Phytochemical-Mediated Biosynthesis of Silver Nanoparticles from Strobilanthes glutinosus: Exploring Biological Applications
Abstract
1. Introduction
2. Results
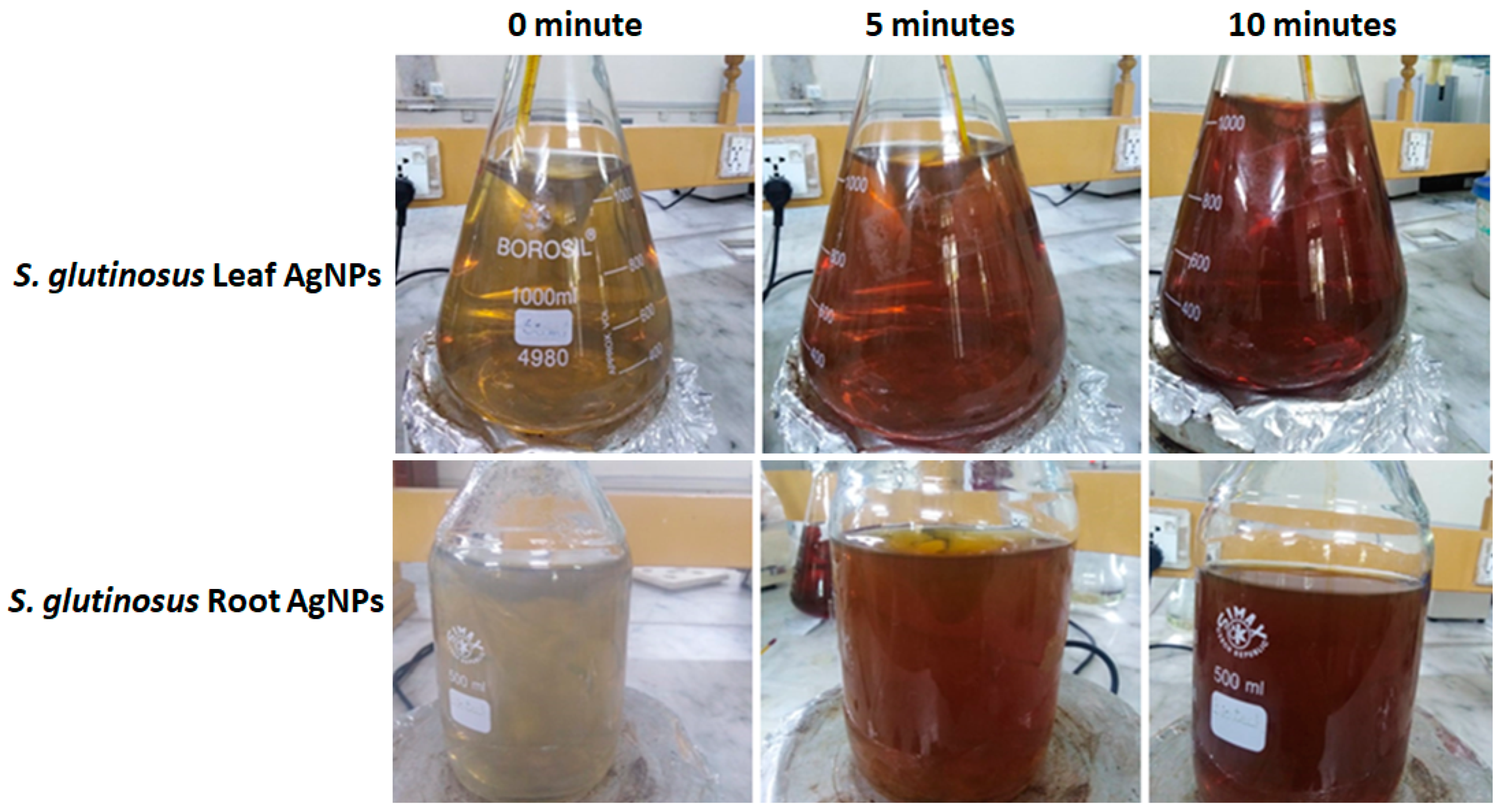
2.1. Optical Observations
2.2. UV–Vis Analysis
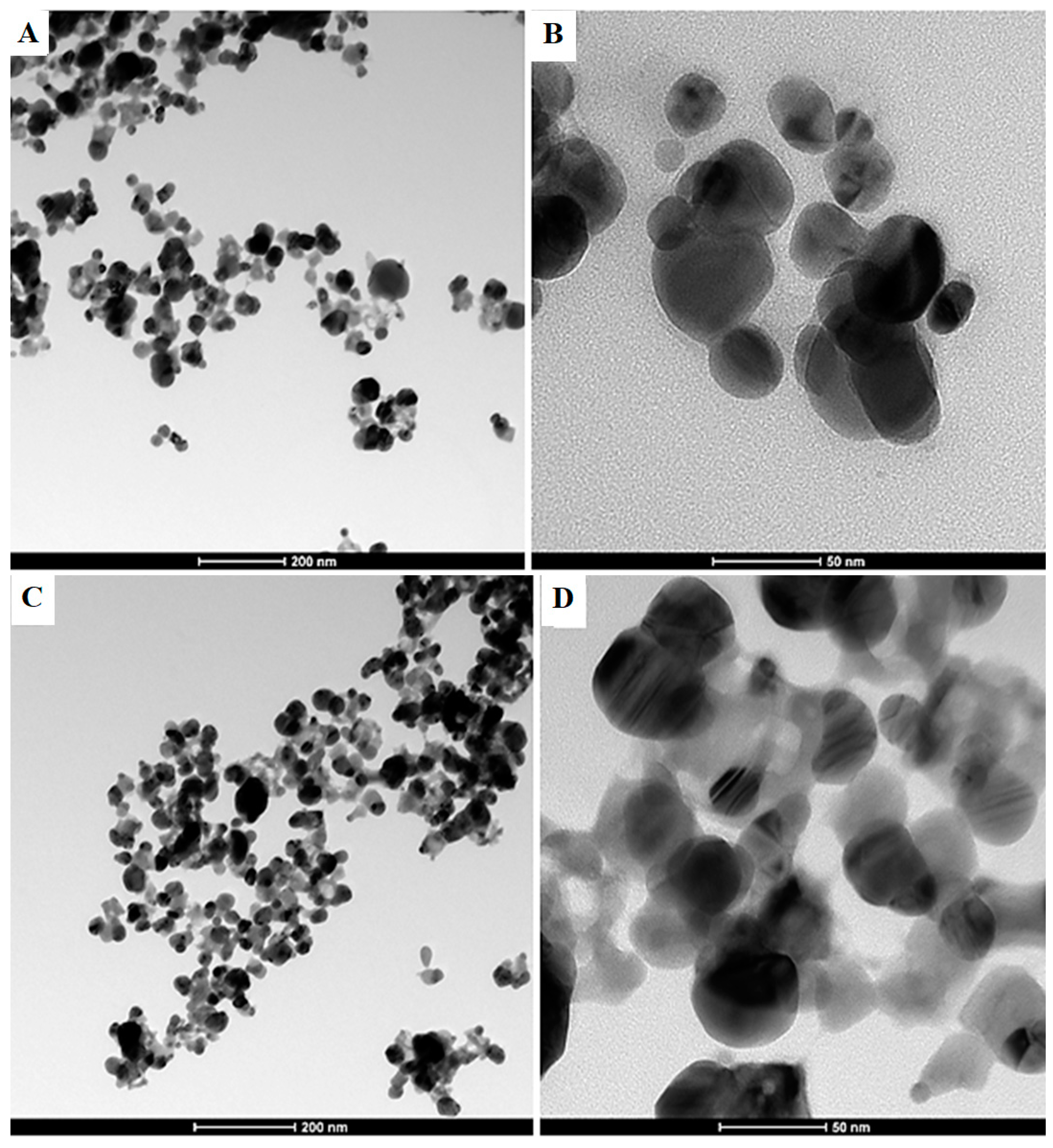
2.3. Transmission Electron Microscopy
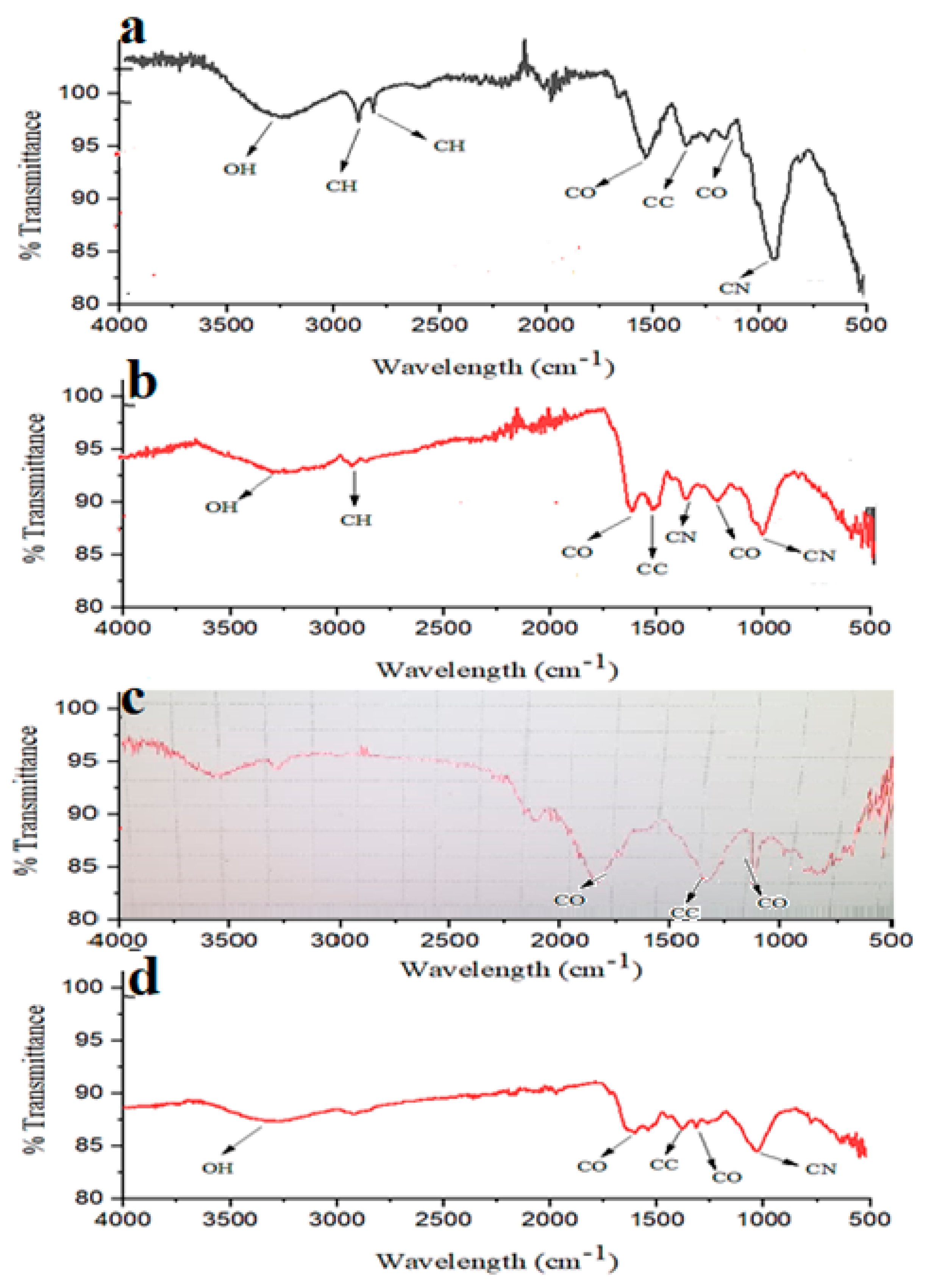
2.4. FTIR Analysis
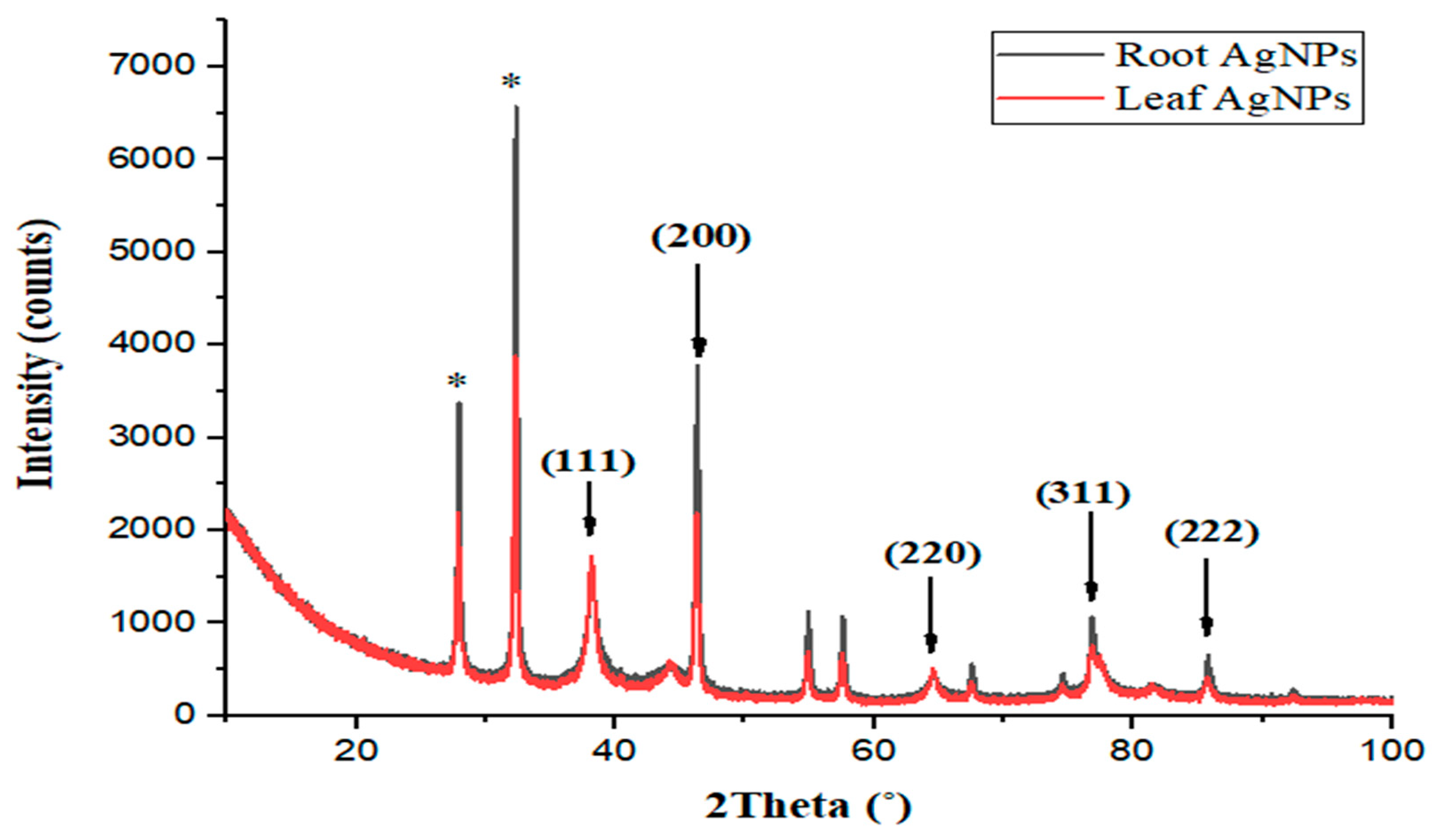
2.5. X-ray Diffraction Analysis
2.6. Antibacterial Activity
2.7. Phytochemical Analysis
2.7.1. Total Phenolic Content
2.7.2. Total Flavonoid Content
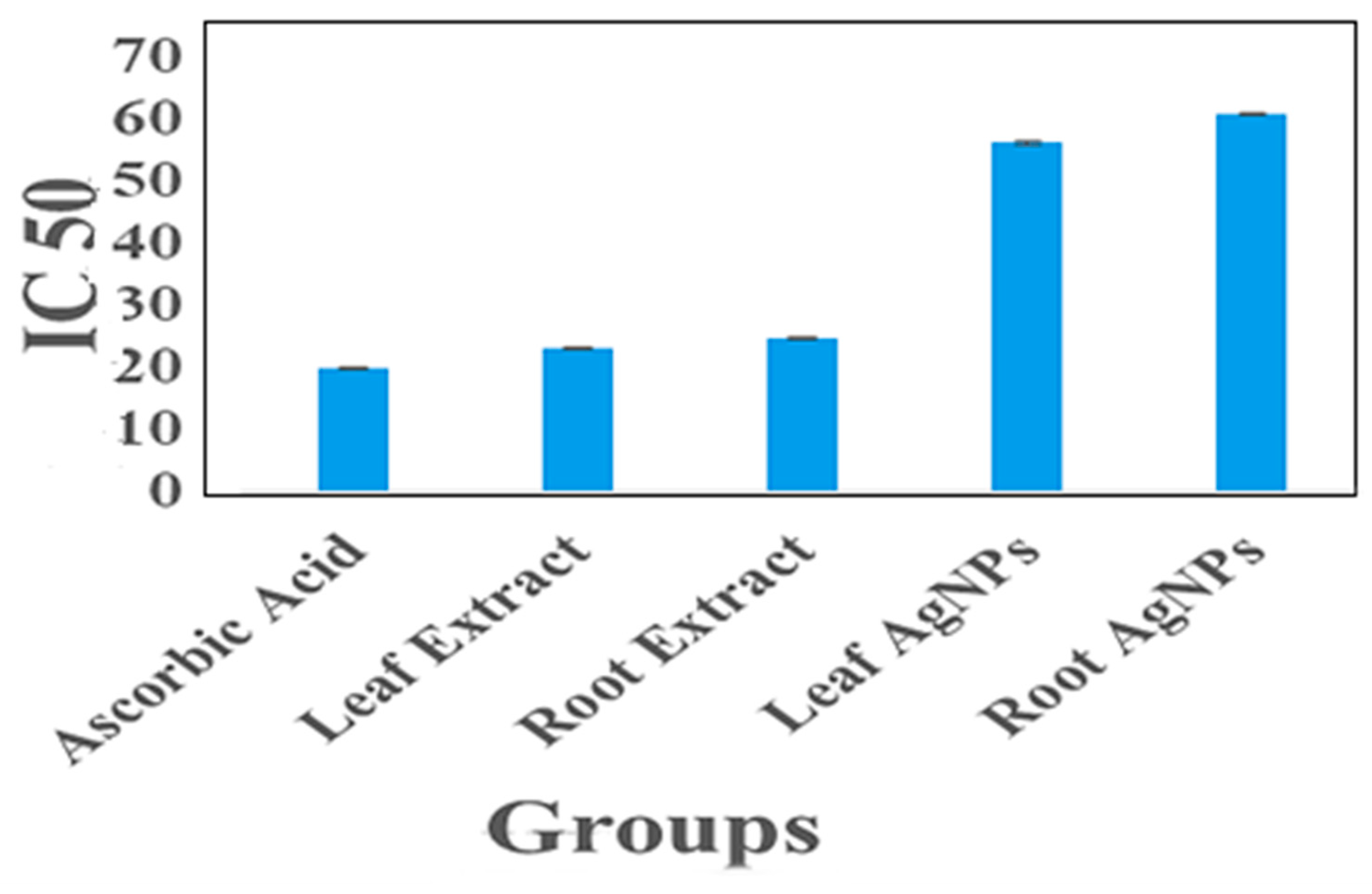
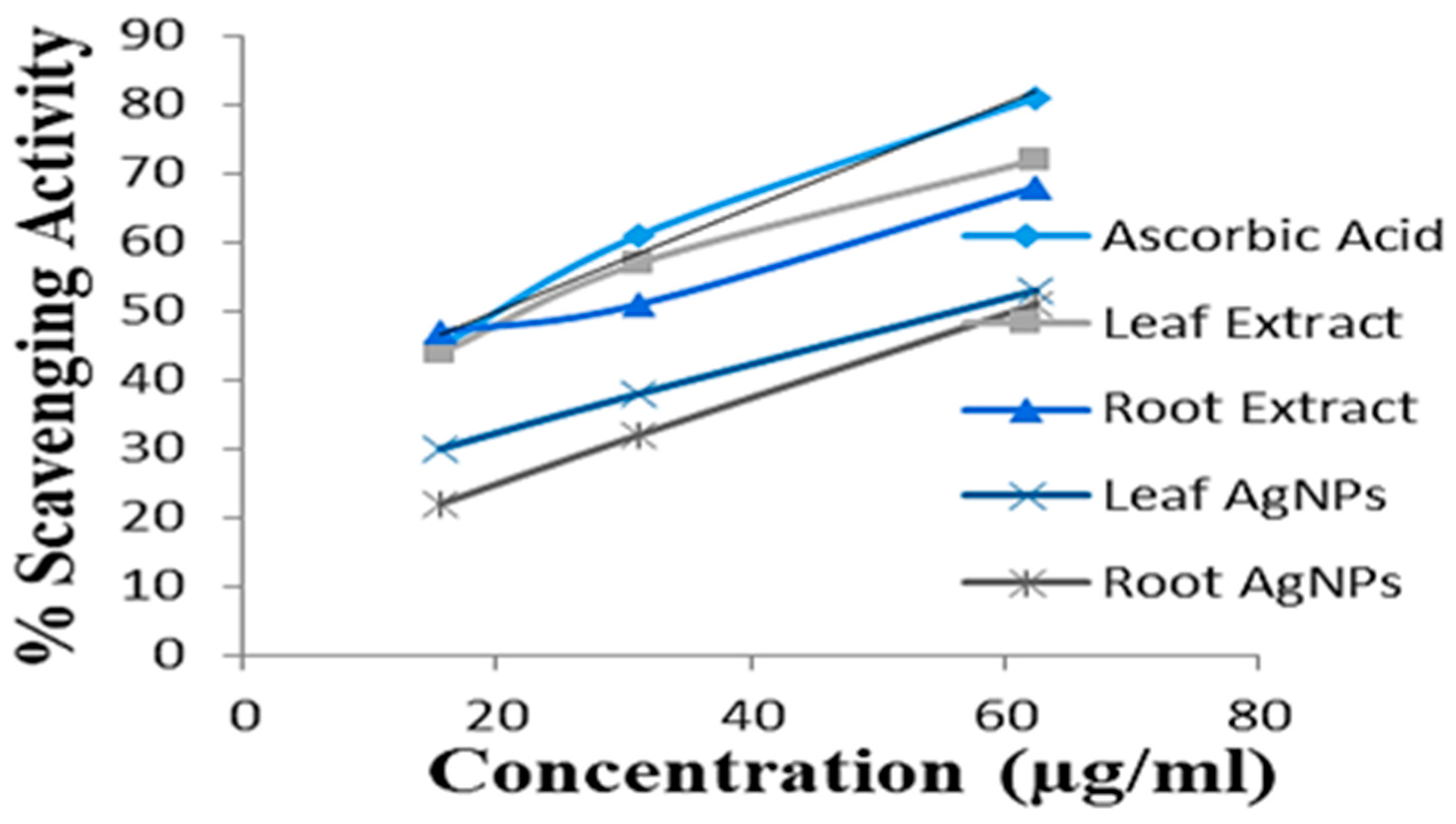
2.8. Antioxidant Activity
3. Materials and Methods
3.1. Green Synthesis of AgNPs from Leaf Extract
3.2. Green Synthesis of AgNPs from Root Extract
3.3. Antibacterial Activity of Strobilanthes Glutinous Synthesized AgNPs
3.4. Antioxidant Activity of Strobilanthes Glutinous Synthesized AgNPs
3.5. Determination of Total Phenolic Content
3.6. Determination of Total Flavonoid Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rautela, A.; Rani, J. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form follows function: Nanoparticle shape and its implications for nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- Chakraborty, B.; Gan-Or, G.; Duan, Y.; Raula, M.; Weinstock, I.A. Visible-Light-Driven Water Oxidation with a Polyoxometalate-Complexed Hematite Core of 275 Iron Atoms. Angew. Chem. Int. Ed. 2019, 58, 6584–6589. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Singh, M.; Manikandan, S.; Kumaraguru, A. Nanoparticles: A new technology with wide applications. Res. J. Nanosci. Nanotechnol. 2011, 1, 1–11. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Deng, N. Silver nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Naveas, N.; Manso-Silván, M.; Carmona, E.; Garrido, K.; Hernández-Montelongo, J.; Recio-Sánchez, G. Green synthesized silver nanoparticles decorated on nanostructured porous silicon as an efficient platform for the removal of organic dye methylene blue. Green Chem. Lett. Rev. 2022, 15, 108–115. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; López-Cota, A.G.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Quintero-Reyes, I.E.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon 2021, 7, e06923. [Google Scholar] [CrossRef] [PubMed]
- Afreen, A.; Ahmed, R.; Mehboob, S.; Tariq, M.; Alghamdi, H.A.; Zahid, A.A.; Ali, I.; Malik, K.; Hasan, A. Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mater. Res. Express 2020, 7, 075404. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef]
- Qidwai, A.; Kumar, R.; Dikshit, A. Green synthesis of silver nanoparticles by seed of Phoenix sylvestris L. and their role in the management of cosmetics embarrassment. Green Chem. Lett. Rev. 2018, 11, 176–188. [Google Scholar] [CrossRef]
- Punjabi, K.; Choudhary, P.; Samant, L.; Mukherjee, S.; Vaidya, S.; Chowdhary, A. Biosynthesis of nanoparticles: A review. Int. J. Pharm. Sci. Rev. Res 2015, 30, 219–226. [Google Scholar]
- Szczepańska, E.; Bielicka-Giełdoń, A.; Niska, K.; Strankowska, J.; Żebrowska, J.; Inkielewicz-Stępniak, I.; Łubkowska, B.; Swebocki, T.; Skowron, P.; Grobelna, B. Synthesis of silver nanoparticles in context of their cytotoxicity, antibacterial activities, skin penetration and application in skincare products. Supramol. Chem. 2020, 32, 207–221. [Google Scholar] [CrossRef]
- Vigneswari, S.; Amelia, T.S.M.; Hazwan, M.H.; Mouriya, G.K.; Bhubalan, K.; Amirul, A.-A.A.; Ramakrishna, S. Transformation of biowaste for medical applications: Incorporation of biologically derived silver nanoparticles as antimicrobial coating. Antibiotics 2021, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.-V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, Y.; Zhao, X.-F.; Tang, Y.-Q.; Li, Y.-Z.; Xiao, Y.-X.; Hu, Z.-Y.; Su, B.-L.; Yang, X.-Y. Hierarchical TiO2 microsphere assembled from nanosheets with high photocatalytic activity and stability. Chem. Phys. Lett. 2020, 739, 136989. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef]
- Ajaib, M.; Ishtiaq, M.; Shafi, F.; Bhatti, K.; Zahid, M. Antimicrobial and antioxidant analysis of Strobilanthes glutinous: An unexplored medicinal plant. Biosci. Res. 2020, 17, 1521–1534. [Google Scholar]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Kumar, A.; Vishnuprasad, C.N.; Patra, J.K. Photo-mediated optimized synthesis of silver nanoparticles using the extracts of outer shell fibre of Cocos nucifera L. fruit and detection of its antioxidant, cytotoxicity and antibacterial potential. Saudi J. Biol. Sci. 2021, 28, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C 2020, 117, 111266. [Google Scholar] [CrossRef]
- Jebril, S.; Jenana, R.K.B.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.; Prasad, N.; Dhananjaya, B.; Yallappa, S.; Satish, S. Synthesis of silver nanoparticles by endosymbiont Pseudomonas fluorescens CA 417 and their bactericidal activity. Enzym. Microb. Technol. 2016, 95, 128–136. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Priyamvada, B.; Khanna, V.G.; Kumar, D.K.; Sujin, P. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Devaraj, P.; Kumari, P.; Aarti, C.; Renganathan, A. Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against MCF-7 cell line. J. Nanotechnol. 2013, 2013, 598328. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Dixit, A.; Das, S.; Jyoti, A.; Kaushik, S. Biogenic synthesis of silver nanoparticles and its potential application in prevention of acute ear infections. J. Pharm. Sci. Res. 2017, 9, 14. [Google Scholar]
- Ahmed, S.F.; Islam, K.Z.; Khan, M.R. Relationship between inflation and stock market returns: Evidence from Bangladesh. Daffodil Int. Univ. J. Bus. Econ. 2015, 9, 1–12. [Google Scholar]
- Devanesan, S.; AlSalhi, M.S. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int. J. Nanomed. 2021, 16, 3343. [Google Scholar] [CrossRef] [PubMed]
- Kandakumar, S.; Sathya, V.; Manju, V. Synthesis and characterization of silver nanoparticles using Hydnocarpus alpina, its application as A potent antimicrobial and antioxidant agent—A novel study. Int. J. Chem. Tech. Res 2014, 6, 4770–4776. [Google Scholar]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. 2011, 6, 434. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Hussien, H.T.; Saleem, M.M. Biosynthesis of silver nanoparticles synthesized by Aspergillus flavus and their antioxidant, antimicrobial and cytotoxicity properties. Bull. Mater. Sci. 2015, 38, 639–644. [Google Scholar] [CrossRef]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carson, L.; Bandara, S.; Joseph, M.; Green, T.; Grady, T.; Osuji, G.; Weerasooriya, A.; Ampim, P.; Woldesenbet, S. Green synthesis of silver nanoparticles with antimicrobial properties using Phyla dulcis plant extract. Foodborne Pathog. Dis. 2020, 17, 504–511. [Google Scholar] [CrossRef]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the effect of the different parts of Morinda citrifolia L.(noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- Hussein, E.A.M.; Mohammad, A.A.-H.; Harraz, F.A.; Ahsan, M.F. Biologically synthesized silver nanoparticles for enhancing tetracycline activity against Staphylococcus aureus and Klebsiella pneumoniae. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Subashini, J.; Gopiesh Khanna, V.; Kannabiran, K. Anti-ESBL activity of silver nanoparticles biosynthesized using soil Streptomyces species. Bioprocess Biosyst. Eng. 2014, 37, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M. Effect of silver nanoparticles on biofilm formation and EPS production of multidrug-resistant Klebsiella pneumoniae. BioMed Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Akhtar, N.; Mir, T.u.G.; Singh, R.; Jha, P.K.; Mallik, S.K.; Sinha, S.; Tripathi, S.K.; Jain, A.; Jha, A. Targeting apoptotic pathway of cancer cells with phytochemicals and plant-based nanomaterials. Biomolecules 2023, 13, 194. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sakravarthi, C.; Kritika, T.; Arul Kirubakaran, M.; Sarada, D. Evaluation of antioxidant and wound healing potentials of Sphaeranthus amaranthoides Burm. f. BioMed Res. Int. 2013, 2013, 607109. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C 2019, 101, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.; Reddy, S.G.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2018, 46, 1138–1148. [Google Scholar] [CrossRef]
- Sharif, M.S.; Hameed, H.; Waheed, A.; Tariq, M.; Afreen, A.; Kamal, A.; Mahmoud, E.A.; Elansary, H.O.; Saqib, S.; Zaman, W. Biofabrication of Fe3O4 Nanoparticles from Spirogyra hyalina and Ajuga bracteosa and Their Antibacterial Applications. Molecules 2023, 28, 3403. [Google Scholar] [CrossRef] [PubMed]


| Groups | Linear Regression Equation | Mean Absorbance of Plant Extract Solution (Y) | Concentration of GAE in Plant Sample (X) | Total Contents Calculated from Equation = (X × V)/m |
|---|---|---|---|---|
| TPC of leaf extract | Y= 0.0004x + 0.0913 | 0.842 ± 0.02 | 1.877 | 8 |
| TPC of root extract | Y= 0.0004x + 0.0913 | 0.311 ± 0.01 | 0.5 | 1 |
| TPC of Leaf AgNPs | Y= 0.0004x + 0.0913 | 0.377 ± 0.02 | 1.78 | 21 |
| TPC of Root ANPs | Y= 0.0004x + 0.0913 | 0.335 ± 0.04 | 0.6 | 26 |
| Groups | Linear Regression Equation | Mean Absorbance of Plant Extract Solution (Y) | Concentration of GAE in Plant Sample (X) | Total Contents Calculated from Equation = (X × V)/m |
|---|---|---|---|---|
| TFC of leaf extract | Y = 0.0032x + 0.0575 | 1.41 ± 0.02 | 422.65 | 16.906 |
| TFC of root extract | Y = 0.0032x + 0.0575 | 1.96 ± 0.04 | 594.5 | 15.84 |
| TFC of leaf AgNPs | Y = 0.0032x + 0.0575 | 1.63 ± 0.02 | 482.6 | 482.6 |
| TFC of root ANPs | Y = 0.0032x + 0.0575 | 1.67 ± 0.04 | 504.2 | 504.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, R.; Ijaz, S.; Hameed, H.; Nazish, M.; Sharif, M.S.; Afreen, A.; Alarjani, K.M.; Elshikh, M.S.; Mehboob, S.; Abdul Razak, S.; et al. Phytochemical-Mediated Biosynthesis of Silver Nanoparticles from Strobilanthes glutinosus: Exploring Biological Applications. Micromachines 2023, 14, 1372. https://doi.org/10.3390/mi14071372
Javed R, Ijaz S, Hameed H, Nazish M, Sharif MS, Afreen A, Alarjani KM, Elshikh MS, Mehboob S, Abdul Razak S, et al. Phytochemical-Mediated Biosynthesis of Silver Nanoparticles from Strobilanthes glutinosus: Exploring Biological Applications. Micromachines. 2023; 14(7):1372. https://doi.org/10.3390/mi14071372
Chicago/Turabian StyleJaved, Rabia, Shumaila Ijaz, Hajra Hameed, Moona Nazish, Muhammad Shakeeb Sharif, Afshan Afreen, Khaloud Mohammed Alarjani, Mohamed S. Elshikh, Saadia Mehboob, Sarah Abdul Razak, and et al. 2023. "Phytochemical-Mediated Biosynthesis of Silver Nanoparticles from Strobilanthes glutinosus: Exploring Biological Applications" Micromachines 14, no. 7: 1372. https://doi.org/10.3390/mi14071372
APA StyleJaved, R., Ijaz, S., Hameed, H., Nazish, M., Sharif, M. S., Afreen, A., Alarjani, K. M., Elshikh, M. S., Mehboob, S., Abdul Razak, S., Waheed, A., Ahmed, R., & Tariq, M. (2023). Phytochemical-Mediated Biosynthesis of Silver Nanoparticles from Strobilanthes glutinosus: Exploring Biological Applications. Micromachines, 14(7), 1372. https://doi.org/10.3390/mi14071372









