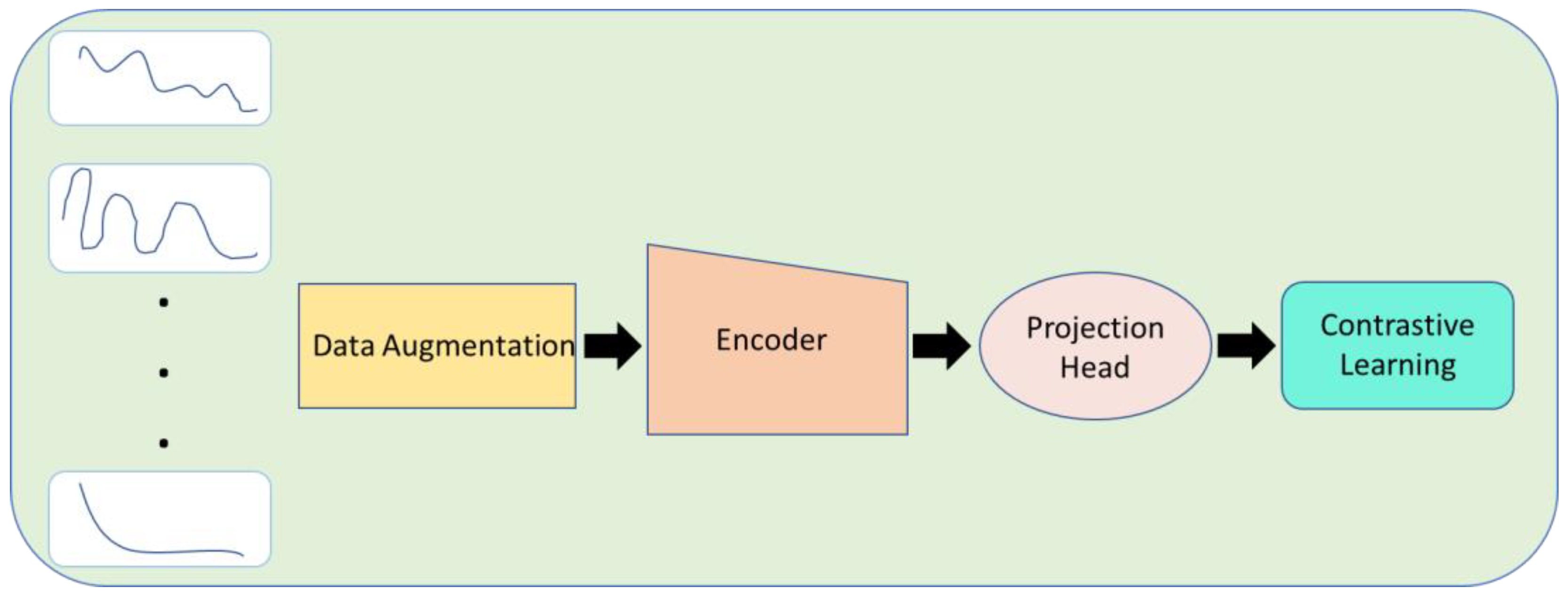
3.3. Contrastive Learning
3.4. Real-Time Interpretation of Biosensor Measurements with Machine Learning
4. Applications of Machine Learning-Enhanced Biosensors for SARS-CoV-2 Testing
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.; Ko, A.I.; Malik, A.A.; Wang, D.; Wang, M. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Pondaven-Letourmy, S.; Alvin, F.; Boumghit, Y.; Simon, F. How to perform a nasopharyngeal swab in adults and children in the COVID-19 era. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Gautier, N.; Godet, T.; Parabère, S.; Pereira, B.; Meunier, E.; Gerbaud, L.; Lesens, O.; Henquell, C.; Beytout, J. Nasopharyngeal swab-induced pain for SARS-CoV-2 screening: A randomised controlled trial of conventional and self-swabbing. Eur. J. Pain 2021, 25, 924–929. [Google Scholar] [CrossRef]
- Cismaru, C.A.; Chira, S.; Cismaru, G.L.; Nutu, A.M.; Netea, M.G.; Berindan-Neagoe, I. Assessment of the frequency of coughing and sneezing triggered by nasopharyngeal swabbing in the pandemic setting. Sci. Rep. 2022, 12, 10874. [Google Scholar]
- Nacher, M.; Mergeay-Fabre, M.; Blanchet, D.; Benois, O.; Pozl, T.; Mesphoule, P.; Sainte-Rose, V.; Vialette, V.; Toulet, B.; Moua, A. Diagnostic accuracy and acceptability of molecular diagnosis of COVID-19 on saliva samples relative to nasopharyngeal swabs in tropical hospital and extra-hospital contexts: The COVISAL study. PLoS ONE 2021, 16, e0257169. [Google Scholar] [CrossRef] [PubMed]
- Staffing, O.Y. Hospital medicine management in the time of COVID-19: Preparing for a sprint and a marathon. J. Hosp. Med. 2020, 15, 305. [Google Scholar]
- Khalid, A.; Ali, S. COVID-19 and its Challenges for the Healthcare System in Pakistan. Asian Bioeth. Rev. 2020, 12, 551–564. [Google Scholar] [CrossRef]
- Herrera, L.A.; Hidalgo-Miranda, A.; Reynoso-Noverón, N.; Meneses-García, A.A.; Mendoza-Vargas, A.; Reyes-Grajeda, J.P.; Vadillo-Ortega, F.; Cedro-Tanda, A.; Peñaloza, F.; Frías-Jimenez, E. Saliva is a reliable and accessible source for the detection of SARS-CoV-2. Int. J. Infect. Dis. 2021, 105, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tobik, E.R.; Kitfield-Vernon, L.B.; Thomas, R.J.; Steel, S.A.; Tan, S.H.; Allicock, O.M.; Choate, B.L.; Akbarzada, S.; Wyllie, A.L. Saliva as a sample type for SARS-CoV-2 detection: Implementation successes and opportunities around the globe. Expert Rev. Mol. Diagn. 2022, 22, 519–535. [Google Scholar] [CrossRef]
- Majam, M.; Msolomba, V.; Scott, L.; Stevens, W.; Marange, F.; Kahamba, T.; Venter, F.; Conserve, D.F. Self-sampling for SARS-CoV-2 diagnostic testing by using nasal and saliva specimens: Protocol for usability and clinical evaluation. JMIR Res. Protoc. 2021, 10, e24811. [Google Scholar]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial intelligence (AI) and internet of medical things (IoMT) assisted biomedical systems for intelligent healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 Sensing at Point-of-Care and Artificial Intelligence for Intelligent COVID-19 Management. ACS Appl. Bio Mater. 2020, 3, 7306–7325. [Google Scholar] [CrossRef]
- Sadak, O.; Sadak, F.; Yildirim, O.; Iverson, N.; Qureshi, R.; Talo, M.; Ooi, C.P.; Acharya, U.R.; Gunasekaran, S.; Alam, T. Electrochemical Biosensing and Deep Learning-based Approaches in the Diagnosis of COVID-19: A Review. IEEE Access 2022, 10, 98633–98648. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Liu, T.; Luo, Y.; Loh, X.J.; Chen, X. Machine Learning-Reinforced Noninvasive Biosensors for Healthcare. Adv. Healthc. Mater. 2021, 10, 2100734. [Google Scholar] [CrossRef]
- Abduljalil, J.M. Laboratory diagnosis of SARS-CoV-2: Available approaches and limitations. New Microbes New Infect. 2020, 36, 100713. [Google Scholar] [CrossRef]
- Maia, R.; Carvalho, V.; Faria, B.; Miranda, I.; Catarino, S.; Teixeira, S.; Lima, R.; Minas, G.; Ribeiro, J. Diagnosis Methods for COVID-19: A Systematic Review. Micromachines 2022, 13, 1349. [Google Scholar] [CrossRef]
- Flower, B.; Brown, J.C.; Simmons, B.; Moshe, M.; Frise, R.; Penn, R.; Kugathasan, R.; Petersen, C.; Daunt, A.; Ashby, D. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax 2020, 75, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Raffle, A.E. Lateral flow tests cannot rule out SARS-CoV-2 infection. BMJ 2020, 371, m4787. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Theel, E.S.; Slev, P.; Wheeler, S.; Couturier, M.R.; Wong, S.J.; Kadkhoda, K. The role of antibody testing for SARS-CoV-2: Is there one? J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Reliability of SARS-CoV-2 serological testing for influencing public health policies: A reappraisal. Eur. J. Intern. Med. 2023, 108, 102–103. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef]
- Vásquez, V.; Orozco, J. Detection of COVID-19-related biomarkers by electrochemical biosensors and potential for diagnosis, prognosis, and prediction of the course of the disease in the context of personalized medicine. Anal. Bioanal. Chem. 2023, 415, 1003–1031. [Google Scholar] [PubMed]
- Imran, S.; Ahmadi, S.; Kerman, K. Electrochemical biosensors for the detection of SARS-CoV-2 and other viruses. Micromachines 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R. Development of point-of-care biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Sohan, A.S.M.M.F.; Lin, X. Microfluidics-Based POCT for SARS-CoV-2 Diagnostics. Micromachines 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Sippy, R.; Sacoto, F. Assessing critical gaps in COVID-19 testing capacity: The case of delayed results in Ecuador. BMC Public Health 2021, 21, 637. [Google Scholar] [CrossRef] [PubMed]
- Savela, E.S.; Viloria Winnett, A.; Romano, A.E.; Porter, M.K.; Shelby, N.; Akana, R.; Ji, J.; Cooper, M.M.; Schlenker, N.W.; Reyes, J.A. Quantitative SARS-CoV-2 viral-load curves in paired saliva samples and nasal swabs inform appropriate respiratory sampling site and analytical test sensitivity required for earliest viral detection. J. Clin. Microbiol. 2022, 60, e01785-21. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.; Ditelberg, S.; Dutta, S.; Littlehale, N.; Cheng, A.; Kupczewski, K.; McVay, D.; Riedel, S.; Kirby, J.E.; Arnaout, R. Saliva is comparable to nasopharyngeal swabs for molecular detection of SARS-CoV-2. Microbiol. Spectr. 2021, 9, e00162-21. [Google Scholar] [CrossRef]
- Masson, J.-F. Consideration of sample matrix effects and “biological” noise in optimizing the limit of detection of biosensors. ACS Sens. 2020, 5, 3290–3292. [Google Scholar] [CrossRef] [PubMed]
- Hassibi, A.; Vikalo, H.; Hajimiri, A. On noise processes and limits of performance in biosensors. J. Appl. Phys. 2007, 102, 14909. [Google Scholar] [CrossRef]
- Kuswandi, B.; Ensafi, A.A. Perspective—Paper-based biosensors: Trending topic in clinical diagnostics developments and commercialization. J. Electrochem. Soc. 2019, 167, 37509. [Google Scholar]
- Schackart, K.E., III; Yoon, J.-Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors 2021, 21, 5519. [Google Scholar]
- Rong, Y.; Padron, A.V.; Hagerty, K.J.; Nelson, N.; Chi, S.; Keyhani, N.O.; Katz, J.; Datta, S.P.A.; Gomes, C.; McLamore, E.S. Post hoc support vector machine learning for impedimetric biosensors based on weak protein–ligand interactions. Analyst 2018, 143, 2066–2075. [Google Scholar] [CrossRef]
- Kim, H.; Seong, W.; Rha, E.; Lee, H.; Kim, S.K.; Kwon, K.K.; Park, K.-H.; Lee, D.-H.; Lee, S.-G. Machine learning linked evolutionary biosensor array for highly sensitive and specific molecular identification. Biosens. Bioelectron. 2020, 170, 112670. [Google Scholar] [CrossRef]
- Fortunati, S.; Giliberti, C.; Giannetto, M.; Bolchi, A.; Ferrari, D.; Donofrio, G.; Bianchi, V.; Boni, A.; De Munari, I.; Careri, M. Rapid Quantification of SARS-CoV-2 Spike Protein Enhanced with a Machine Learning Technique Integrated in a Smart and Portable Immunosensor. Biosensors 2022, 12, 426. [Google Scholar] [CrossRef]
- Gecgel, O.; Ramanujam, A.; Botte, G.G. Selective Electrochemical Detection of SARS-CoV-2 Using Deep Learning. Viruses 2022, 14, 1930. [Google Scholar] [CrossRef]
- Rosandi, V.A.; Linda, T.M.; Agustirandi, B.; Umar, L. Simple Amperometric Biosensor for Sucrose Concentration Measurement Based on Principal Component Analysis. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 2049, p. 12048. [Google Scholar]
- Ertl, P.; Mikkelsen, S.R. Electrochemical biosensor array for the identification of microorganisms based on lectin− lipopolysaccharide recognition. Anal. Chem. 2001, 73, 4241–4248. [Google Scholar] [CrossRef]
- Naik, G.R.; Guo, Y.; Nguyen, H. A new approach to improve the quality of biosensor signals using Fast Independent Component Analysis: Feasibility study using EMG recordings. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 1927–1929. [Google Scholar]
- Xu, F.; Jin, Z.; Zou, S.; Chen, C.; Song, Q.; Deng, S.; Xiao, W.; Zhang, X.; Jia, A.; Tang, Y. EuNPs-mAb fluorescent probe based immunochromatographic strip for rapid and sensitive detection of porcine epidemic diarrhea virus. Talanta 2020, 214, 120865. [Google Scholar]
- Ambartsumyan, O.; Gribanyov, D.; Kukushkin, V.; Kopylov, A.; Zavyalova, E. SERS-based biosensors for virus determination with oligonucleotides as recognition elements. Int. J. Mol. Sci. 2020, 21, 3373. [Google Scholar] [CrossRef] [PubMed]
- Ayer, S.; Zhao, W.; Davis, C.E. Differentiation of Proteins and Viruses Using Pyrolysis Gas Chromatography Differential Mobility Spectrometry (PY/GC/DMS) and Pattern Recognition. IEEE Sens. J. 2008, 8, 1586–1592. [Google Scholar] [CrossRef]
- van den Oord, A.; Li, Y.; Vinyals, O. Representation learning with contrastive predictive coding. arXiv 2018, arXiv:1807.03748. [Google Scholar]
- Le-Khac, P.H.; Healy, G.; Smeaton, A.F. Contrastive Representation Learning: A Framework and Review. IEEE Access 2020, 8, 193907–193934. [Google Scholar] [CrossRef]
- Kiyasseh, D.; Zhu, T.; Clifton, D.A. CLOCS: Contrastive Learning of Cardiac Signals Across Space, Time, and Patients. arXiv 2020, arXiv:2005.13249. [Google Scholar]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Goh, H.; Dogrusoz, K.; Tuzel, O.; Azemi, E. Subject-Aware Contrastive Learning for Biosignals. arXiv 2020, arXiv:2007.04871. [Google Scholar]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Alonso, G.A.; Gutiérrez, J.M.; Marty, J.-L.; Muñoz, R. Implementation of the Discrete Wavelet Transform Used in the Calibration of the Enzymatic Biosensors; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Harrison, R.R. A low-power integrated circuit for adaptive detection of action potentials in noisy signals. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003; IEEE: New York, NY, USA, 2003; Volume 4, pp. 3325–3328. [Google Scholar]
- Rieger, R.; Taylor, J.T. An adaptive sampling system for sensor nodes in body area networks. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 183–189. [Google Scholar] [CrossRef]
- Mehrani, M.; Attarzadeh, I.; Hosseinzadeh, M. Sampling rate prediction of biosensors in wireless body area networks using deep-learning methods. Simul. Model. Pract. Theory 2020, 105, 102101. [Google Scholar] [CrossRef]
- Alhussein, D.A.; Idrees, A.K.; Harb, H. Energy-Saving Adaptive Sampling Mechanism for Patient Health Monitoring Based IoT Networks. In Proceedings of the New Trends in Information and Communications Technology Applications: 5th International Conference, NTICT 2021, Baghdad, Iraq, 17–18 November 2021; Proceedings 5. Springer: Berlin/Heidelberg, Germany, 2021; pp. 163–175. [Google Scholar]
- Beduk, D.; Ilton de Oliveira Filho, J.; Beduk, T.; Harmanci, D.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; et al. “All In One” SARS-CoV-2 variant recognition platform: Machine learning-enabled point of care diagnostics. Biosens. Bioelectron. X 2022, 10, 100105. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Dong, J.; Wang, N.; Huang, H.; Xu, H.; Xia, C. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: Role of deep-learning-based ct diagnosis and insights from two cases. Korean J. Radiol. 2020, 21, 505–508. [Google Scholar] [CrossRef]
- Potter, C.J.; Hu, Y.; Xiong, Z.; Wang, J.; McLeod, E. Point-of-care SARS-CoV-2 sensing using lens-free imaging and a deep learning-assisted quantitative agglutination assay. Lab Chip 2022, 22, 3744–3754. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, J.; Puyal, E.L.; Kontaxis, S.; Sun, S.; Locatelli, P.; Dineley, J.; Pokorny, F.B.; Costa, G.D.; Leocani, L.; et al. Fitbeat: COVID-19 estimation based on wristband heart rate using a contrastive convolutional auto-encoder. Pattern Recognit. 2022, 123, 108403. [Google Scholar] [CrossRef]
- Gudivada, V.N.; Ding, J.; Apon, A. Data Quality Considerations for Big Data and Machine Learning: Going Beyond Data Cleaning and Transformations Big Data Management View project Transforming Programmers to Professional Software Engineers View project Data Quality Considerations for Big Data. Int. J. Adv. Softw. 2017, 10, 1–20. [Google Scholar]
- Picard, S.; Chapdelaine, C.; Cappi, C.; Gardes, L.; Jenn, E.; Lefevre, B.; Soumarmon, T. Ensuring Dataset Quality for Machine Learning Certification. In Proceedings of the 2020 IEEE International Symposium on Software Reliability Engineering Workshops (ISSREW), Coimbra, Portugal, 12–15 October 2020; pp. 275–282. [Google Scholar] [CrossRef]
- O’neil, C. Weapons of Math Destruction: How Big Data Increases Inequality and Threatens Democracy; Crown: New York, NY, USA, 2017; ISBN 0553418831. [Google Scholar]
- Esterhuizen, J.A.; Goldsmith, B.R.; Linic, S. Interpretable machine learning for knowledge generation in heterogeneous catalysis. Nat. Catal. 2022, 5, 175–184. [Google Scholar] [CrossRef]
- Rudin, C.; Chen, C.; Chen, Z.; Huang, H.; Semenova, L.; Zhong, C. Interpretable machine learning: Fundamental principles and 10 grand challenges. Stat. Surv. 2022, 16, 1–85. [Google Scholar] [CrossRef]
- Vellido, A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput. Appl. 2020, 32, 18069–18083. [Google Scholar] [CrossRef]

| Biomarker | Mechanism | Forms of Collected Data | ML Algorithm | Ref |
|---|---|---|---|---|
| Spike protein | Electrical | Current | DNN | [56] |
| Spike protein | Electrical | Current | SVM | [37] |
| SARS-CoV-2 pseudovirus | Optical | Images | CNN | [58] |
| Heart rate | Optical | HRV signals | Contrastive CAE | [59] |
| Spike protein | Electrical | Current | SVM, RF, ABC, DTC, CNN | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgas, A.; Georgas, K.; Hristoforou, E. Advancements in SARS-CoV-2 Testing: Enhancing Accessibility through Machine Learning-Enhanced Biosensors. Micromachines 2023, 14, 1518. https://doi.org/10.3390/mi14081518
Georgas A, Georgas K, Hristoforou E. Advancements in SARS-CoV-2 Testing: Enhancing Accessibility through Machine Learning-Enhanced Biosensors. Micromachines. 2023; 14(8):1518. https://doi.org/10.3390/mi14081518
Chicago/Turabian StyleGeorgas, Antonios, Konstantinos Georgas, and Evangelos Hristoforou. 2023. "Advancements in SARS-CoV-2 Testing: Enhancing Accessibility through Machine Learning-Enhanced Biosensors" Micromachines 14, no. 8: 1518. https://doi.org/10.3390/mi14081518
APA StyleGeorgas, A., Georgas, K., & Hristoforou, E. (2023). Advancements in SARS-CoV-2 Testing: Enhancing Accessibility through Machine Learning-Enhanced Biosensors. Micromachines, 14(8), 1518. https://doi.org/10.3390/mi14081518








