Continuous On-Chip Cell Washing Using Viscoelastic Microfluidics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication
2.2. Sample Preparation
2.3. Experimental Procedure and Post Analysis
3. Results and Discussion
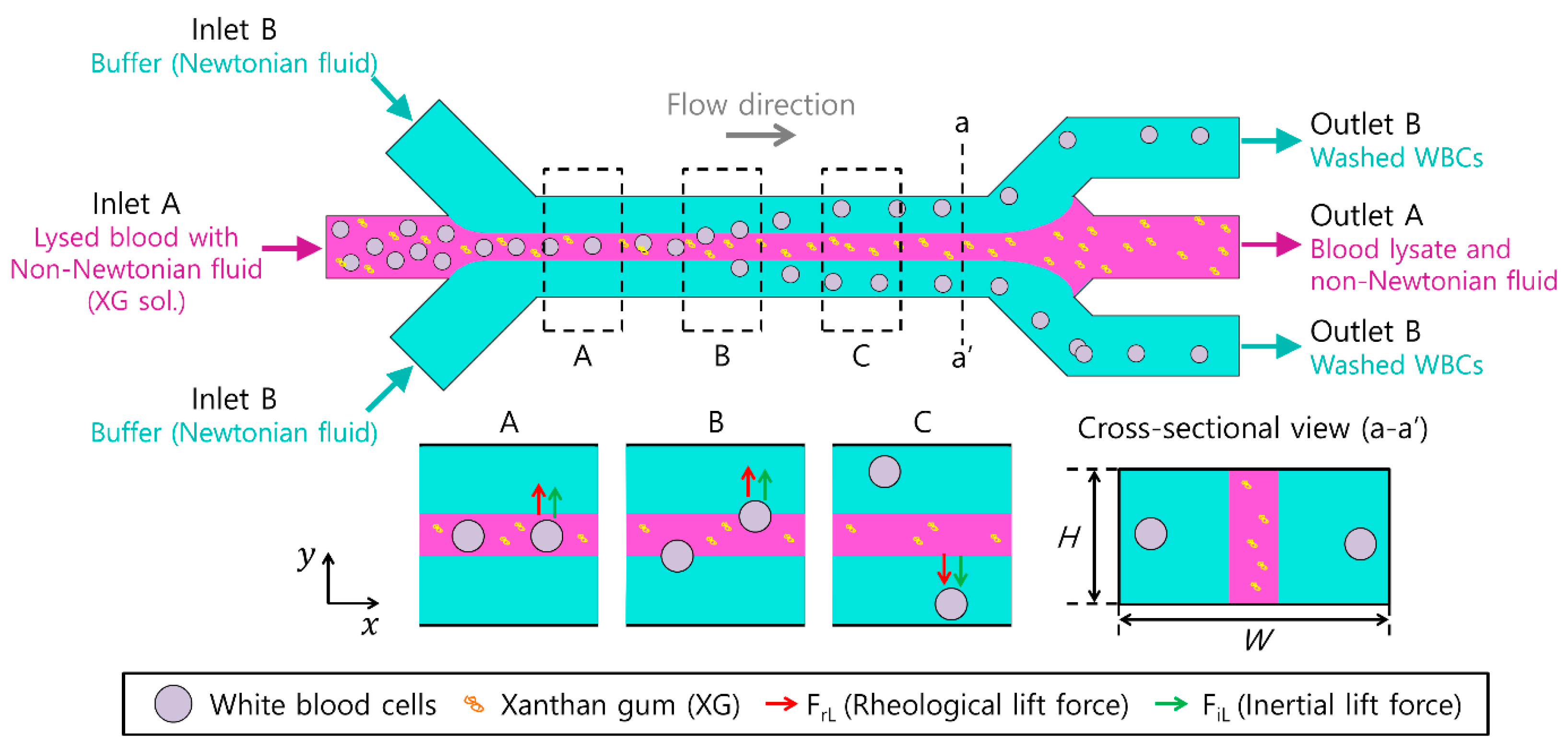
3.1. Working Principle
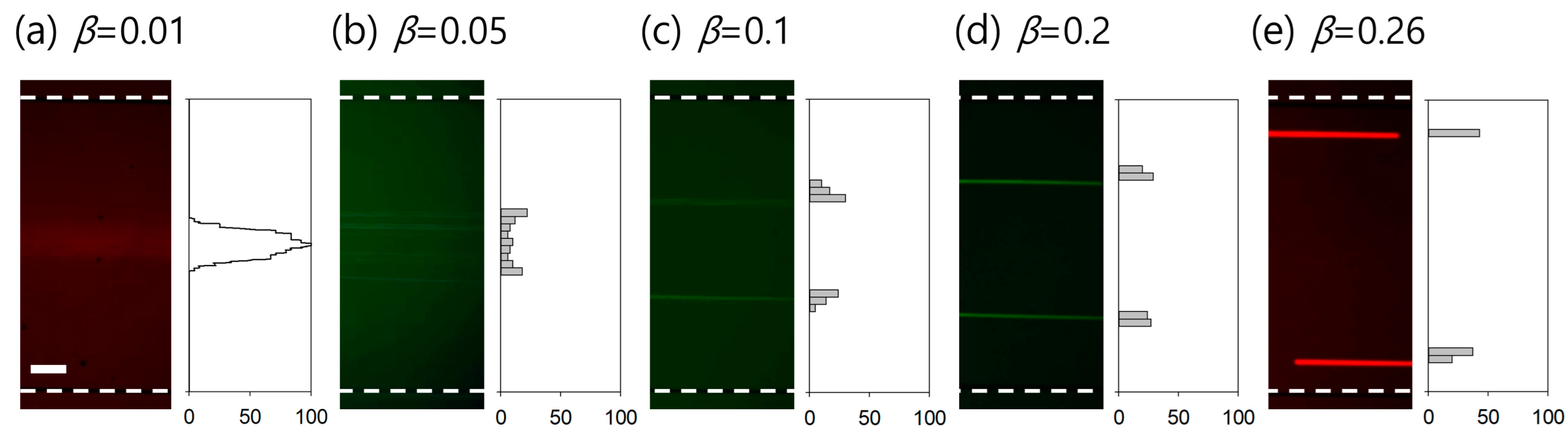
3.2. Effect of Viscoelasticity on Lateral Migration of Particles
3.3. Determination of Critical Blockage Ratio for Efficient Washing
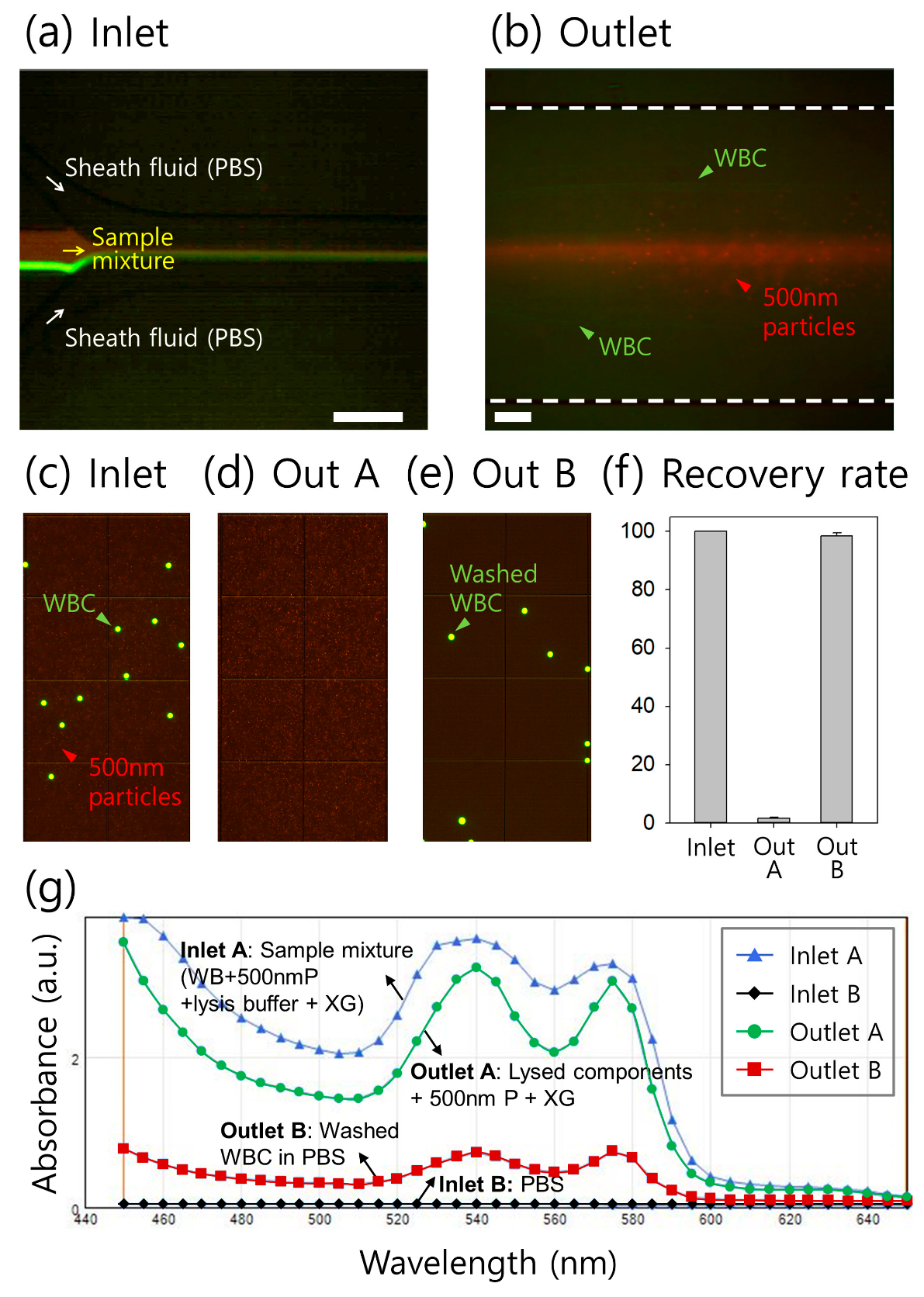
3.4. Clinical Application of Continuous On-Chip Washing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dineva, M.A.; Mahilum-Tapay, L.; Lee, H. Sample preparation: A challenge in the development of point-of-care nucleic acid-based assays for resource-limited settings. Analyst 2007, 132, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Cohen, K.S.; Scadden, D.T.; Jain, R.K. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat. Protoc. 2007, 2, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Bacsa, B.; Kappe, C.O. Rapid solid-phase synthesis of a calmodulin-binding peptide using controlled microwave irradiation. Nat. Protoc. 2007, 2, 2222–2227. [Google Scholar] [CrossRef]
- Park, S.; Zhang, Y.; Wang, T.-H.; Yang, S. Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab Chip 2011, 11, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Tornay, R.; Braschler, T.; Demierre, N.; Steitz, B.; Finka, A.; Hofmann, H.; Hubbell, J.A.; Renaud, P. Dielectrophoresis-based particle exchanger for the manipulation and surface functionalization of particles. Lab Chip 2008, 8, 267–273. [Google Scholar] [CrossRef]
- Nilsson, A.; Petersson, F.; Jönsson, H.; Laurell, T. Acoustic control of suspended particles in micro fluidic chips. Lab Chip 2004, 4, 131–135. [Google Scholar] [CrossRef]
- Petersson, F.; Nilsson, A.; Jönsson, H.; Laurell, T. Carrier Medium Exchange through Ultrasonic Particle Switching in Microfluidic Channels. Anal. Chem. 2005, 77, 1216–1221. [Google Scholar] [CrossRef]
- Augustsson, P.; Persson, J.; Ekström, S.; Ohlin, M.; Laurell, T. Decomplexing biofluids using microchip based acoustophoresis. Lab Chip 2009, 9, 810–818. [Google Scholar] [CrossRef]
- Augustsson, P.; Åberg, L.B.; Swärd-Nilsson, A.-M.K.; Laurell, T. Buffer medium exchange in continuous cell and particle streams using ultrasonic standing wave focusing. Microchim. Acta 2009, 164, 269–277. [Google Scholar] [CrossRef]
- Peyman, S.A.; Iles, A.; Pamme, N. Rapid on-chip multi-step (bio)chemical procedures in continuous flow—Manoeuvring particles through co-laminar reagent streams. Chem. Commun. 2008, 1220–1222. [Google Scholar] [CrossRef]
- Peyman, S.A.; Iles, A.; Pamme, N. Mobile magnetic particles as solid-supports for rapid surface-based bioanalysis in continuous flow. Lab Chip 2009, 9, 3110–3117. [Google Scholar] [CrossRef]
- Morton, K.J.; Loutherback, K.; Inglis, D.W.; Tsui, O.K.; Sturm, J.C.; Chou, S.Y.; Austin, R.H. Crossing microfluidic streamlines to lyse, label and wash cells. Lab Chip 2008, 8, 1448–1453. [Google Scholar] [CrossRef]
- Gossett, D.R.; Tse, H.T.K.; Dudani, J.S.; Goda, K.; Woods, T.A.; Graves, S.W.; Di Carlo, D. Inertial Manipulation and Transfer of Microparticles Across Laminar Fluid Streams. Small 2012, 8, 2757–2764. [Google Scholar] [CrossRef]
- Shi, X.; Tan, W.; Lu, Y.; Cao, W.; Zhu, G. A needle tip CCEA microfluidic device based on enhanced Dean flow for cell washing. Microsystems Nanoeng. 2021, 7, 81. [Google Scholar] [CrossRef]
- Bogseth, A.; Zhou, J.; Papautsky, I. Evaluation of Performance and Tunability of a Co-Flow Inertial Microfluidic Device. Micromachines 2020, 11, 287. [Google Scholar] [CrossRef]
- D’avino, G.; Maffettone, P.; Greco, F.; Hulsen, M. Viscoelasticity-induced migration of a rigid sphere in confined shear flow. J. Non-Newton. Fluid Mech. 2010, 165, 466–474. [Google Scholar] [CrossRef]
- Leshansky, A.M.; Bransky, A.; Korin, N.; Dinnar, U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys. Rev. Lett. 2007, 98, 234501. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.; Kim, D.; Jung, H.; Shin, S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab Chip 2012, 12, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Namgung, B.; Lim, C.T.; Bae, J.-E.; Leo, H.L.; Cho, K.S.; Kim, S. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J. Chromatogr. A 2015, 1406, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Back, S.M.; Hwang, M.H.; Lee, D.-H.; Choi, H.; Nam, J. Sheathless High-Throughput Circulating Tumor Cell Separation Using Viscoelastic non-Newtonian Fluid. Micromachines 2019, 10, 462. [Google Scholar] [CrossRef]
- Nam, J.; Jang, W.S.; Lim, C.S. Non-electrical powered continuous cell concentration for enumeration of residual white blood cells in WBC-depleted blood using a viscoelastic fluid. Talanta 2019, 197, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, H.; Jee, H.; Choo, S.; Yang, M.; Park, S.; Lee, K.; Park, H.; Lim, C.; Nam, J. High-Throughput Cell Concentration Using A Piezoelectric Pump in Closed-Loop Viscoelastic Microfluidics. Micromachines 2021, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.; Lim, H.; Kim, T.E.; Park, J.; Park, K.B.; Park, C.; Lim, C.S.; Nam, J. A Continuous Microfluidic Concentrator for High-Sensitivity Detection of Bacteria in Water Sources. Micromachines 2022, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, J.Y.; Choo, S.; Lee, C.; Han, B.J.; Lim, C.S.; Nam, J. Separation and Washing of Candida Cells from White Blood Cells Using Viscoelastic Microfluidics. Micromachines 2023, 14, 712. [Google Scholar] [CrossRef]
- Ha, B.; Park, J.; Destgeer, G.; Jung, J.H.; Sung, H.J. Transfer of Microparticles across Laminar Streams from Non-Newtonian to Newtonian Fluid. Anal. Chem. 2016, 88, 4205–4210. [Google Scholar] [CrossRef]
- Yuan, D.; Tan, S.H.; Sluyter, R.; Zhao, Q.; Yan, S.; Nguyen, N.T.; Guo, J.; Zhang, J.; Li, W. On-Chip Microparticle and Cell Washing Using Coflow of Viscoelastic Fluid and Newtonian Fluid. Anal. Chem. 2017, 89, 9574–9582. [Google Scholar] [CrossRef]
- Kang, K.; Lee, S.S.; Hyun, K.; Lee, S.J.; Kim, J.M. DNA-based highly tunable particle focuser. Nat. Commun. 2013, 4, 2567. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, J.; Tian, F.; Chang, J.; Zhang, W.; Sun, J. λ-DNA- and Aptamer-Mediated Sorting and Analysis of Extracellular Vesicles. J. Am. Chem. Soc. 2019, 141, 3817–3821. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Hua, D.; Gao, S.; Zhang, M.; Ma, W.; Huang, C. A novel xanthan gum-based conductive hydrogel with excellent mechanical, biocompatible, and self-healing performances. Carbohydr. Polym. 2020, 247, 116743. [Google Scholar] [CrossRef]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef]
- Li, D.; Xuan, X. Fluid rheological effects on particle migration in a straight rectangular microchannel. Microfluid. Nanofluidics 2018, 22, 49. [Google Scholar] [CrossRef]
- Li, D.; Xuan, X. The motion of rigid particles in the Poiseuille flow of pseudoplastic fluids through straight rectangular microchannels. Microfluid. Nanofluidics 2019, 23, 54. [Google Scholar] [CrossRef]
- Li, D.; Shao, X.; Bostwick, J.B.; Xuan, X. Particle separation in xanthan gum solutions. Microfluid. Nanofluidics 2019, 23, 125. [Google Scholar] [CrossRef]
- Shankaran, H.; Alexandridis, P.; Neelamegham, S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 2003, 101, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.P.; Leal, L.G. Inertial migration of rigid spheres in two-dimensional unidirectional flows. J. Fluid Mech. 1974, 65, 365–400. [Google Scholar] [CrossRef]
- Li, D.; Lu, X.; Xuan, X. Viscoelastic Separation of Particles by Size in Straight Rectangular Microchannels: A Parametric Study for a Refined Understanding. Anal. Chem. 2016, 88, 12303–12309. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Chen, X.; Shan, L.; Tian, Y.; Hu, G. Size-Based Separation of Particles and Cells Utilizing Viscoelastic Effects in Straight Microchannels. Anal. Chem. 2015, 87, 6041–6048. [Google Scholar] [CrossRef]
- Kang, D.; Song, J.M.; Yeom, E. Design of microfluidic viscometer based on pressure estimation. J. Vis. 2019, 22, 25–34. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.-K. Microfluidic Rheometer for Characterization of Protein Unfolding and Aggregation in Microflows. Small 2010, 6, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.C.; Yeom, E. Microfluidic method for measuring viscosity using images from smartphone. Opt. Lasers Eng. 2018, 104, 237–243. [Google Scholar] [CrossRef]
- Choi, J.; Hyun, J.-C.; Yang, S. On-chip Extraction of Intracellular Molecules in White Blood Cells from Whole Blood. Sci. Rep. 2015, 5, 15167. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003, 9, 727–728. [Google Scholar] [CrossRef]
- Laxmi, V.; Joshi, S.S.; Agrawal, A. Extracting white blood cells from blood on microfluidics platform: A review of isolation techniques and working mechanisms. J. Micromech. Microeng. 2022, 32, 053001. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Water retention and controlled release of KCl by using microwave-assisted green synthesis of xanthan gum-cl-poly (acrylic acid)/AgNPs hydrogel nanocomposite. Polym. Bull. 2020, 77, 4867–4893. [Google Scholar] [CrossRef]
- Bruijns, B.; Tiggelaar, R.; Gardeniers, H. Dataset of the absorption, emission and excitation spectra and fluorescence intensity graphs of fluorescent cyanine dyes for the quantification of low amounts of dsDNA. Data Brief 2017, 10, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, Z.; Zeng, C.; Nie, G. Specific absorption spectra of hemoglobin at different PO2 levels: Potential noninvasive method to detect PO2 in tissues. J. Biomed. Opt. 2012, 17, 125002. [Google Scholar] [CrossRef]
- Hou, H.W.; Gan, H.Y.; Bhagat, A.A.S.; Li, L.D.; Lim, C.T.; Han, J. A microfluidics approach towards high-throughput pathogen removal from blood using margination. Biomicrofluidics 2012, 6, 024115–2411513. [Google Scholar] [CrossRef]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.-W.; Bhagat, A.A.S.; Irwin, D.; Lau, D.P.; Lim, A.S.T.; Lim, K.H.; Krisna, S.S.; Lim, W.-T.; et al. Clinical Validation of an Ultra High-Throughput Spiral Microfluidics for the Detection and Enrichment of Viable Circulating Tumor Cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.-W.; Bhagat, A.A.S.; Lim, W.-T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Hupert, M.L.; Jackson, J.M.; Wang, H.; Witek, M.A.; Kamande, J.; Milowsky, M.I.; Whang, Y.E.; Soper, S.A. Arrays of high-aspect ratio microchannels for high-throughput isolation of circulating tumor cells (CTCs). Microsyst. Technol. 2014, 20, 1815–1825. [Google Scholar] [CrossRef] [PubMed]

| XG Concentration (ppm) | (mPa∙s) 1 | (mPa∙s) 2 | λ (ms) |
|---|---|---|---|
| 50 | 478 | 0.95 | 76.5 |
| 100 | 698 | 1.5 | 92.5 |
| 250 | 790 | 2.3 | 172.5 |
| 500 | 892 | 4 | 313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Kim, M.; Kim, Y.; Choo, S.; Kim, T.E.; Han, J.; Han, B.J.; Lim, C.S.; Nam, J. Continuous On-Chip Cell Washing Using Viscoelastic Microfluidics. Micromachines 2023, 14, 1658. https://doi.org/10.3390/mi14091658
Lim H, Kim M, Kim Y, Choo S, Kim TE, Han J, Han BJ, Lim CS, Nam J. Continuous On-Chip Cell Washing Using Viscoelastic Microfluidics. Micromachines. 2023; 14(9):1658. https://doi.org/10.3390/mi14091658
Chicago/Turabian StyleLim, Hyunjung, Minji Kim, Yeongmu Kim, Seunghee Choo, Tae Eun Kim, Jaesung Han, Byoung Joe Han, Chae Seung Lim, and Jeonghun Nam. 2023. "Continuous On-Chip Cell Washing Using Viscoelastic Microfluidics" Micromachines 14, no. 9: 1658. https://doi.org/10.3390/mi14091658
APA StyleLim, H., Kim, M., Kim, Y., Choo, S., Kim, T. E., Han, J., Han, B. J., Lim, C. S., & Nam, J. (2023). Continuous On-Chip Cell Washing Using Viscoelastic Microfluidics. Micromachines, 14(9), 1658. https://doi.org/10.3390/mi14091658







