3.1. Optimal Bonding Conditions
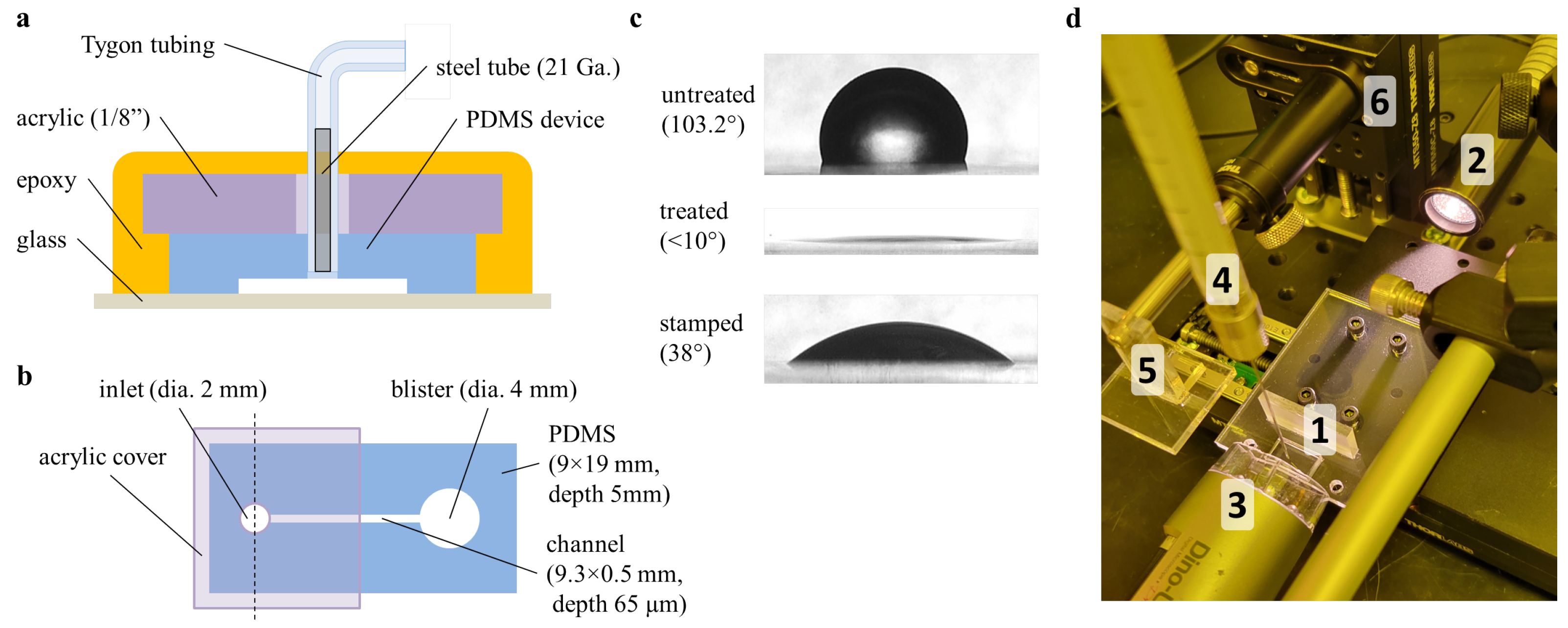
Contact angle measurements serve as a useful proxy for eventual bond strength. Plasma treatment of the surface renders it hydrophilic, so a lower deionized water contact angle correlates with more-effective surface treatment. Likewise, the contact angle is inversely proportional to bond strength. However, for stamped samples, an increased contact angle is ideal as it indicates reduced bonding in the valve area.
The effects of oxygen plasma treatment for bonding on non-stamped PDMS have been previously investigated [
2]. It was shown that the contact angle reaches a minimum near a plasma treatment power of 20 W over a total exposure time of 30 s. However, the accuracy of telescopic contact angle goniometers in general decreases significantly at lower contact angles [
14], and our setup was unable to detect any statistically significant difference in angles resulting from the reasonable range of treatment conditions on unstamped samples. Droplets deposited on treated surfaces exhibited strong wetting with near-zero contact angles. These results were significantly lower than those of the previous study [
2], though others have also assessed the contact angle under plasma treatment to be too near zero to report any other result [
12].
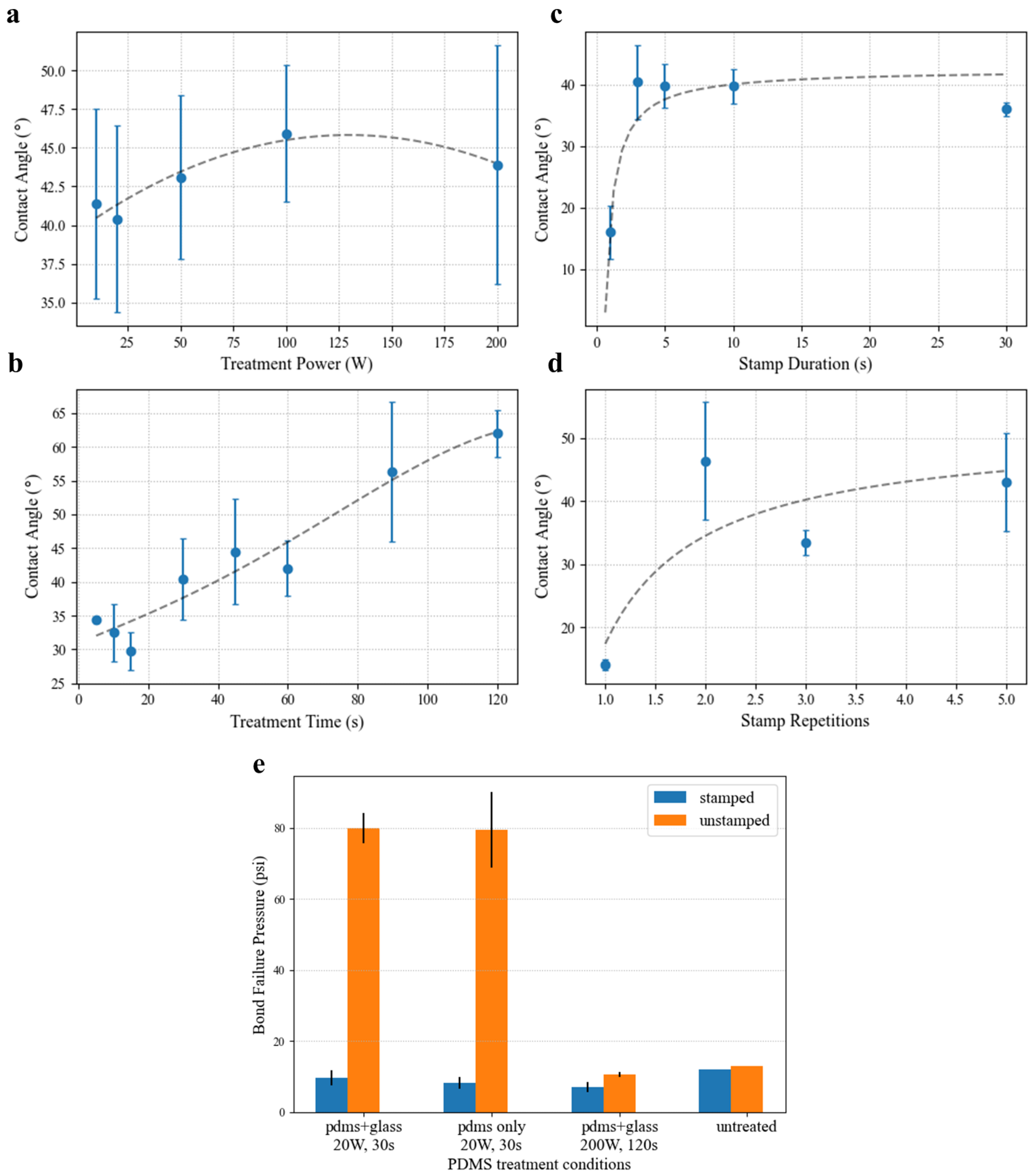
Figure 5 shows the results of oligomer stamping on the PDMS bond strength via treatment with oxygen plasma. The effectiveness of the stamping approach is clearly demonstrated, with the contact angle recovering nearly 40° for most treatment conditions. As shown in
Figure 5a, for 30 s treatments at 500 mT, the measured contact angle shows little dependence on the treatment power. While the average values generally show constant contact angle recovery compared to the previously published unstamped results (i.e., a similar shape with a minimum near 20 W), the standard deviations of the measurements (N ≥ 3 drops, 6 angles) are quite large. Though attempts have been made to control as many variables in the stamping process as possible (i.e., duration, pressure, angle) through automation, the effect on the surface treatment still seems to be quite variable. One difference that was observed between samples that is particularly difficult control is the manner in which the stamp and surface under test separate. For the large surfaces necessary for droplet spreading, the PDMS samples tended to peel apart in a wavefront that originated at inconsistent locations throughout these experiments. As a result, some areas of the samples may have experienced more mechanical perturbance, as the final region of contact often sprang apart elastically.
Contact angle results examining the effect of plasma treatment time with source power of 20 W (
Figure 5b) showed a much stronger dependency. Again, the minimum contact angle matches the previously reported minimum for unstamped PDMS near 30 s. This minimum is expected for unstamped PDMS, as insufficient exposure produces fewer of the silanol chains needed for bonding, and increased overexposure results in the formation of a brittle silica-like layer [
17,
18]. Oligomer stamping likely acts by removing or reorienting the activated surface groups and should not be able to reverse the formation of the silica layer. Rather, its action seems to be proportional to the concentration of activated surface groups, resulting in similar contact angle curve shapes.
The stamp contact duration was shown to play a significant part in the effectiveness of the oligomer stamping approach in selectively removing the PDMS surface treatment.
Figure 5c shows that under test conditions of 20 W and treatment time of 30 s, the optimal stamping time should exceed 1 s, but contact lasting longer than 3 s offers no further benefit. While the mechanism of oligomer stamping does not seem to occur instantaneously, contact angle testing in
Figure 5d shows that the action can be repeated. Making stamp contact with the treated surface multiple times further decreased contact angle in the contacted area. However, this approach showed diminishing returns after 3 repeated stamps. So long as alignment can be retained between successive contacts (requiring both the stamp and the device to be well-fixed on their backings), multistamping is an effective way to increase the bond strength margin between the treated and stamped areas without compromising spatial resolution.
Blister bursting tests assessing bonding strength (
Figure 5e) confirm contact angle results, showing an 8× decrease in bond strength between treated and stamped samples for the optimal bonding conditions of 20 W and 30 s. Interestingly, treatment of the glass substrate seems to have little effect on the final bond strength. This investigation found that plasma-treating the substrate seems primarily to decrease the variability in the bonding process. This advantage could likely be attributed to removal of any possible surface contamination by plasma treatment rather than to surface activation. Burst testing further demonstrated that the conditions at which the stamped contact angles were greatest (high treatment power and long exposure) are unsuitable for device bonding. In fact, both stamped and unstamped samples exposed to 200 W oxygen plasma for 2 min exhibited poorer substrate adhesion than untreated PDMS.
Oligomer stamping is clearly an effective means of spatial patterning of plasma surface treatment on PDMS. For optimal recovery of non-bonding areas, stamps should contact the surface for more than one second, and contact should be repeated at least three times. Following these recommendations, sufficient removal of the surface treatment to prevent bonding can be achieved for any treatment conditions. Thus, for normally closed PDMS valves, the surfaces should be treated to optimize the performance of the bonding surfaces rather than to ensure valve release.
3.2. Valve Performance
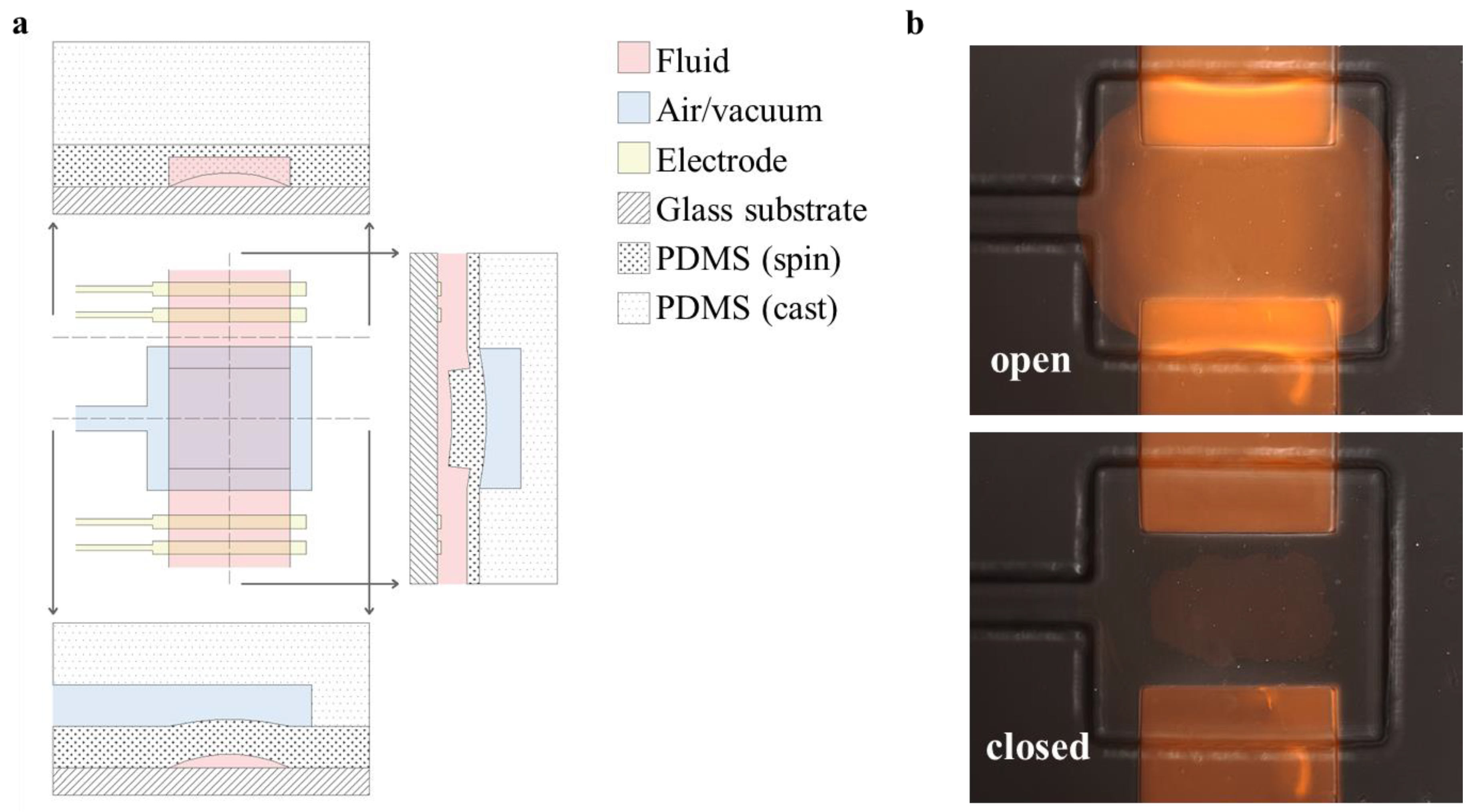
Fabrication on glass is ideally suited to integrate planar microelectrodes into valved microfluidic devices. In the presence of a highly conductive medium, such as DPBS, the electrical impedance seen across the valve site serves to characterize the effectiveness of the valve sealing [
19], and the impedance ratio between the open and closed states of the valve signifies the electrical isolation it provides at each frequency.
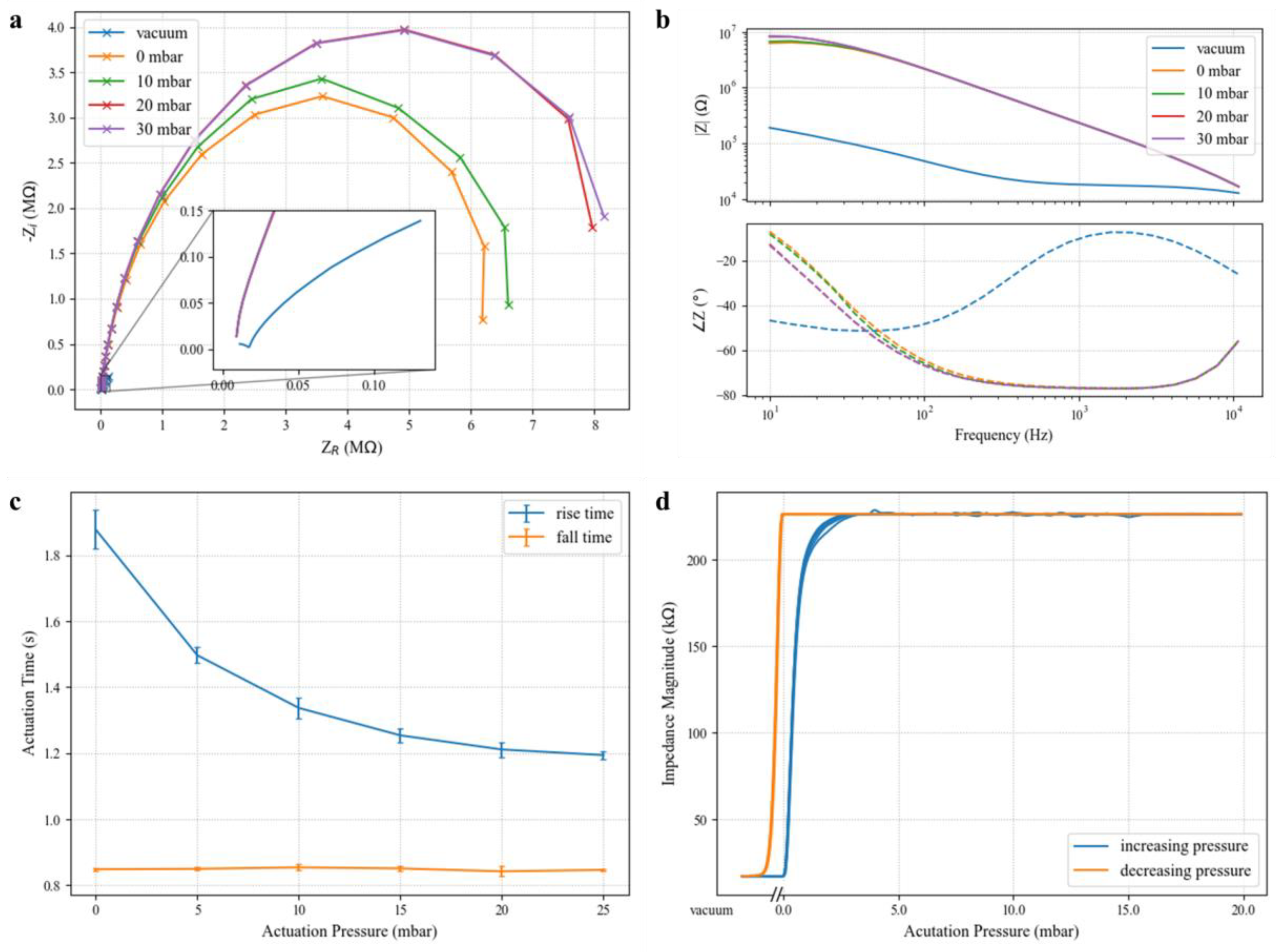
Figure 6a,b show the characteristic complex impedance spectra for a representative valve at various actuation pressures. At low frequencies, the valve impedance magnitude exceeds 8 M
for positive actuation pressures greater than 10 mbar when the valve is sealed. In contrast, the measured impedance magnitude for the valve in the open state (vacuum) is only 190.0 k
at 10 Hz, a 35-fold difference. At 30 mbar actuation pressure, the valve in the closed state exhibits a maximum of 55.6-times greater impedance magnitude over the open state, showing that such normally open valves are suitable to provide reversible electrical isolation of different microfluidic compartments.
In the closed state, the valve impedance curves are bandwidth-limited by the cabling (BNC) connecting benchtop instruments to the device under test. The closed valve impedance closely matches a single-pole model with a cutoff frequency of 31 Hz. At 30 mbar, it can be modeled by a resistor and capacitor in parallel with equivalent values of 8.2 M and 0.62 nF, respectively. Resistive load tests have verified that this parasitic capacitance is introduced in the cabling rather than the device or interconnect. The valve barrier then represents a constant resistance over the biologically relevant spectrum. Even in its resting state (0 mbar), the valve represents an extracted resistance of 6.4 M, making these devices useful for applications characterizing analytes across a broad frequency range (e.g., dielectric spectroscopy flow cytometry) or those using high-frequency stimulation.
Ionic conduction in the open valve state also experiences significant degradation across the measured frequency range. The vacuum curve shape in
Figure 6a is reminiscent of the characteristics of a series connection of two parallel resistor–capacitor pairs. This model matches previously studied electrical models for the electrode–fluid interface, where a large capacitance arises from the electric double layer formed by free ionic species at the interface, and a resistance models redox reactions responsible for charge transfer [
20,
21].
Valve actuation time is shown to be a function of the pneumatic actuating pressure in
Figure 6c. The rise time corresponds to the impedance magnitude rise observed when positive pressure is applied to a valve’s pneumatic chamber and is calculated as the time difference between 10% and 90% of the total impedance rise after 60 s. As would be expected, driving the system at higher pressures causes more rapid deflection of the elastomer membrane, resulting in shorter rise times. However, diminishing returns in rise time are observed beyond 20 mbar, and valves should be operated at the minimum acceptable pressure level to preserve longevity. Fall times (the time to drop from 90% of the sealed impedance to 10% once vacuum is applied) do not exhibit dependency on the previous actuation pressure since all valves are well-sealed after 60 s, and an identical vacuum pressure is applied in all cases. Presumably, decreased vacuum strength would result in increased falling valve actuation times, but this response was not investigated.
Figure 6b shows the valve hysteresis for a 1 kHz signal as a function of the actuation pressure. We posit that this hysteresis effect is primarily mechanical in nature. When vacuum is released on the valve, the elastic membrane begins sealing immediately, achieving a 10-fold impedance increase with less than 1 mbar of applied pressure. The valve impedance plateaus at less than 5 mbar of positive pressure, indicating that the membrane has completely sealed against the substrate. Once sealed, the elastomer is in a low-energy state, and electrical isolation is retained upon decreasing the actuation pressure back to zero. For low-power solutions that rely on solenoid valves to apply pressure, this result implies that a scheme whereby the actuation pressure is briefly pulsed instead of held continuously is feasible.
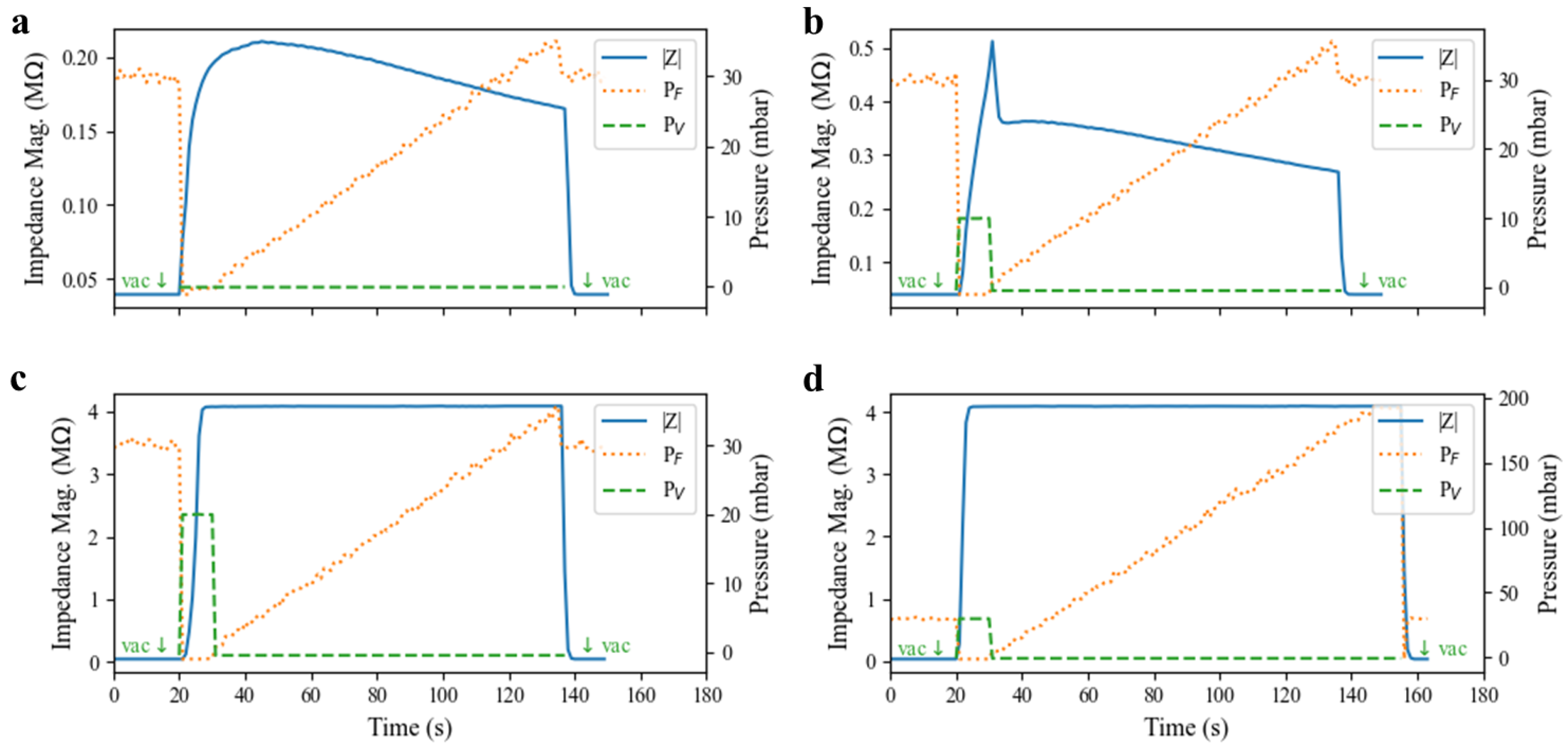
The valve dynamics are explored in
Figure 7, and such a pulsed actuation scheme is demonstrated. The ability of the valve to create and maintain a complete seal in the presence of positive pressure applied to the fluid channel was tested. A test valve was first closed for 10 s using varying actuation pressures (0, 10, 20, and 30 mbar) before the pneumatic pressure on the valve was released and positive pressure on the fluid channel flowing into the valve was increased linearly every 3 s. The electrical impedance was measured across the valve to monitor the seal quality and any leakage current. In
Figure 7a, when zero positive pressure was applied to the valve, its elastomeric membrane slowly returned to its resting state against the substrate, resulting in a gradual increase in impedance. However, the force generated by the elastomer relaxation was likely insufficient to expel all of the conductive fluid under the valve area, resulting in a poor seal and a small fluid leakage current under the valve. The leakage current expanded at higher driving pressures, resulting in declining electrical impedance. Valve impedance decreased to 90 percent of its maximum value at less than 24 mbar, suggesting that for operation without positive pressures, some amount of fluid leakage must be acceptable (e.g., a peristaltic pump or control of flowing cell suspensions) or the device must operate at very low pressure. At 10 mbar actuation pressure (
Figure 7b), the valve impedance rose quickly, but it was still insufficient to form a complete seal in the 10 s actuation window. A small leakage current was still present, as evidenced by the similarity of the post-actuation curve shape with that of the 0 mbar case.
Figure 7b indicates a trade-off between the actuation pressure and actuation window in order to form complete seals in this pulsed control scheme. For a 10 s actuation window, 20 mbar actuation pressure is sufficient to fully seal the channel, as evidenced by
Figure 7c. Further,
Figure 7d demonstrates that for a 10 s pulse of pressure at 30 mbar, the valve forms a sufficient seal to the substrate to withstand a 200 mbar flow pressure (the full range of the experimental pressure controller) without any leakage current forming. Presumably, these valves can withstand even greater fluid pressure given sufficient actuation pressure and time to form a tight seal to the substrate.
3.3. Limitations and Future Work
Traditional lithographic alignment processes are not optimized for “soft” materials. PDMS alignment for bonding in particular presents a variety of challenges, namely (1) the possibility of layers stretching or drooping, (2) challenges related to leveling soft surfaces, and (3) more-pronounced thermal contraction.
The elastic nature of PDMS permits stretching, warping, and drooping of thin layers if not rigidly backed during alignment. Ideally, layers should be cured against transparent backing and not released until alignment is complete. Care must be taken, however, to ensure that the backing itself is either sufficiently light or well-balanced enough that it does not exert significant levering force on the device after alignment while the bond is developing.
Contrary to normal photoresist alignment processes, where cross-linked photoresist may be contacted to the mask in the stage-leveling step prior to alignment, the treated PDMS surface must not contact its bond target prior to final contact. Further, the compressibility of PDMS makes leveling procedurally difficult, as a freely rotating stage may compress the device rather than adjusting its angle of alignment.
If the challenges of (1) and (2) are addressed and good alignment is achieved, further issues can arise due to the trapping of air. When both surfaces are leveled, contact can occur at multiple points across the device, causing air pockets to arise in the bonding interface. The gas permeability of PDMS can help evacuate some of these air pockets during the heated curing phase, but they often result in areas of decreased bond strength.
Many of these challenges could be addressed through the continued development of a customized alignment and plasma treatment rig. An ideal tool might operate similarly to a mask aligner, where the UV lamp is replaced by a plasma source. The ability to align soft layers and fix their position during plasma treatment would alleviate many of the noted limitations of this approach. Practically, alignment challenges, rather than bond strength, can lead to low yield of valved devices. In theory, spatial resolution of the approach is limited only by lithographic resolution and alignment quality.
3.4. Conclusions
A spatially selective bonding method has been demonstrated to fabricate normally closed microfluidic valves on glass substrates. This oligomer stamping technique had not previously been demonstrated on glass and prevents the need to actuate valves during the bonding process, thereby increasing fabrication reliability and enabling large-scale integration. The oligomer stamping process itself has been examined in detail using contact angle measurements to predict bonding strength. The stamping mechanism was found to produce a roughly constant recovery of the PDMS surface hydrophobicity over a wide range of exposure parameters. Further, the oligomer interaction with activated surface groups does not occur instantaneously; rather, stamping to remove plasma surface treatment should maintain contact for greater than 3 s and may be repeated to ensure maximum recovery of hydrophobicity. Blister burst testing showed that, in practice, this stamping technique is sufficient to prevent bonding under any surface treatment conditions, so oxygen plasma treatment should be optimized for efficient bonding of the device area rather than minimum bonding in the valve area, i.e., 20 W exposure for 30 s.
The elastomeric valves fabricated using this optimization require only two PDMS layers (and no additional chemicals or fabrication tools) and permit convenient integration of electrodes into a microfluidic device. Integrated electrodes enable monitoring of valve behavior among a number of other useful applications. Here, electrical impedance measurements through the valves have demonstrated reversible electrical isolation beyond 8 M at exceptionally low actuation pressures (less than 30 mbar). Further, a pulsed actuation scheme was explored, in which positive pressure is applied briefly to seal the valve against the substrate before releasing pneumatic pressure to conserve operational energy. Electrical measurement verified that valves pulsed in this manner at 30 mbar for 10 s were able to withstand channel back-pressures greater than 200 mbar without leaking. The presented microcontact method of valve fabrication is suitable for dense integration of many normally closed elastomeric valves on glass substrates, enabling applications where optical imaging or electrode integration are critical.