Field Effect Transistor with Nanoporous Gold Electrode
Abstract
1. Introduction
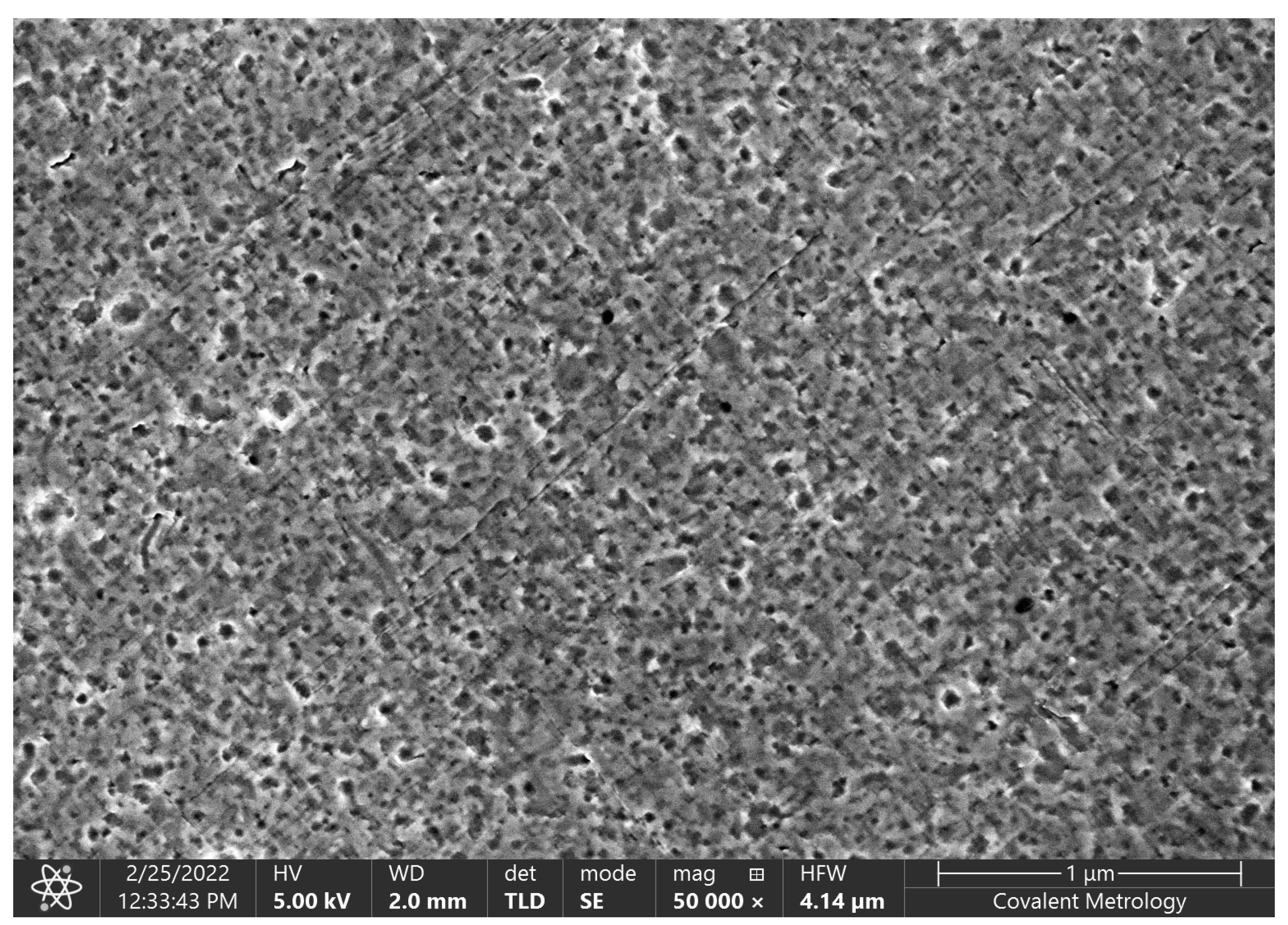
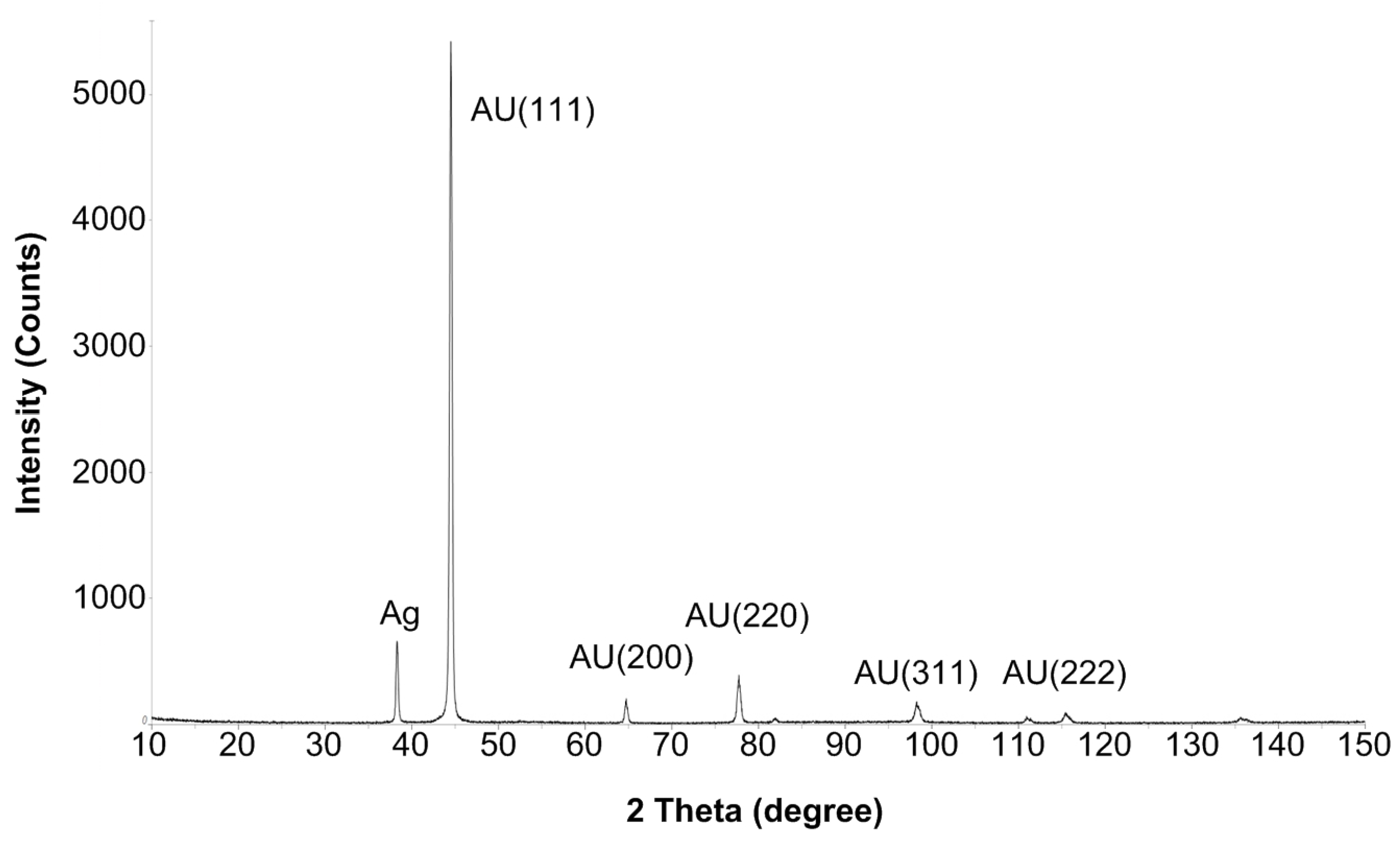
2. Fabrication/Assembly of the Prototype MOSFETs
3. Theory of the MOSFET with NPG Gate Electrode
4. Experimental Results
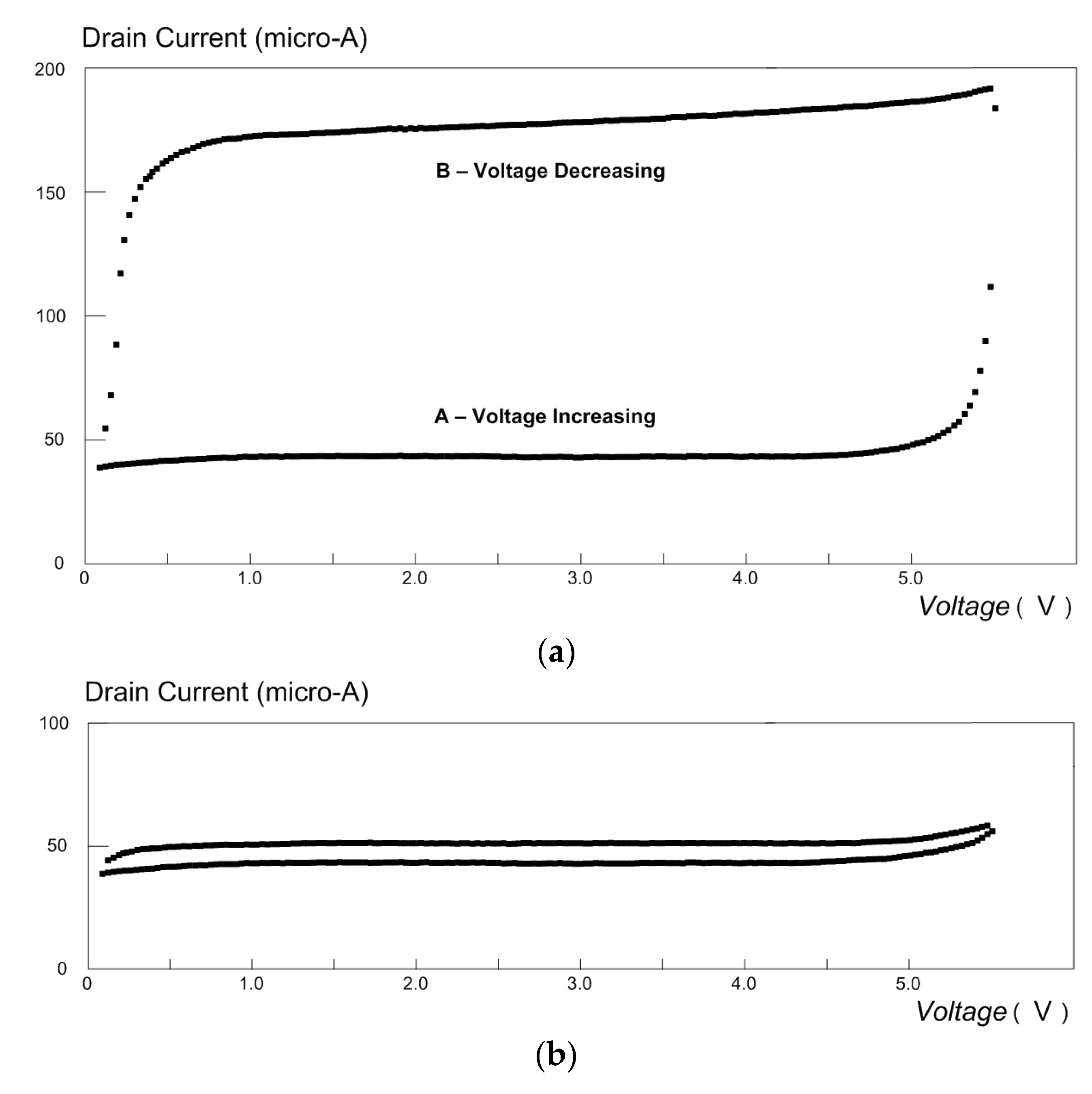
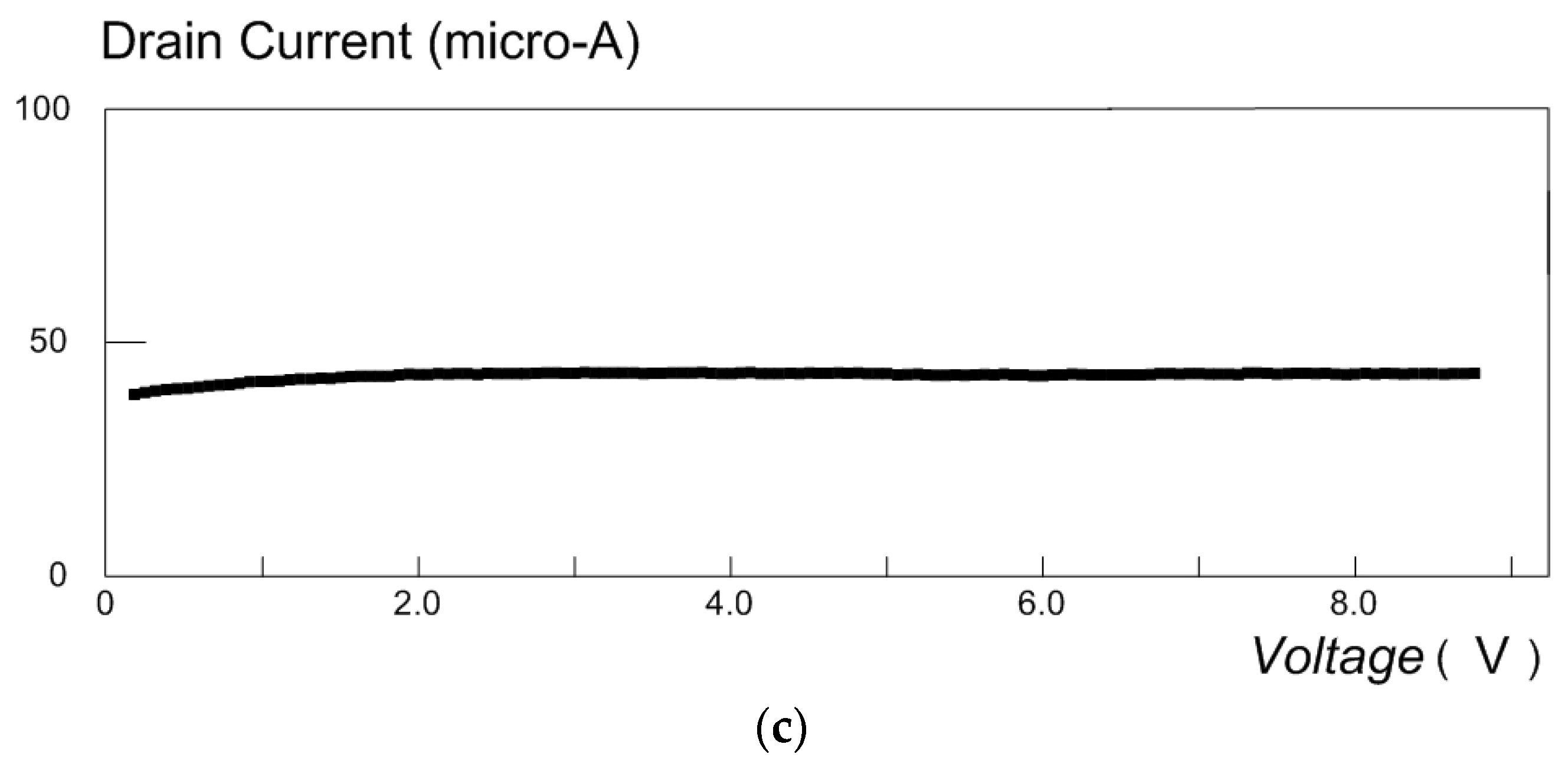
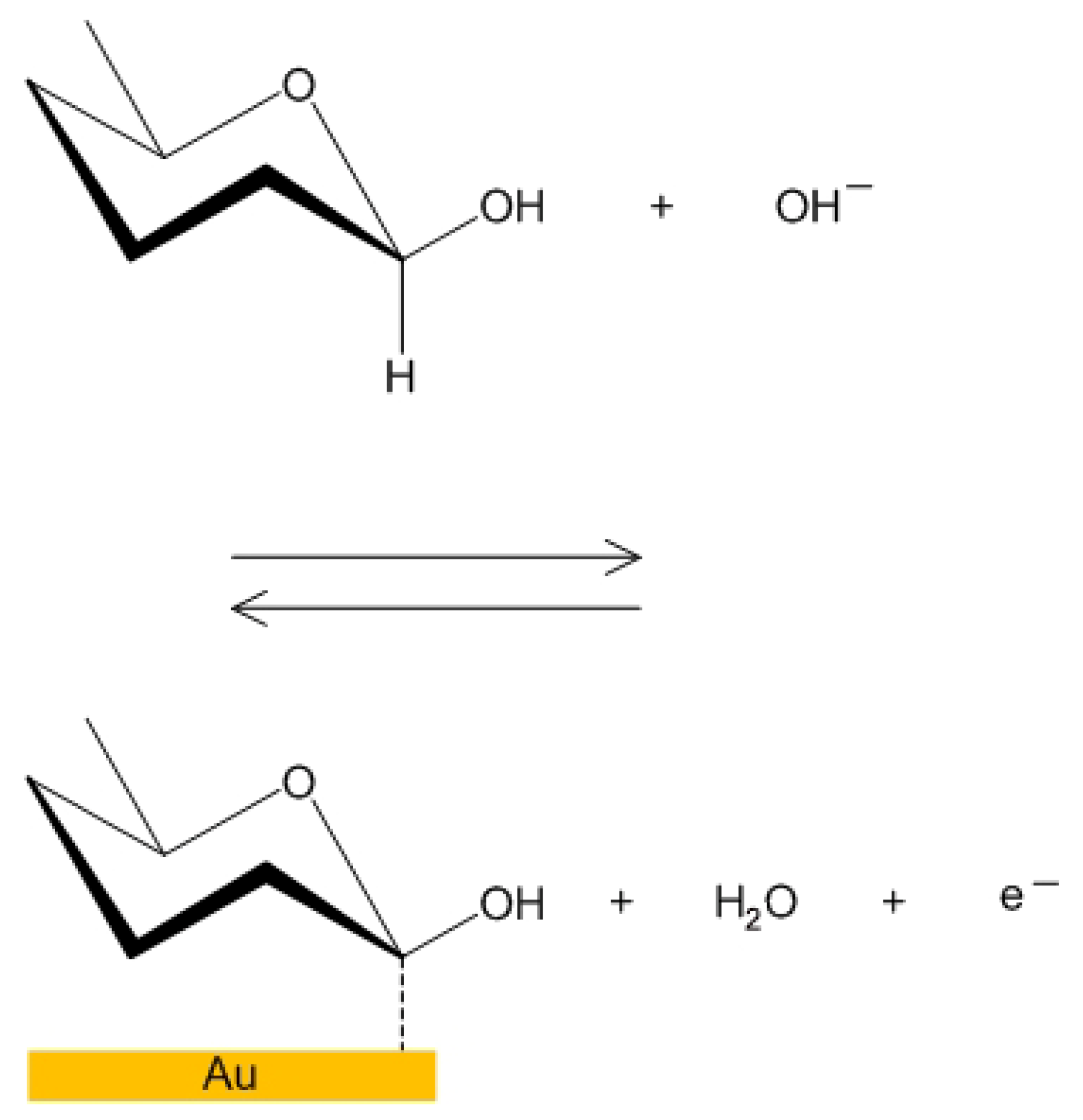
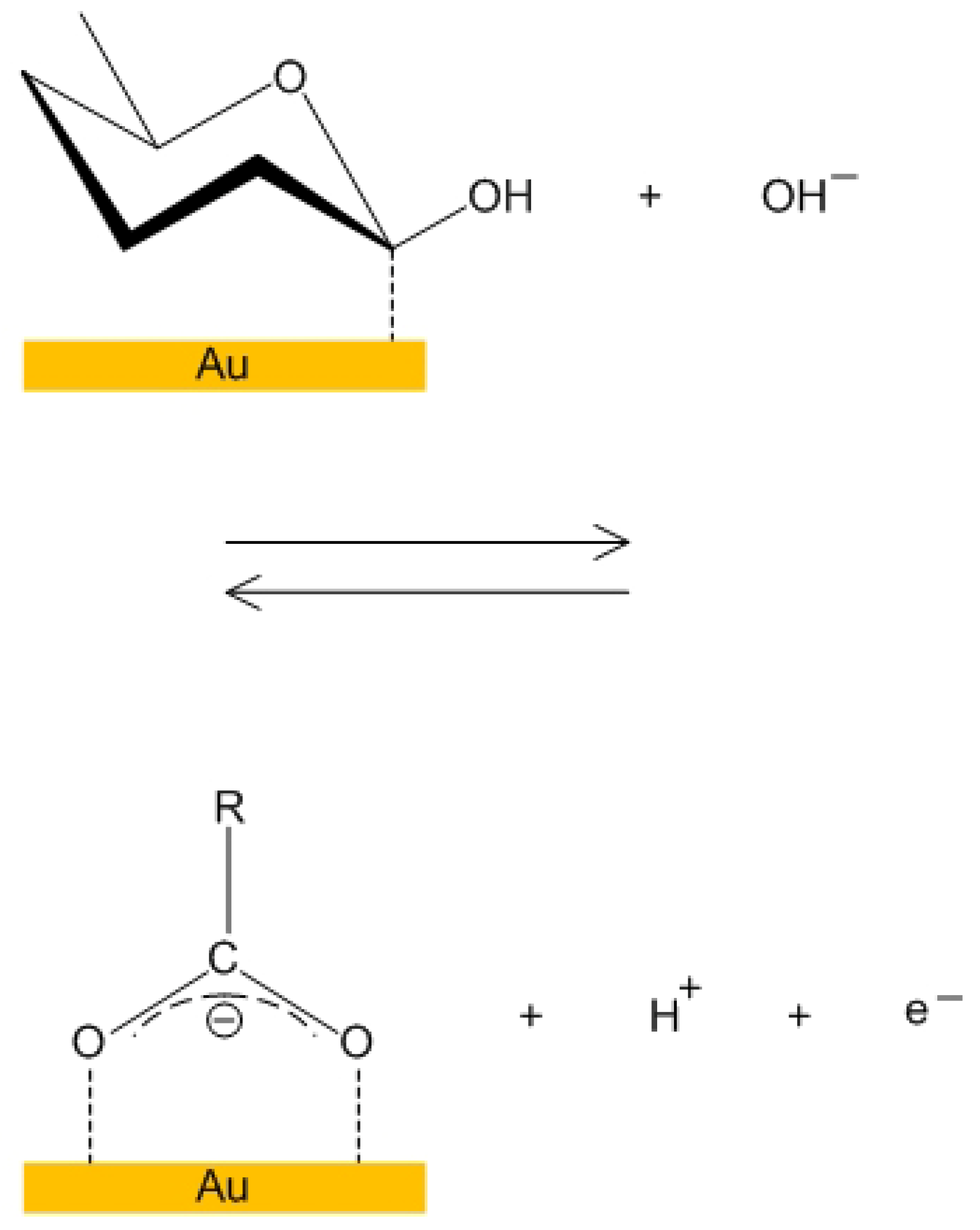
4.1. Cyclic Voltammetry for MOSFET in a Glucose Solution
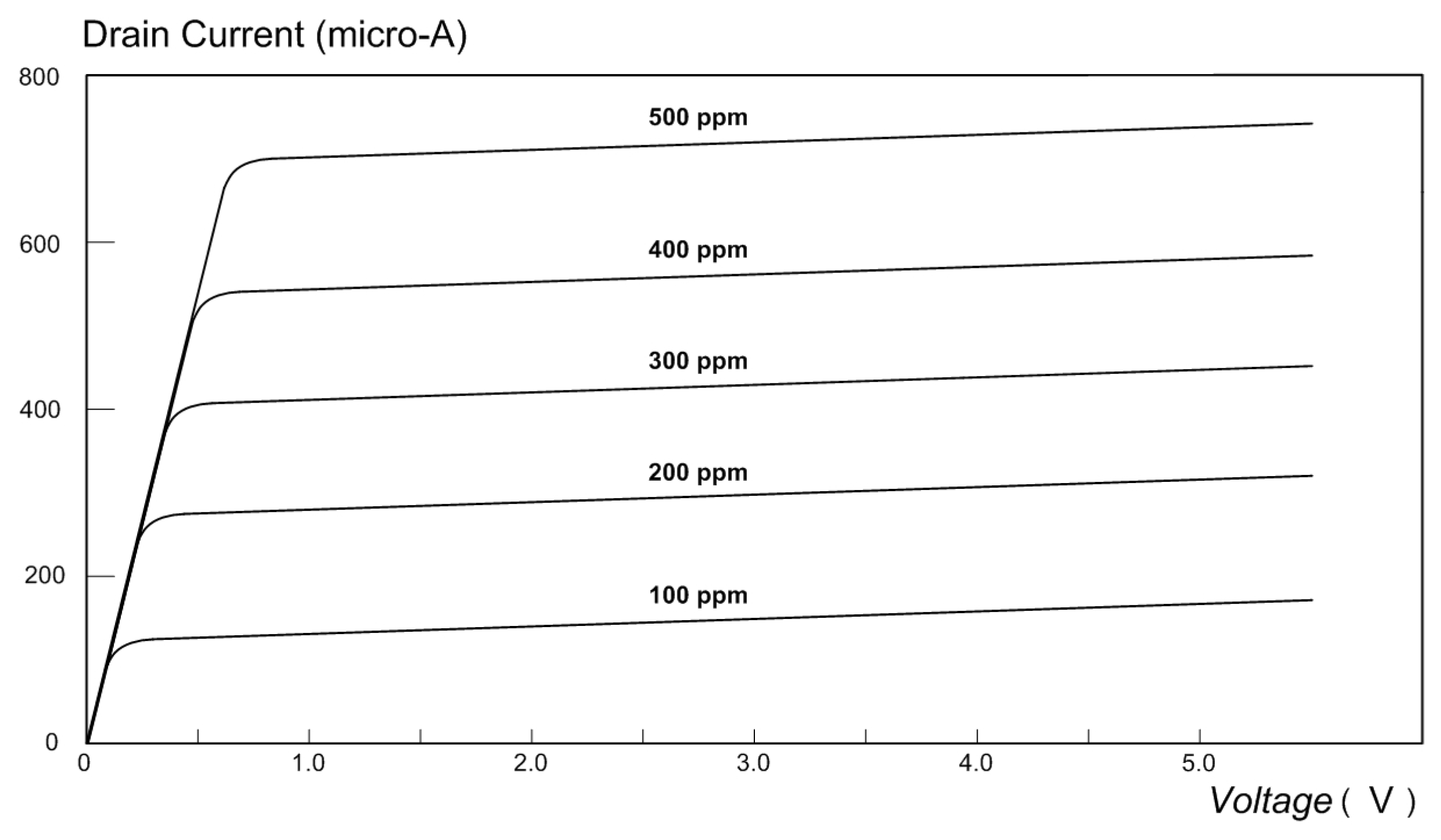
4.2. Detection of Carbon Monoxide
5. Discussion and Comparison of the NPG MOSFET to the ZnO MOSFET
- Deposition of the gate electrode: only atomic layer deposition (ALD) technique is known to be practical for the task of successfully depositing ZnO nanowires as a gate electrode in a MOSFET. ALD, however, is an expensive technique that is not suited for mass production. By comparison, the NPG gate electrode can be easily deposited by sputtering as described earlier. Sputtering is a low-cost technique that is suitable for mass production.
- Leakage currents: The leakage currents in ZnO MOSFETs are known to be high because of the degradation of the oxide layer during the growth process of the ZnO nanowires. The gate-source leakage current, for example, is typically in the order of a few hundred nA. In the present MOSFET, the leakage current is substantially lower because the NPG layer is deposited by sputtering. The gate-source leakage current in the present MOSFET was measured with a Tektronix DMM4020 multimeter and was found to be approximately 1 nA, which is substantially less than the leakage current in ZnO MOSFETs.
- Sensitivity: The sensitivity of a MOSFET that is fitted with a chemically sensitive gate electrode is given by the following equation [30]:where is the change in the conductivity of the MOSFET, is the initial conductivity, is the permittivity of free space and is the relative permittivity of the oxide layer, is the surface potential, is the density of charge states at the surface, is the electron’s charge, is a geometry dependent parameter, is the concentration of donor atoms, and is the thickness of the oxide layer. The sensitivity of the ZnO MOSFET was determined experimentally by Ditshego [28] to be approximately 75%. For the new NPG MOSFET, the conductivity was measured first without the presence of a chemical analyte and was subsequently measured during the presence of carbon monoxide gas (concentration: 500 ppm). It was found that the conductivity increases to approximately 300% when the carbon monoxide gas is present. (It should be pointed out, however, that the ZnO MOSFET was not tested in the present work, and the data that were relied upon are the only published data [30]).
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van der Zalm, J.; Chen, S.; Huang, W.; Chen, A. Review: Recent Advances in the Development of Nanoporous Au for Sensing Applications. J. Electrochem. Soc. 2020, 167, 037532. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Collinson, M.M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 692484. [Google Scholar] [CrossRef]
- Chen, L.; Lang, X.; Fujita, T.; Chen, M. Nanoporous gold for enzyme-free electrochemical glucose sensors. Scr. Mater. 2011, 65, 17–20. [Google Scholar] [CrossRef]
- Li, Q.; Cui, S.; Yan, X. Electrocatalytic oxidation of glucose on nanoporous gold membranes. J. Solid State Electrochem. 2012, 16, 1099–1104. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, M.; Li, H.; Pan, Y.; Si, P. Non-Enzymatic Glucose Sensors based on Controllable Nanoporous Gold/Copper Oxide Nanohybrids. Talanta 2014, 125, 366–371. [Google Scholar] [CrossRef]
- Cao, Q.; Feng, J.; Lu, H.; Zhang, H.; Zhang, F.; Zeng, H. Surface-enhanced Raman scattering using nanoporous gold on suspended silicon nitride waveguides. Opt. Express 2018, 26, 24614–24620. [Google Scholar] [CrossRef]
- Wittstock, A.; Biener, J.; Baumer, M. Nanoporous Gold: A New Material for Catalytic and Sensor Applications. Phys. Chem. Chem. Phys. 2010, 40, 12919–12930. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Salaj-Kosla, U.; Belochapkine, S.; Mac Aodha, D.; Leech, D.; Ding, Y.; Magner, E. Characterization of Nanoporous Gold Electrodes for Bioelectrochemical Applications. Langmuir 2012, 28, 2251–2261. [Google Scholar] [CrossRef]
- Fujita, T.; Qian, L.-H.; Inoke, K.; Erlebacher, J.; Chen, M.-W. Three-Dimensional Morphology of Nanoporous Gold. Appl. Phys. Lett. 2008, 92, 251902. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Baumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Baumer, M. On the Origin of the Catalytic Activity of Gold Nanoparticles for Low-Temperature CO Oxidation. J. Catal. 2004, 223, 232–235. [Google Scholar]
- Zhang, L.; Chang, H.; Hirata, A.; Wu, H.; Xue, Q.-K.; Chen, M. Nanoporous Gold Based Optical Sensor for Sub-ppt Detection of Mercury Ions. ACS Nano 2013, 7, 4595–4600. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Liu, Z.; Puumala, E.; Chen, A. Sensitive electrochemical detection of Hg(II) via a FeOOH modified nanoporous gold microelectrode. Sensors Actuators B Chem. 2019, 287, 517–525. [Google Scholar] [CrossRef]
- Cha, S.N.; Jang, J.E.; Choi, Y.; Amaratunga, G.A.J.; Ho, G.W.; Welland, M.E.; Hasko, D.G.; Kang, D.J.; Kim, J.M. High performance ZnO nanowire field effect transistor using self-aligned nanogap gate electrodes. Appl. Phys. Lett. 2006, 89, 263102. [Google Scholar] [CrossRef]
- Sultan, S.S.M. Top-Down Fabrication and Characterization of Zinc Oxide Nanowire Field Effect Transistors. Ph.D. Thesis, University of Southampton, Southampton, UK, April 2013. [Google Scholar]
- Hsu, C.; Tsai, T. Fabrication of fully transparent indium-doped ZnO nanowire field-effect transistors on ITO/glass substrates. J. Electrochem. Soc. 2011, 158, K20–K23. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.W.; Zhao, J.; Goh, G.K.L.; Chen, L.; Liew, L.L.; Qiu, J.; Hwang, Y.H. Comparison of the hydrothermal and VPT grown ZnO nanowire field effect transistors. Int. J. Nanosci. 2010, 9, 317–320. [Google Scholar] [CrossRef]
- Heo, Y.W.; Tien, L.C.; Kwon, Y.; Norton, D.P.; Pearton, S.J.; Kang, B.S.; Ren, F. Depletion-mode ZnO nanowire field-effect transistor. Appl. Phys. Lett. 2004, 85, 2274–2276. [Google Scholar] [CrossRef]
- Ng, H.T.; Han, J.; Yamada, T.; Nguyen, P.; Chen, Y.P.; Meyyappan, M. Single Crystal Nanowire Vertical Surround-Gate Field-Effect Transistor. Nano Lett. 2004, 4, 1247–1252. [Google Scholar] [CrossRef]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef]
- Pachauri, V.; Ingebrandt, S. Biologically sensitive field-effect transistors: From ISFETs to NanoFETs. Essays Biochem. 2016, 60, 81–90. [Google Scholar] [CrossRef]
- Neamen, D. An Introduction to Semiconductor Devices; McGraw Hill: New York, NY, USA, 2006. [Google Scholar]
- Li, Y.; Song, Y.-Y.; Yang, C.; Xia, X.-H. Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem. Commun. 2007, 9, 981–988. [Google Scholar] [CrossRef]
- Pasta, M.; La Mantia, F.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochim. Acta 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Bakhoum, E.G.; Cheng, M.H.M. Miniature Carbon Monoxide Detector Based on Nanotechnology. IEEE Trans. Instrum. Meas. 2012, 62, 240–245. [Google Scholar] [CrossRef]
- Ditshego, N.M.J. Zinc Oxide Nanowire Field Effect Transistor Used as a pH Sensor. Int. J. Electr. Electron. Eng. Telecommun. 2022, 11, 162–166. [Google Scholar] [CrossRef]



| Parameter | ZnO MOSFET | NPG MOSFET |
|---|---|---|
| Deposition of gate electrode | ALD | Sputtering |
| Leakage current | few hundred nA | 1 nA |
| Sensitivity | 75% | 300% or higher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhoum, E.G.; Zhang, C. Field Effect Transistor with Nanoporous Gold Electrode. Micromachines 2023, 14, 1135. https://doi.org/10.3390/mi14061135
Bakhoum EG, Zhang C. Field Effect Transistor with Nanoporous Gold Electrode. Micromachines. 2023; 14(6):1135. https://doi.org/10.3390/mi14061135
Chicago/Turabian StyleBakhoum, Ezzat G., and Cheng Zhang. 2023. "Field Effect Transistor with Nanoporous Gold Electrode" Micromachines 14, no. 6: 1135. https://doi.org/10.3390/mi14061135
APA StyleBakhoum, E. G., & Zhang, C. (2023). Field Effect Transistor with Nanoporous Gold Electrode. Micromachines, 14(6), 1135. https://doi.org/10.3390/mi14061135








