Highly Uniform Spherical MoO2-MoO3/Polypyrrole Core-Shell Nanocomposite as an Optoelectronic Photodetector in UV, Vis, and IR Domains
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Ppy Preparation
2.3. MoO2-MoO3/Polypyrrole Core-Shell Thin Film Photodetector
2.4. The Electrical Measurements for the Constructed MoO2-MoO3/Ppy Core-Shell Thin Film Photodetector
3. Results and Discussion
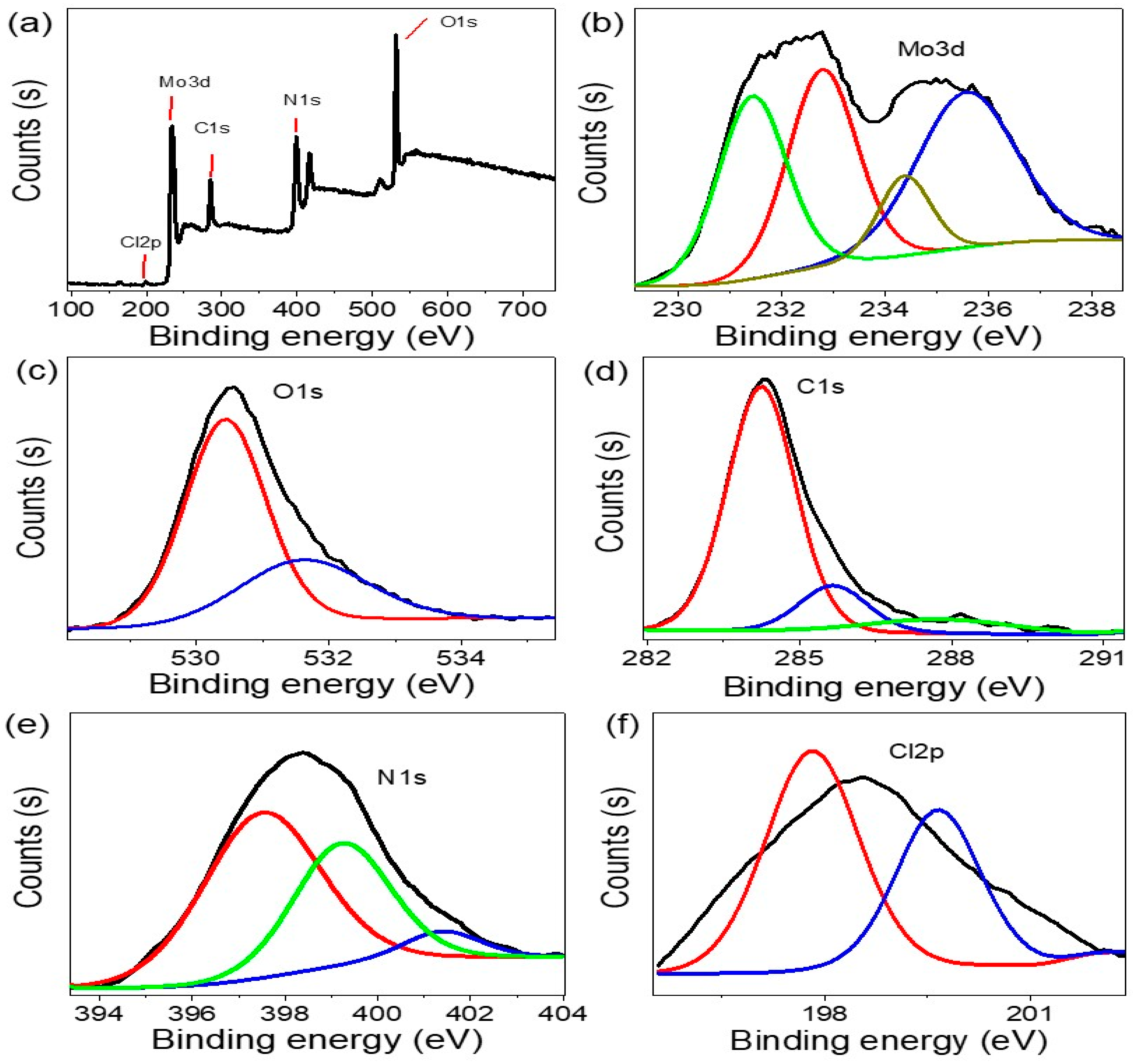
3.1. Analyses Processes
3.2. Electrical Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Cui, Y.; Deng, H.; Wang, J.; Hong, R.; Hu, S.; Hou, H.; Dong, Y.; Wang, H.; Chen, J.; et al. Bioresorbable Thin-Film Silicon Diodes for the Optoelectronic Excitation and Inhibition of Neural Activities. Nat. Biomed. Eng. 2022, 7, 486–498. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhang, Y.L.; Pan, C.; Dou, J.Y.; Li, Z.Z.; Tian, Z.N.; Mao, J.W.; Chen, Q.D.; Sun, H.B. Miniature Optoelectronic Compound Eye Camera. Nat. Commun. 2022, 13, 5634. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Hinton, H.; Jung, W.B.; Lee, M.H.; Kim, C.; Park, M.; Lee, S.K.; Park, S.; Ham, D. In-Sensor Optoelectronic Computing Using Electrostatically Doped Silicon. Nat. Electron. 2022, 5, 519–525. [Google Scholar] [CrossRef]
- Khan, S.; Primavera, B.A.; Chiles, J.; McCaughan, A.N.; Buckley, S.M.; Tait, A.N.; Lita, A.; Biesecker, J.; Fox, A.; Olaya, D.; et al. Superconducting Optoelectronic Single-Photon Synapses. Nat. Electron. 2022, 5, 650–659. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.; Dang, B.; Bao, L.; Xu, L.; Cheng, C.; Yang, Z.; Huang, R.; Yang, Y. An Optoelectronic Synapse Based on α-In2Se3 with Controllable Temporal Dynamics for Multimode and Multiscale Reservoir Computing. Nat. Electron. 2022, 5, 761–773. [Google Scholar] [CrossRef]
- Magdy, A.; El-Shaer, A.; EL-Farrash, A.H.; Salim, E. Influence of Corona Poling on ZnO Properties as N-Type Layer for Optoelectronic Devices. Sci. Rep. 2022, 12, 21489. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, P.; Gao, P.; Tong, J.; Li, J.; Wang, E.; Wang, C.; Xia, Y. Effect of Fluorine Atoms on Optoelectronic, Aggregation and Dielectric Constants of 2,1,3-Benzothiadiazole-Based Alternating Conjugated Polymers. Dye. Pigment. 2021, 193, 109486. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. Self-Powered UV–Visible Photodetectors Based on ZnO/Cu2O Nanowire/Electrolyte Heterojunctions. J. Alloys Compd. 2016, 675, 325–330. [Google Scholar] [CrossRef]
- Naldoni, A.; Guler, U.; Wang, Z.; Marelli, M.; Malara, F.; Meng, X.; Besteiro, L.V.; Govorov, A.O.; Kildishev, A.V.; Boltasseva, A.; et al. Broadband Hot-Electron Collection for Solar Water Splitting with Plasmonic Titanium Nitride. Adv. Opt. Mater. 2017, 5, 1601031. [Google Scholar] [CrossRef]
- Ma, L.; Li, Z.; Chen, B.; Zheng, X.; Xie, H.; Ji, C.; Zhan, X.; Liu, Y.; Chen, X. Comparative studies on frontier orbitals, molecular packing and optoelectronic properties of ambipolar polymer semiconductors based on bisthiophene-fused diketopyrrolopyrrole. Org. Electron. 2023, 122, 106909. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Shaban, M.; Mohamed, S.H.; Al-Ghamdi, A.F.; Alzaid, M.; Elsayed, A.M.; Mourad, A.H.I.; Amin, M.A.; Boukherroub, R.; Abdelazeez, A.A.A.; et al. Highly Crystalline Hexagonal PbI2 Sheets on Polyaniline/Antimony Tin Oxide Surface as a Novel and Highly Efficient Photodetector in UV, Vis, and near IR Regions. Polym. Adv. Technol. 2022, 33, 3977–3987. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Ibrahim, M.; Alshammari, K.; Taha, T.A.M. Processing polymer film nanocomposites of polyvinyl chloride—Polyvinylpyrrolidone and MoO3 for optoelectronic applications. Opt. Laser Technol. 2024, 168, 109833. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Alshammari, K.; Allam, N.K.; Taha, T.A. PVC/PVP/SrTiO3 polymer blend nanocomposites as potential materials for optoelectronic applications. Results Phys. 2023, 44, 106173. [Google Scholar] [CrossRef]
- Abdelhamied, M.M.; Atta, A.; Abdelreheem, A.M.; Farag, A.T.M.; El Okr, M.M. Synthesis and Optical Properties of PVA/PANI/Ag Nanocomposite Films. J. Mater. Sci. Mater. Electron. 2020, 31, 22629–22641. [Google Scholar] [CrossRef]
- Fulari, A.V.; Thanh Duong, N.; Anh Nguyen, D.; Jo, Y.; Cho, S.; Young Kim, D.; Shrestha, N.K.; Kim, H.; Im, H. Achieving Direct Electrophoretically Deposited Highly Stable Polymer Induced CsPbBr3 Colloidal Nanocrystal Films for High-Performance Optoelectronics. Chem. Eng. J. 2021, 433, 133809. [Google Scholar] [CrossRef]
- Atta, A.; Abdeltwab, E.; Negm, H.; Al-Harbi, N.; Rabia, M.; Abdelhamied, M.M. Fabrication of Polypyrrole/Graphene Oxide Polymer Nanocomposites and Evaluation of Their Optical Behavior for Optoelectronic Applications. J. Inorg. Organomet. Polym. Mater. 2023. accepted. [Google Scholar] [CrossRef]
- Atta, A.; Abdeltwab, E.; Negm, H.; Al-Harbi, N.; Rabia, M.; Abdelhamied, M.M. Characterization and Linear/Non-Linear Optical Properties of Polypyrrole/NiO for Optoelectronic Devices. Inorg. Chem. Commun. 2023, 152, 110726. [Google Scholar] [CrossRef]
- Rabia, M.; Elsayed, A.M.; Alnuwaiser, M.A. Preparation and Characterization of Polyhedron Mn(III) Oxide/-β-Mn(IV) Oxide/Poly-o-Chloroaniline Porous Nanocomposite for Electroanalytical Photon Detection. Processes 2023, 11, 2375. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Arda, E. Determination of Optical Band Gap Energies of CS/MWCNT Bio-Nanocomposites by Tauc and ASF Methods. Synth. Met. 2020, 269, 116539. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A Facile Method for Tauc Exponent and Corresponding Electronic Transitions Determination in Semiconductors Directly from UV–Vis Spectroscopy Data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Bhogi, A.; Srinivas, B.; Shareefuddin, M.; Kistaiah, P. Band Gap Determination by Tauc’s, ASF and DASF Methods of Alkaline Earth Modified Lithium Borate Glasses Co-Doped with Transition Metal Ions. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Rabia, M.; Elsayed, A.M.; Salem, A.M.; Abdallah Alnuwaiser, M. Highly Uniform Multi-Layers Reduced Graphene Oxide/Poly-2-Aminobenzene-1-Thiol Nanocomposite as a Promising Two Electrode Symmetric Supercapacitor under the Effect of Absence and Presence of Porous-Sphere Polypyrrole Nanomaterial. Micromachines 2023, 14, 1424. [Google Scholar] [CrossRef]
- Sen, S.K.; Dutta, S.; Khan, M.R.; Manir, M.S.; Dutta, S.; Al Mortuza, A.; Razia, S.; Hakim, M.A. Characterization and Antibacterial Activity Study of Hydrothermally Synthesized H-MoO3 Nanorods and α-MoO3 Nanoplates. BioNanoScience 2019, 9, 873–882. [Google Scholar] [CrossRef]
- Kumar, N.; Sengwa, R.J. Optical characterization of PVDF/PMMA/OMMT hybrid polymer nanocomposite films for futuristic optoelectronic devices. Optik 2023, 287, 171163. [Google Scholar] [CrossRef]
- Li, N.; Sheng, H.; Sun, Y.; Wang, J. Spectroscopic study on size-dependent optoelectronics of N-type ultra-high conductive polymer PBFDO. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 298, 122744. [Google Scholar] [CrossRef] [PubMed]
- Noothongkaew, S.; Thumthan, O.; An, K.S. Minimal Layer Graphene/TiO2 Nanotube Membranes Used for Enhancement of UV Photodetectors. Mater. Lett. 2018, 218, 274–279. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Alkallas, F.H.; Trabelsi, A.B.G.; AlFaify, S.; Shkir, M.; Alrebdi, T.A.; Almugren, K.S.; Kusmatsev, F.V.; Rabia, M. Photodetection Enhancement via Graphene Oxide Deposition on Poly 3-Methyl Aniline. Micromachines 2023, 14, 606. [Google Scholar] [CrossRef] [PubMed]
- Alruqi, M.; Rabia, M.; Elsayed, A.M.; Hanafi, H.A.; Shaban, M.; Hamid, M.M.A. Development of Polypyrrole/Ni(OH)2-NiO Core-Shell Nanocomposite as an Optoelectronic Device. J. Appl. Polym. Sci. 2023, 140, e53833. [Google Scholar] [CrossRef]
- Masoumi, S.; Nabiyouni, G.; Ghanbari, D. Photo-degradation of azo dyes: Photo catalyst and magnetic investigation of CuFe2O4–TiO2 nanoparticles and nanocomposites. J. Mater.Sci. Mater. Electron. 2016, 27, 9962–9975. [Google Scholar] [CrossRef]
- Lan, T.; Fallatah, A.; Suiter, E.; Padalkar, S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose. Sensors 2017, 17, 1944. [Google Scholar] [CrossRef]
- Chen, Z.; Ci, H.; Tan, Z.; Dou, Z.; Chen, X.-D.; Liu, B.; Liu, R.; Lin, L.; Cui, L.; Gao, P.; et al. Growth of 12-Inch Uniform Monolayer Graphene Film on Molten Glass and Its Application in PbI2-Based Photodetector. Nano Res. 2019, 12, 1888–1893. [Google Scholar] [CrossRef]
- Tan, W.C.; Shih, W.H.; Chen, Y.F. A Highly Sensitive Graphene-Organic Hybrid Photodetector with a Piezoelectric Substrate. Adv. Funct. Mater. 2014, 24, 6818–6825. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, P.; Hu, K.; Teng, F.; Chen, H.; Fang, X. Scalable-Production, Self-Powered TiO2 Nanowell-Organic Hybrid UV Photodetectors with Tunable Performances. ACS Appl. Mater. Interfaces 2016, 8, 33924–33932. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant Improvement of the Performance of ZnO Nanowire Photodetectors by Au Nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Costas, A.; Florica, C.; Preda, N.; Apostol, N.; Kuncser, A.; Nitescu, A.; Enculescu, I. Radial Heterojunction Based on Single ZnO-CuxO Core-Shell Nanowire for Photodetector Applications. Sci. Rep. 2019, 9, 5553. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Vura, S.; Rathkanthiwar, S.; Muralidharan, R.; Raghavan, S.; Nath, D.N. Demonstration of High-Responsivity Epitaxial β-Ga2O3/GaN Metal-Heterojunction-Metal Broadband UV-A/UV-C Detector. Appl. Phys. Express 2018, 11, 064101. [Google Scholar] [CrossRef]
- Wang, S.B.; Hsiao, C.H.; Chang, S.J.; Lam, K.T.; Wen, K.H.; Hung, S.C.; Young, S.J.; Huang, B.R. A CuO Nanowire Infrared Photodetector. Sens. Actuators A Phys. 2011, 171, 207–211. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, K.; Teng, F.; Fang, X. Novel UV–Visible Photodetector in Photovoltaic Mode with Fast Response and Ultrahigh Photosensitivity Employing Se/TiO2 Nanotubes Heterojunction. Small 2017, 13, 1602448. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mousa, A.M.; Shaker, S.S. Visible-Enhanced Silver-Doped PbI2 Nanostructure/Si Heterojunction Photodetector: Effect of Doping Concentration on Photodetector Parameters. Opt. Quantum Electron. 2019, 51, 362. [Google Scholar] [CrossRef]
- Hong, Q.; Cao, Y.; Xu, J.; Lu, H.; He, J.; Sun, J.L. Self-Powered Ultrafast Broadband Photodetector Based on p-n Heterojunctions of CuO/Si Nanowire Array. ACS Appl. Mater. Interfaces 2014, 6, 20887–20894. [Google Scholar] [CrossRef]


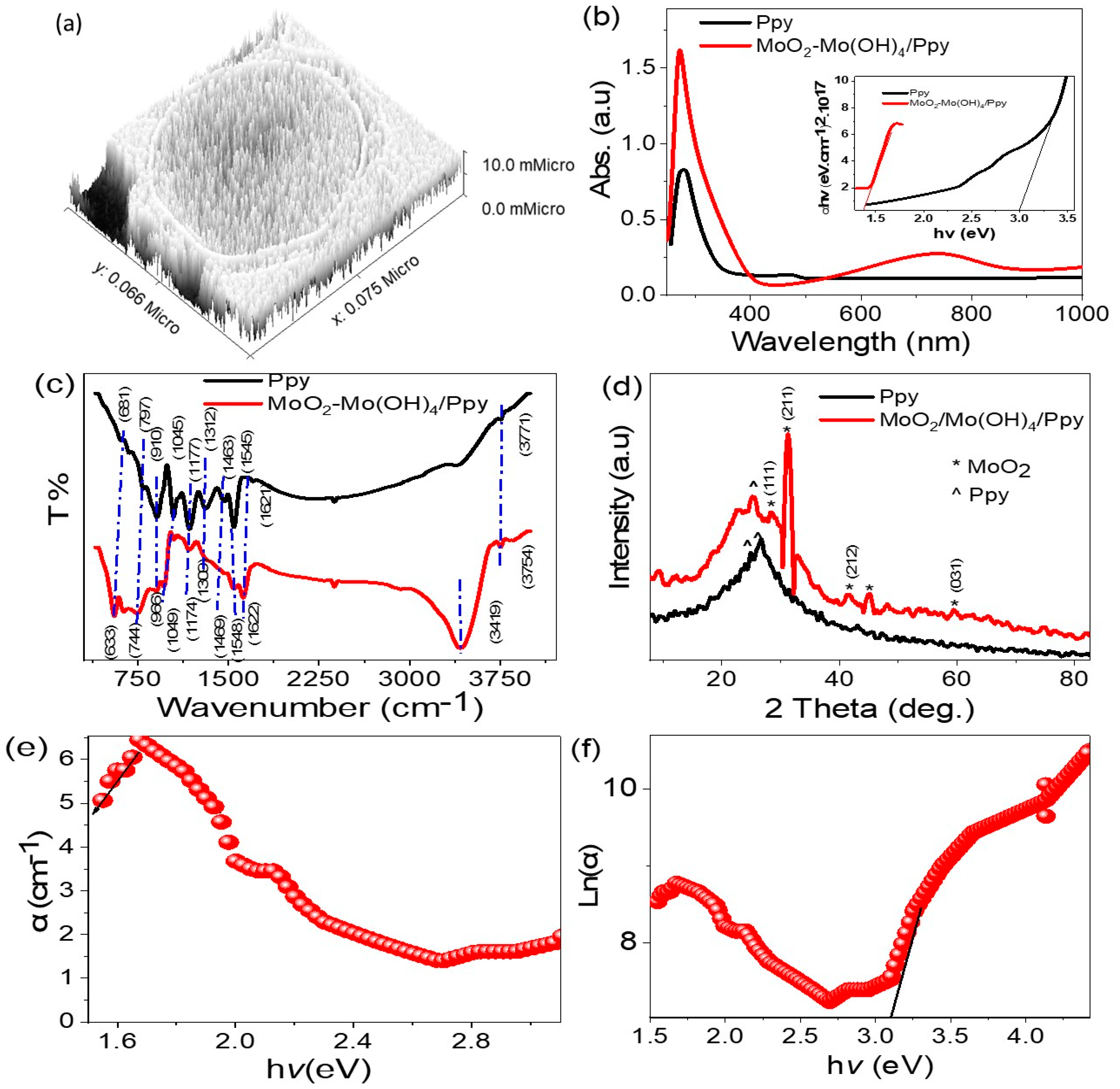



| Materials/Band Position (cm−1) | Characteristic Group | |
|---|---|---|
| Ppy | MoO2-MoO3/Ppy | |
| 3771 | 3754 | N–H |
| 3419 | O–H group | |
| 1621 | 1622 | C=C quinoid |
| 1545 and 1463 | 1548 and 1469 | C=C benzene |
| 1312 | 1309 | C–N [22] |
| 1177 | 1174 | C–H |
| 798, 1045, 910 | 1049 and 996 | Ring vibration |
| Structure | Wavelength (nm) | Bais (V) | R (mAW−1) |
|---|---|---|---|
| GO/Cu2O [30] | 300 | 2 | 0.5 × 10−3 |
| PbI2-graphene [31] | 550 | 2 | NA |
| Graphene/P3HT [32] | 325 | 1 | NA |
| TiO2-PANI [33] | 320 | 0 | 3 × 10−3 |
| ZnO/RGO [34] | 350 | 5 | 1.3 × 10−3 |
| ZnO-CuO [35] | 405 | 1 | 3 × 10−3 |
| Graphene/GaN [36] | 365 | 7 | 3 × 10−3 |
| PbI2-graphene [31] | 550 | 2 | NA |
| CuO nanowires [37] | 390 | 5 | - |
| TiN/TiO2 [9] | 550 | 5 | - |
| ZnO/Cu2O [8] | 350 | 2 | 4 × 10−3 |
| Se/TiO2 [38] | 450 | 1 | 5 × 10−3 |
| PbI2-5%Ag [39] | 532 | 6 | NA |
| CuO/Si Nanowire [40] | 405 | 0.2 | 3.8 × 10−3 |
| MoO2-MoO3/Ppy (this work) | 440 | 2 | 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, A.M.; Alkallas, F.H.; Trabelsi, A.B.G.; Rabia, M. Highly Uniform Spherical MoO2-MoO3/Polypyrrole Core-Shell Nanocomposite as an Optoelectronic Photodetector in UV, Vis, and IR Domains. Micromachines 2023, 14, 1694. https://doi.org/10.3390/mi14091694
Elsayed AM, Alkallas FH, Trabelsi ABG, Rabia M. Highly Uniform Spherical MoO2-MoO3/Polypyrrole Core-Shell Nanocomposite as an Optoelectronic Photodetector in UV, Vis, and IR Domains. Micromachines. 2023; 14(9):1694. https://doi.org/10.3390/mi14091694
Chicago/Turabian StyleElsayed, Asmaa M., Fatemah H. Alkallas, Amira Ben Gouider Trabelsi, and Mohamed Rabia. 2023. "Highly Uniform Spherical MoO2-MoO3/Polypyrrole Core-Shell Nanocomposite as an Optoelectronic Photodetector in UV, Vis, and IR Domains" Micromachines 14, no. 9: 1694. https://doi.org/10.3390/mi14091694
APA StyleElsayed, A. M., Alkallas, F. H., Trabelsi, A. B. G., & Rabia, M. (2023). Highly Uniform Spherical MoO2-MoO3/Polypyrrole Core-Shell Nanocomposite as an Optoelectronic Photodetector in UV, Vis, and IR Domains. Micromachines, 14(9), 1694. https://doi.org/10.3390/mi14091694







