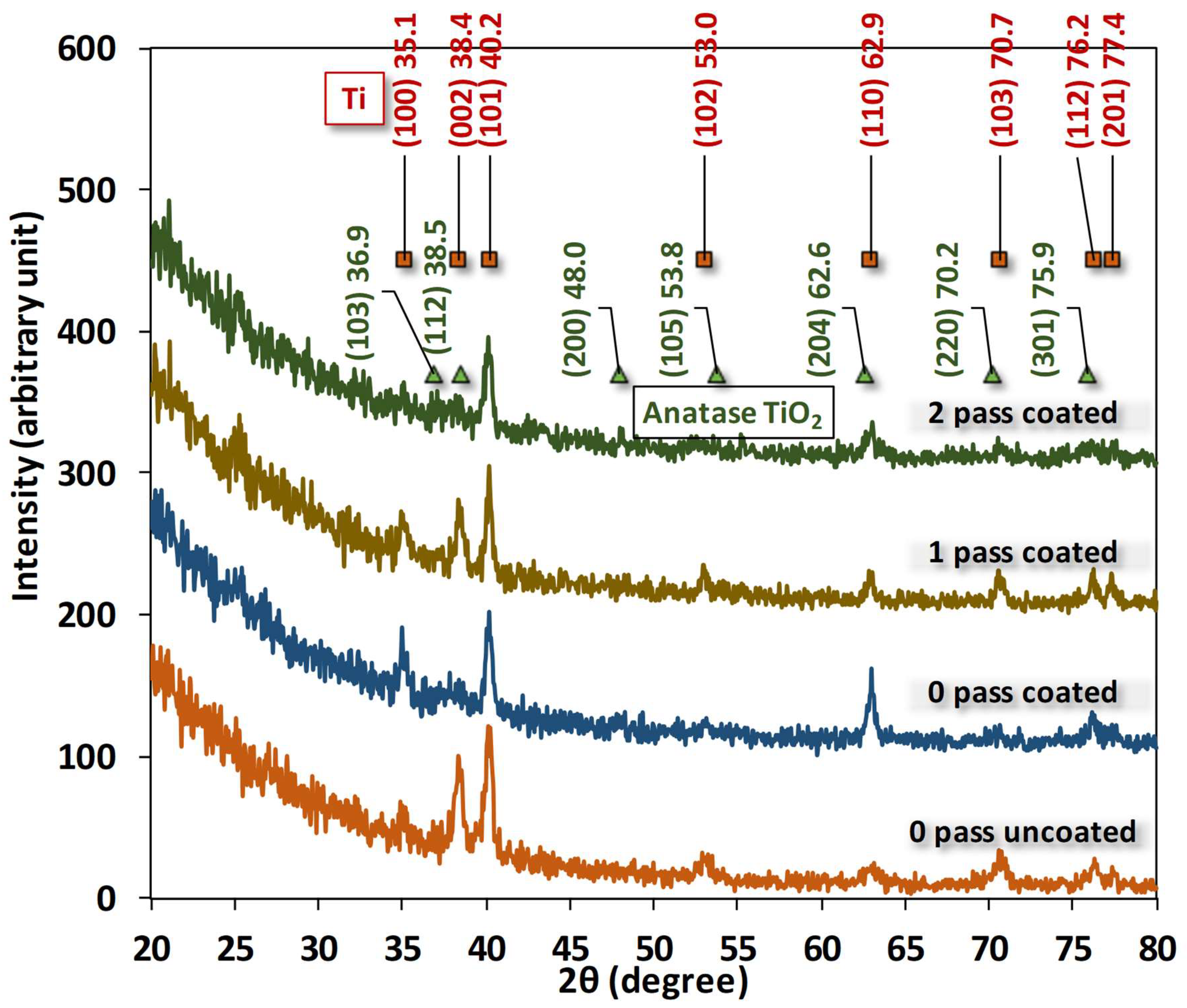
The plot is made by measuring the anodic and cathodic currents on a logarithmic scale as a function of the applied potential. The slope of the Tafel line gives information on the kinetics of electrochemical reactions, and the intersection of the anodic and cathodic Tafel lines gives information about corrosion characteristics. The formula below can be used to determine the corrosion parameters from the Tafel plot.
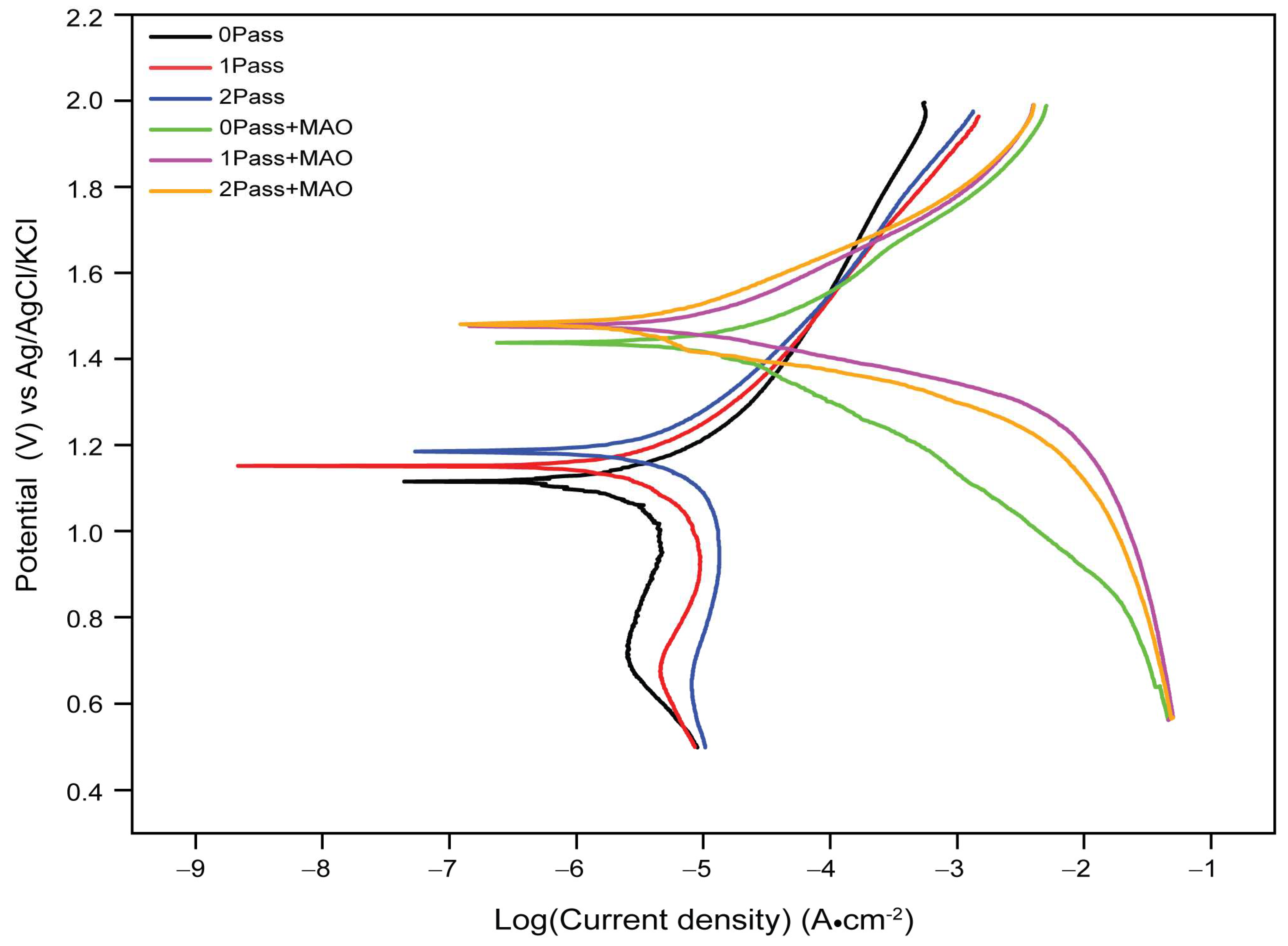
On the other hand, the coated samples show a significant increase in electrode potential values compared to the uncoated samples. The 0-pass coated sample has an electrode potential of 1.42 V, which is significantly higher than that of the uncoated 0-pass sample (1.12 V). The electrode potentials for the 1-pass and 2-pass coated samples are 1.44 V and 1.47 V, respectively; thus, indicating that further coating does fairly provide additional protection against corrosion. This suggests that the coating is highly effective in reducing the corrosion rate of the CP Ti sample.
The results of the Tafel plot analysis suggest that the coating significantly reduces the rate of corrosion compared to the uncoated samples. The increase in electrode potential values of the coated samples indicates that the coating provides a more stable and passive layer, preventing the metallic samples from further corrosion. The effect of additional coating passes appears to be limited, with no significant change in electrode potential observed between 1-pass and 2-pass coated samples. Overall, the results suggest that the coating significantly enhances the corrosion resistance of the metallic samples. The findings could be useful in designing and developing more effective corrosion-resistant coatings for industrial applications.
When testing the titanium samples for corrosion behavior in a 3.5% NaCl electrolyte solution, different variables contribute to the reported decrease or increase in corrosion potentials.
Titanium, when exposed to an electrolyte, has a higher initial corrosion potential than other metals. As a result of the formation of a passive oxidation layer on its surface during the corrosion process, titanium’s corrosion potential decreases. By operating as a barrier and reducing the rate of corrosion, this inert oxide layer prevents further deterioration. Micro-arc oxidation (MAO) enhances the corrosion resistance of titanium by producing a ceramic oxide layer on its surface. The MAO coating enables barrier protection, reduced diffusion of corrosive species, and enhanced passive oxide layer stability. As a result, the titanium’s corrosion potential is decreased relative to both its as-received state and that of titanium that has been substantially deformed.
Corrosion Loss
The data presented in
Table 1 provide a view of the effectiveness of coatings in reducing the corrosion rate of commercially pure titanium samples. This finding is consistent with previous research studies that have examined the impact of coatings on metallic materials. This experiment conducted a similar study on a titanium alloy and discovered that the corrosion resistance of the alloy significantly increased with the use of a protective coating [
81].
In their study, the coating’s thickness and composition had a significant effect on the metal corrosion resistance. However, the effectiveness of the coating in reducing the corrosion rate of the metal depends on the selection of the coating material and method of application. Discovering the most effective coatings for various metallic materials and applications requires additional research.
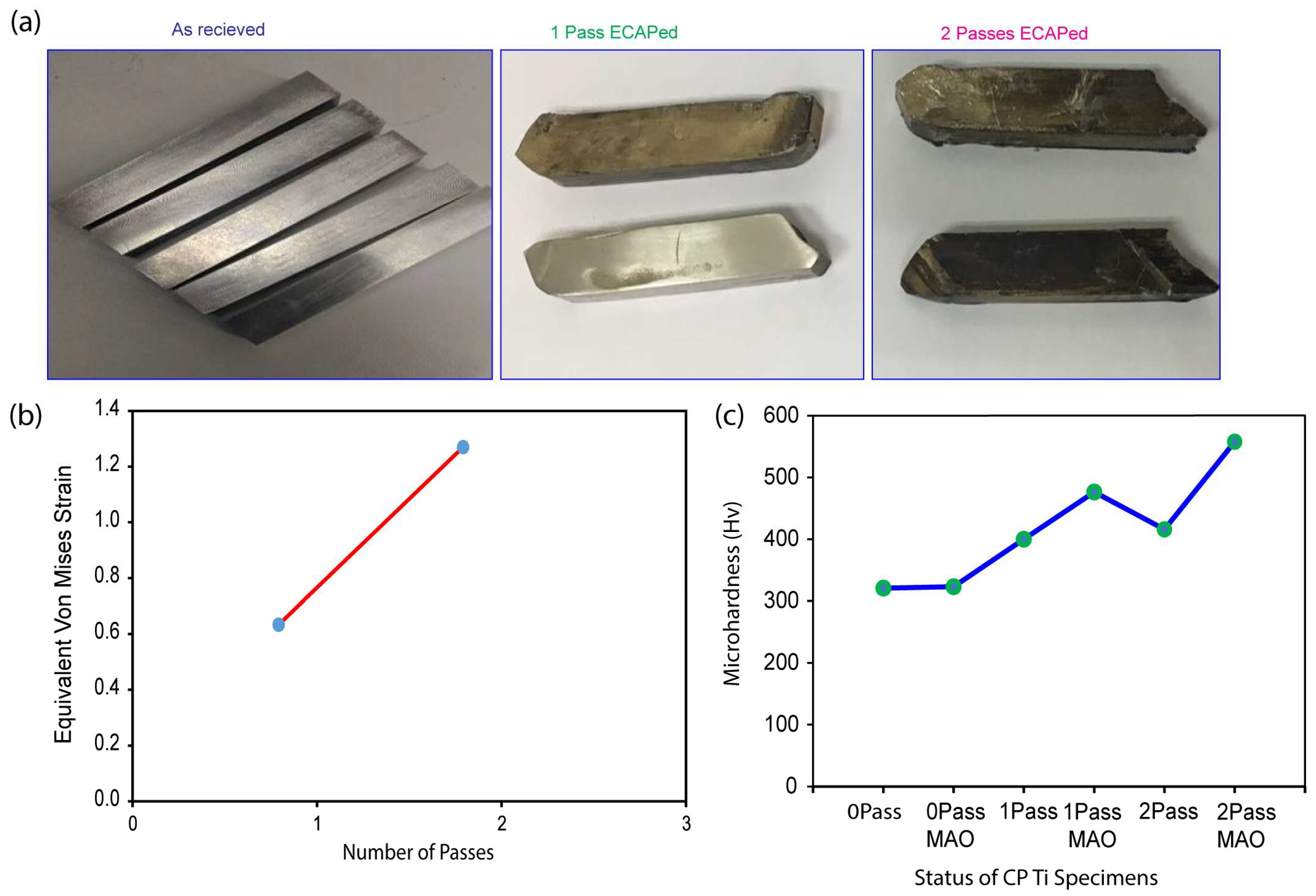
The corrosion loss, expressed in miles per year (mmpy), was used in Equation (1) to calculate the corrosion resistance of the samples. The samples were labeled as follows: 0Pass (untreated), 1Pass (after one ECAP pass), 2Pass (after two ECAP passes), 0PassMAO (after MAO treatment), UFG1MAO (after 1-pass ECAP treatment followed by MAO treatment), and 2PassMAO (after 2-pass ECAP treatment followed by MAO treatment).
According to the corrosion loss measurement data in
Table 1, 2PassMAO had the lowest corrosion rate (0.495 mmpy), followed by 1PassMAO (0.502 mmpy), 0PassMAO (0.510 mmpy), 2Pass (0.610 mmpy), 1Pass (0.649 mmpy), and 0Pass (0.673 mmpy). These findings indicate that increasing titanium’s corrosion resistance can be accomplished by combining ECAP and MAO surface modifications.
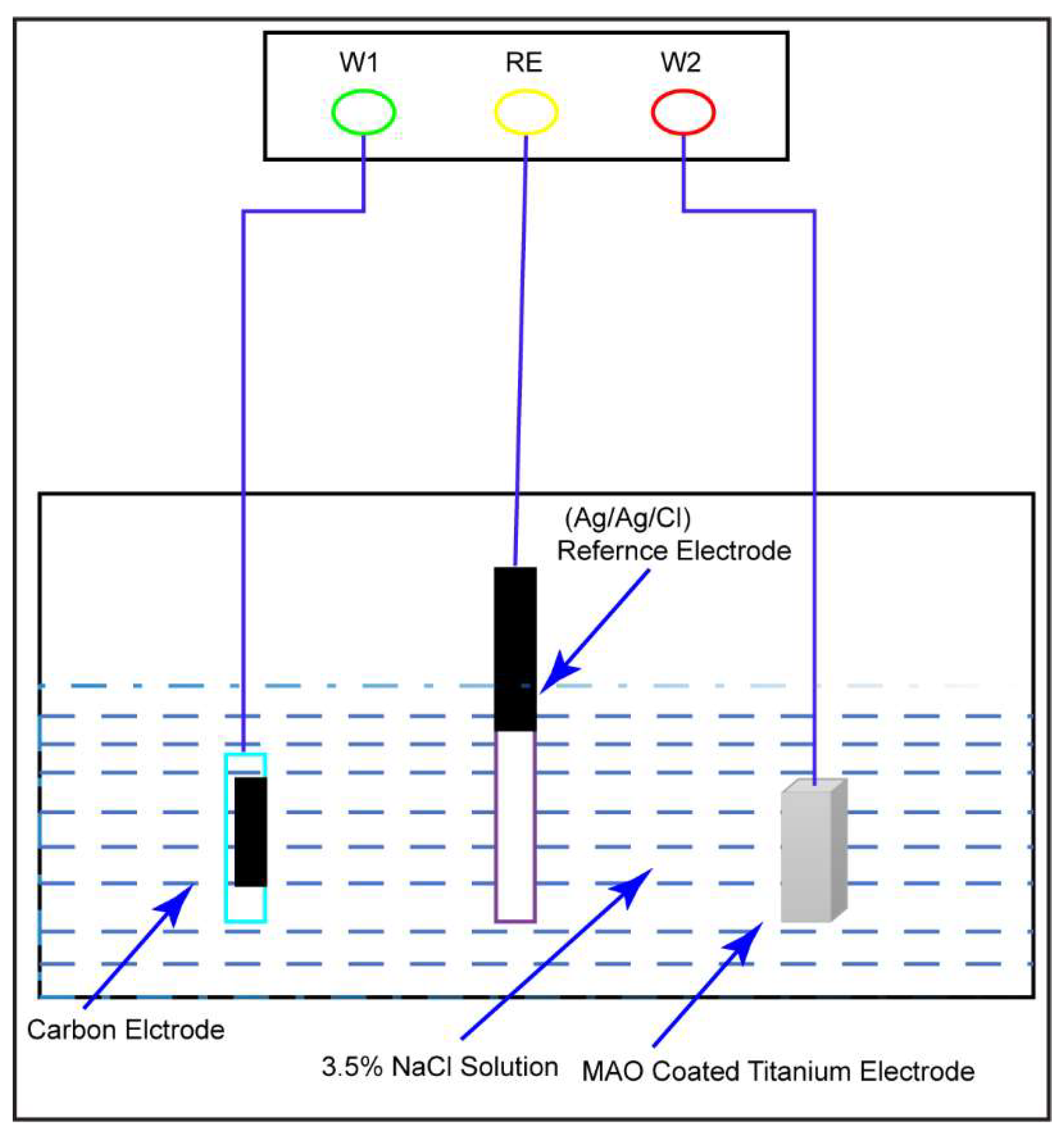
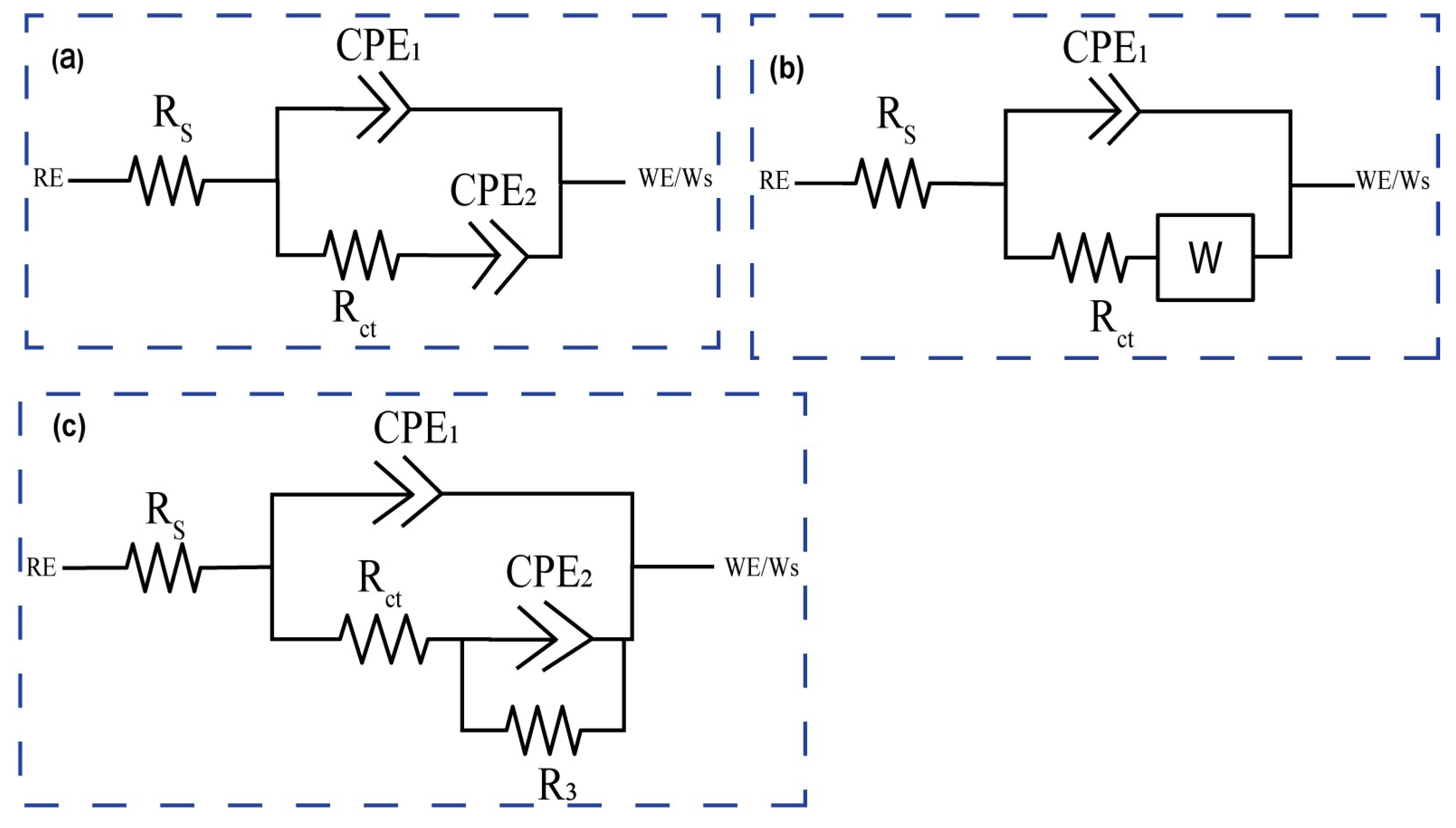
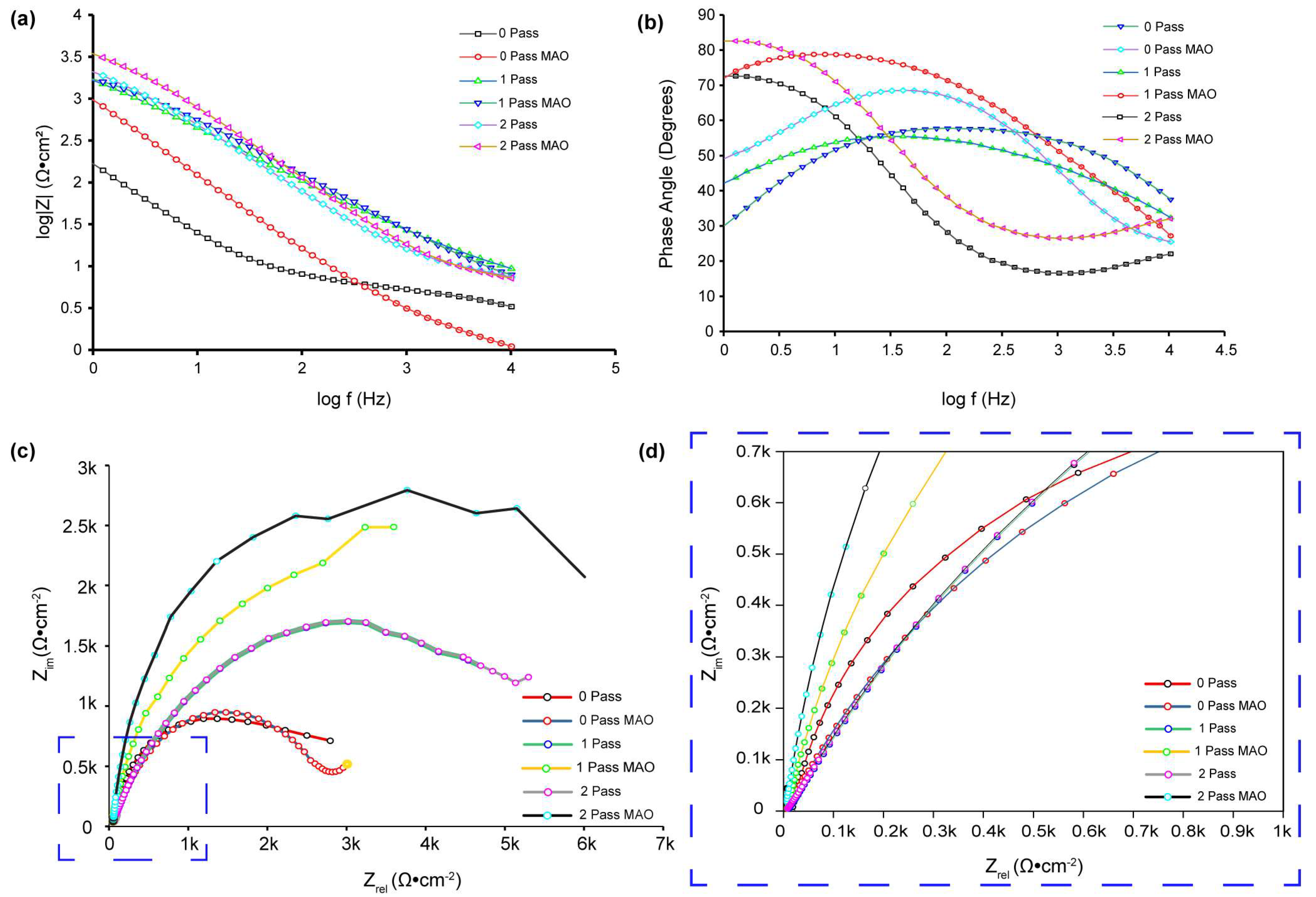
For corrosion analysis, electrochemical impedance spectroscopy (EIS) was utilized. The modified equivalent circuit of Randles was then used to match the gathered impedance data. This circuit model shows different mechanisms at the sample–interface contact. In this investigation, corrosion was analyzed using conventional electrical circuits that are comparable and substantiated by physical evidence. These circuits are illustrated in
Figure 10a–c. In accordance with
Table 2, the as-received, 0PassMAO, 1Pass, and 2Pass samples exhibit the same corrosion behavior as the sample with a natural oxide coating (passive TiO
2 layer), as depicted in
Figure 10a. Due to the diffusion restriction in the corrosion process, a Warburg resistance (W) was applied to the pure titanium sample coated with amorphous hydroxyapatite (HA) in the circuit depicted in
Figure 10b. The circuits in
Figure 10c describe the passive TiO
2 layer and the MAO-coated double layer, respectively, as Rct with the lowest value and R3 with a greater value in cm
−2 are introduced (refer to
Table 2).
The Rs represents the solution or electrolyte resistance, while R
ct represents the electron-transfer resistance; a constant phase element (CPE
1) represents the real double-layer capacitance (C
dl), and a constant phase element (CPE
2) represents the Warburg impedance (Z
w). The electron transfer rate constants (k
et) were computed as follows [
82,
83]:
The results in
Table 2 show that the electron transfer rate constant, k
et, is larger for the 0Pass, 1Pass, and 2Pass samples than for the ECAP- and MAO-treated samples, with the exception of the 2PassMAO sample, which also has a high value of k
et. This is due to the lower R
ct (charge transfer resistance) values seen in these samples. As a result, lower R
ct values lead to higher k
et values, suggesting a more efficient electron transfer mechanism in the samples mentioned.
From
Figure 10c, R3 and CPE
2 are parallel-connected elements that characterize the charge transfer process at the nonporous layer/electrolyte interface by measuring the charge transfer resistance and capacitance of the electric double layer, respectively. The following equation defines a constant phase element (CPE), which represents the capacitive element [
82,
84].
It is evident that constant
, (2πf) radial frequency
, and exponent n are all related. Exponent n is derived from the logarithmic plot of impedance (Z) versus frequency (f). When n = 0, the constant phase element (CPE) functions as a pure resistor, when n = 1 as a pure capacitor, and when n = −1 as an inductor. When n = 0.5, the Warburg impedance (Zw), which is related to mass transport control due to ion diffusion at the electrode-solution interface, is present. CPE is determined by electrode properties (e.g., roughness), relaxation durations at the electrode–electrolyte interface, porosity, and dynamic disturbance associated with diffusion [
85]. In the case of the 0Pass and 2Pass ECAPed samples, when the value of n1 approaches 1, a flawless capacitive behavior is indicated by
Table 2. However, because n2 ranges from 0.52 to 0.91 (instead of the Warburg diffusion), the electrode’s adsorbed coating is porous. Using Equation (5), the electron transfer rate constant (ket) can be calculated. Bode diagrams (phase angle versus log f,
Figure 10b) reveal that the phases are not 90 degrees, as predicted for optimum capacitive behavior, but rather from 50 to 83 degrees. As evidenced by the gradients of the Bode diagrams (log Z vs. log f,
Figure 10a), logarithmic plots of impedance (Z) versus frequency (f) reveal an unchanging slope of −0.78 in the middle-frequency band, exhibiting pseudo-capacitive behavior. At high frequencies, gradients approaching 0 indicate resistant behavior. This is due to the coating on the HA titanium samples’ composition and structure, which makes it excellent for storing and discharging charge during electrochemical operations.
Figure 10c demonstrates the absence of Warburg impedance (W = 0), but
Figure 11 presents experimental data fitted using the same circuit. It properly describes the experimental data and has an approximation error of roughly 3%. In contrast to previous research [
82], fitting the 1PassMAO using the equivalent circuit shown in
Figure 10b did not result in a significant drop in the fitted result when the Warburg element was included, according to the scheme shown in
Figure 11b. The experimental data contain a 4% approximation error. This variance might be caused by a number of factors, such as the experimental apparatus, substrate used, electrolyte solution used, and so on.
The charge transfer resistance (R
ct) at low frequencies is significantly increased by the creation of an MAO coating on the 0Pass, 1Pass, and 2Pass samples, as shown by the EIS data (
Figure 11b,
Table 2).
Both
Figure 11b and
Table 2 show that the 0PassMAO and 1PassMAO samples also show a rise in R
ct at low frequencies, but to an even greater extent, around 10
3 times that of Rs, showing the establishment of a second layer due to the HA coating applied by MAO. The 0Pass, 1Pass, and 2Pass samples exhibit charge transfer resistance values between 58 and 111.72 Ω·cm
−2, whereas the 0PassMAO and 1PassMAO samples vary from 3381.64 to 6187.4 Ω·cm
−2. It is interesting to see that severe plastic deformation and MAO coating seem to have an effect on the charge transfer resistance. Hence these data demonstrate that ECAP technology improves the titanium sample’s resistance to passivation. The polarization resistance of the double layer may be affected by the introduction of a new resistance component, R3 (Rp) polarization resistance, in the 2PassMAO sample (
Figure 10c). In contrast to the exterior porous coating, which has a resistance value of 6187.4 Ω·cm
−2, the inner oxide layer, which functions as a barrier layer, has resistance values of approximately three orders of magnitude greater.
Table 2 shows that the capacitance of the double layer is drastically decreased by hundreds when MAO coating is applied to the 2PassMAO sample, while the resistance is increased by three orders of magnitude. It is also important to note that the charge transfer resistance (Rct) for the 1Pass and 2Pass samples is increased by a factor of 1.5 compared to the as-received 0Pass sample.
Numerous studies have examined the effect of ECAP and MAO surface modification on the corrosion resistance of Ti and its alloys. For example, after the ECAP treatment, the grain size of pure Ti was refined and the density of dislocations was increased, resulting in a substantially improved corrosion resistance [
86]. Additionally, the ECAP treatment and subsequent MAO surface modification improved the corrosion resistance of the Ti-6Al-4V alloy [
75,
86].
Figure 11b demonstrates that the phase angle of the sample treated with 2-pass ECAP and MAO is greater at lower frequencies. A larger phase angle indicates that the system exhibits a greater capacitance. This type of behavior has been linked to the formation of a barrier layer on the surface of the substrate, in this instance, the 2-pass ECAP and MAO-treated sample. By preventing the passage of charge, this protective coating significantly slows down the corrosion process. Thus, a larger phase angle at low frequencies is frequently indicative of a more effective and protective coating. Unlike the UFGMAO sample, due to its higher frequency and lower phase angle, the CGMAO sample is more susceptible to corrosion.
Figure 11a depicts the Bode graphs; the absolute value of Z in UFGMAO titanium is greater at lower frequencies. Interpreting this behavior, a greater absolute value of Z at lower logarithmic frequencies indicates an increased impedance or corrosion resistance. This suggests that the formation of a protective oxidation layer on the surface of the material is responsible for the increased corrosion resistance observed at lower frequencies in titanium treated with two passes of ECAP and MAO. This oxide layer acts as a barrier, reducing corrosion by isolating the metal from its corrosive environment.
The Nyquist plot depicted in
Figure 11c,d (zoomed area selected in blue dashed square box) enables us to conclude that the UFGMAO sample has the highest value of Z imaginary in ohms per centimeter square. Compared to the other samples, this indicates a reduced corrosion rate. It also indicates that MAO and multi-pass ECAP have the potential to enhance CP Tis corrosion resistance.
Figure 11c indicates that the as-received sample is more corroded than the other samples, indicating a higher corrosion rate.
It has been hypothesized that MAO surface modification increases the corrosion resistance of Ti and its alloys via the formation of a protective oxide layer on the surface of the material. The MAO treatment generates surface fissures containing Ca-P minerals that promote the formation of bioactive hydroxyapatite [
87]. The hydroxyapatite layer provides a stable and protective contact between the material and the corrosive environment by acting as a barrier to prevent the diffusion of corrosive ions.
The chi-square value (χ
2) lies within the range from approximately 5.08 × 10
−5 to 2.49 × 10
−3, indicating that the experimental results are consistent with the results of the fitting (see
Table 2). This result demonstrates that the appropriate electrical circuit models were selected for the investigation.
By referring to all the results from the corrosion test, we can roughly report that the impact of equal-channel angular pressing (ECAP) on the corrosion resistance of the titanium samples used in this investigation cannot be overemphasized.
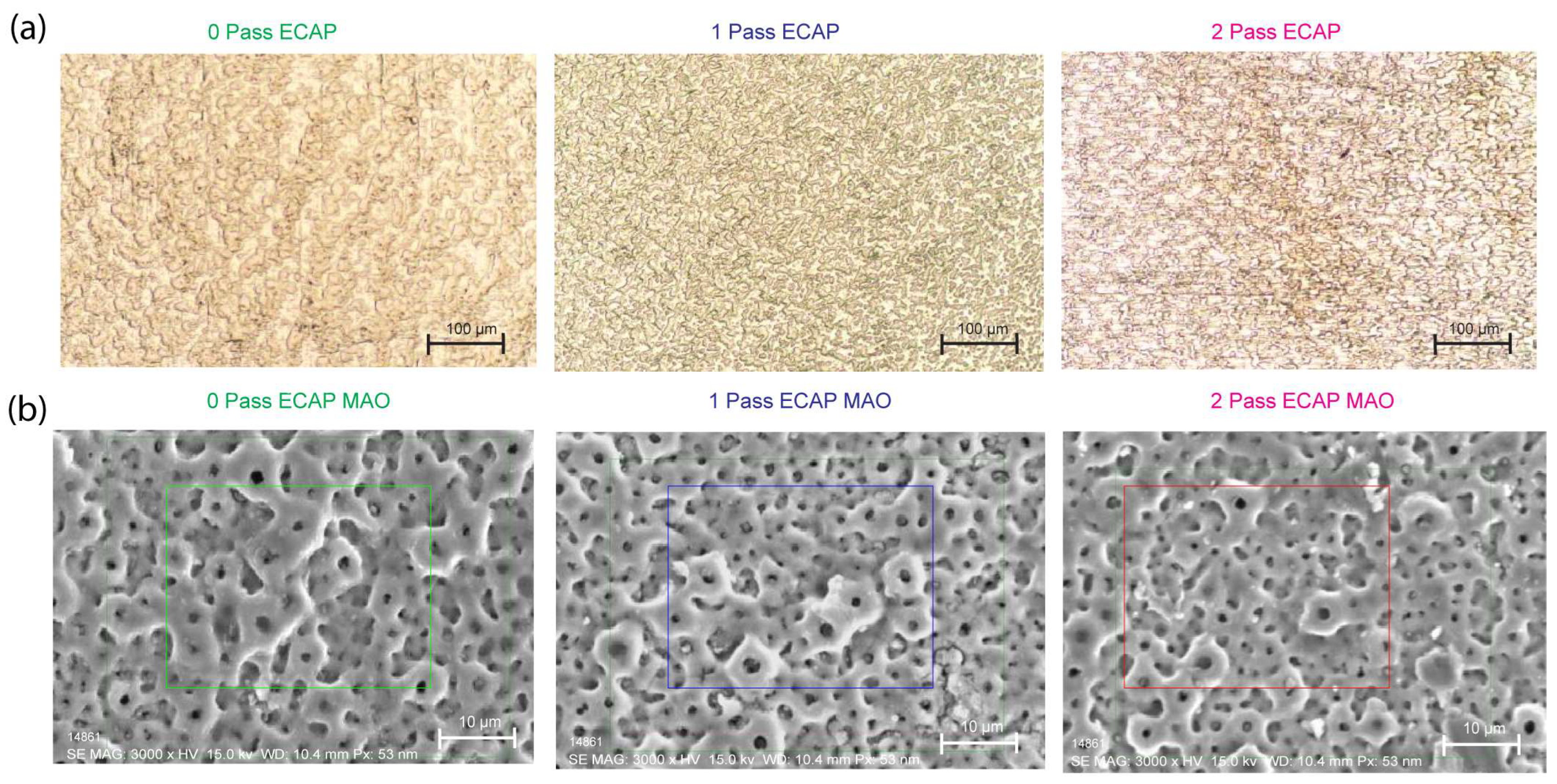
Figure 6a demonstrates that, in comparison to the as-received sample (0Pass CP Ti), the titanium sample exposed to a 2-pass ECAP underwent substantial deformation, resulting in a significant reduction in the grain size and the formation of multiple grain boundaries and defects.
When tested for corrosion behavior, the corrosion resistance (case of a double layer with MAO HA coating applied) and passivation resistance (with a natural coating of TiO
2) of 0-pass, 1-pass, and 2-pass samples without micro-arc oxidation (MAO) coating all increase. The corrosion resistance of ECAPs ultrafine-grained (UFG) titanium exceeds that of coarse-grained (CG) titanium, as demonstrated by testing with simulated saline [
88]. According to a number of studies, the corrosion resistance of UFG/nano-crystalline (NC) titanium is superior to that of titanium in its coarse-grained state. The reduced particle size facilitates the formation of passive coatings on the surface, which in turn improves their adhesion to the crystallographic defects, resulting in enhanced corrosion resistance. Due to the small particle size and numerous grain boundaries, magnesium (Mg) metal is especially susceptible to corrosion.
In conclusion, the ECAP procedure refines titanium’s grain and forms grain boundaries and defects, thereby increasing the metal’s resistance to corrosion. The phenomenon of enhanced corrosion resistance, which is supported by earlier investigations of UFG/NC titanium compared to CG titanium, has been attributed to the faster production and better adhesion of passive coatings on surface crystallographic defects.