SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study
Abstract
1. Introduction
2. Synthesis, Microfabrication and Characterization of SiCNFe Ceramics
2.1. SiCNFe Micromolding
2.2. SiCN Films Fabrication with Use of UV Lithography
2.2.1. Optimal Composition of Liquid Polymer and Possible Substrates
2.2.2. Spin-Coating
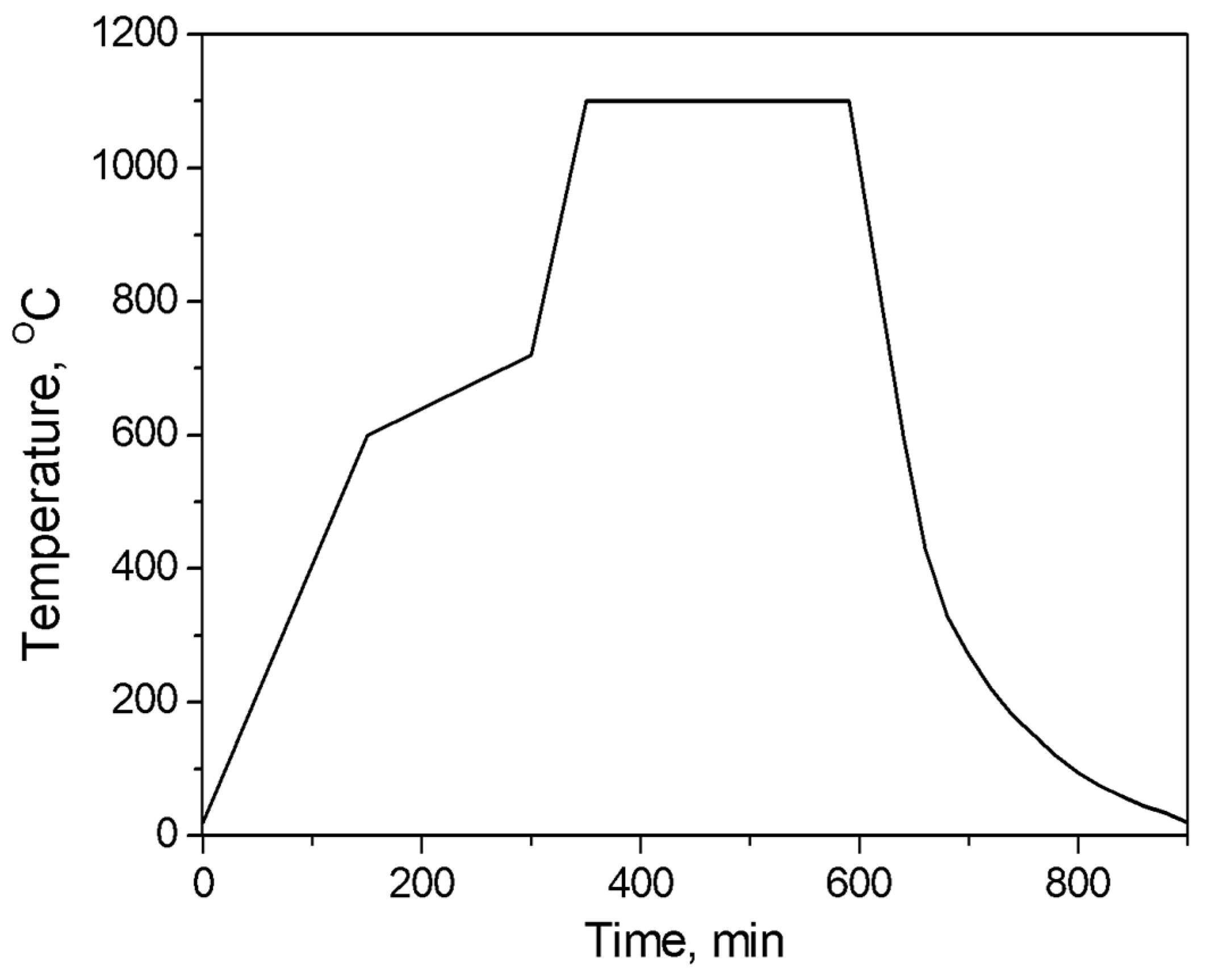
2.2.3. Heat Treatment
2.3. SEM Image of SiCNFe Sample Annealed at 1100 °C
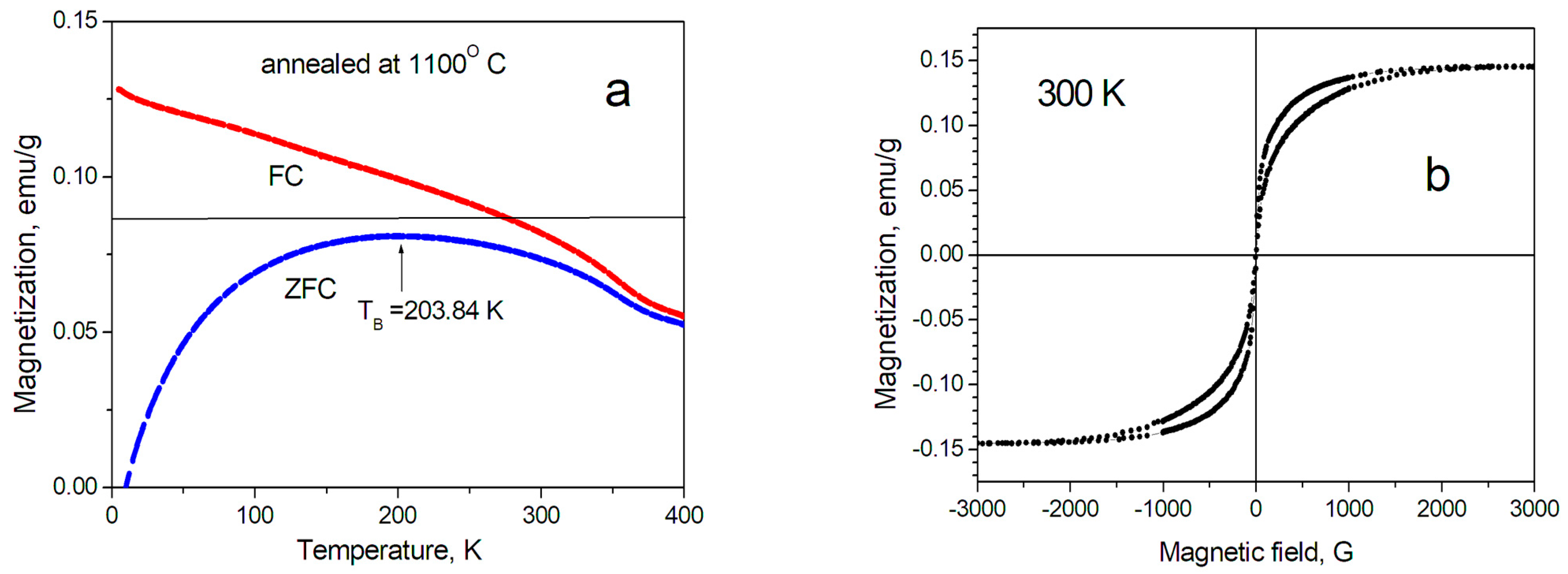
2.4. Magnetic Properties of SiCNFe Annealed at 1100 °C
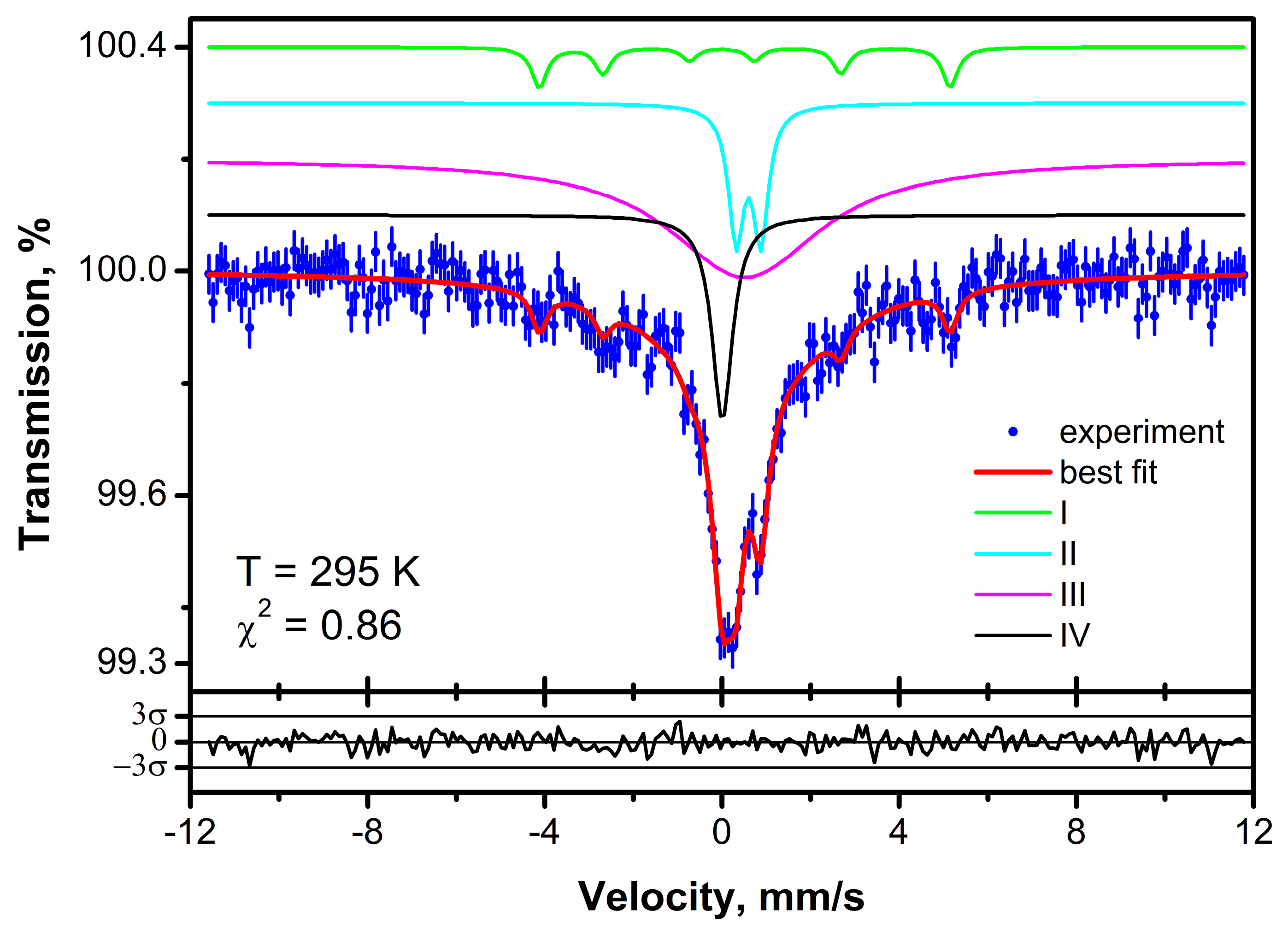
3. Mössbauer Spectrum of SiCNFe Ceramics Annealed at 1100 °C
3.1. The Instrumentation and Analysis
3.2. The Interpretation of Mössbauer Spectrum
3.2.1. Component I
3.2.2. Component II
3.2.3. Component III
3.2.4. Component IV
4. Conclusions
- The rigorous analysis of Mössbauer spectrum of the SiCNFe sample annealed at temperature 1100 °C revealed the presence of α-Fe, Fe5Si3 nanoparticles and, probably, Fe3Si nanoparticles also. However, there are also the traces of iron nitrides (Fe3N, and Fe4N) and Fe3+ ions in octahedral oxygen environment.
- The presence of iron nitride and paramagnetic Fe3+ ions shows that pyrolysis process was not completed in SiCNFe ceramics annealed at 1100 °C.
- The best results for SiCNFe films on the substrate were obtained only for high-speed coating (at 6100 rpm, maximum speed of spin-coating machine and crystalline ZrO2 substrate (100) plane, unpolished).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Hu, W.; Ze, Q.; Sitti, M.; Zhao, R. Multifunctional magnetic soft composites: A review. Multifunct. Mater. 2020, 3, 042003. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.-A.; Zhang, W.; Bright, V.M.; An, L.; Dunn, M.L.; Raj, R. Fabrication of SiCN ceramics MEMS using injectable polymer-precursor technique. Sens. Actuators A Phys. 2001, 89, 64–70. [Google Scholar] [CrossRef]
- Liew, L.-A.; Liu, Y.; Luo, R.; Cross, T.; An, L.; Bright, V.M.; Dunn, M.L.L.; Daily, J.W.; Raj, R. Fabrication of SiCN MEMS by photopolymerization of pre-ceramic polymer. Sens. Actuators A Phys. 2002, 95, 120–134. [Google Scholar] [CrossRef]
- Bright, V.M.; Raj, R.; Dunn, M.L.; Daily, J.W. Injectable Ceramic Microcast Silicon Carbon Nitride (SiCN), Micro Electro Mechanical Systems (MEMS) for Extreme Temperature Environments with Extension: Micro Packages for Nano Devices; AFRL-IF-RS-TR 2004-2; AFRL: New York, NY, USA, 2004. [Google Scholar]
- Liew, L.-A.; Saravanan, R.A.; Bright, V.M.; Dunn, M.L.; Daily, J.W.; Raj, R. Processing and caracterization of silicon carbon-nitride ceramics: Application of electrical properties toward MEMS thermal actuators. Sens. Actuators A Phys. 2003, 103, 171–181. [Google Scholar] [CrossRef]
- Li, Y.-L.; Riedel, R.; Steiger, J.; von Seggem, H. Novel Transparent Polysilazane Glass: Synthesis and Properties. Adv. Eng. Mater. 2000, 2, 290–293. [Google Scholar] [CrossRef]
- Schulz, M. Polymer Derived Ceramics in MEMS/NEMS—A Review on Production Processes and Application. Adv. Appl. Ceram. 2009, 108, 454–460. [Google Scholar] [CrossRef]
- Xia, X.; Yin, J.; Chen, X.; Liu, X.; Huang, Z. Polymer-Derived Si-Based Ceramics: Recent Developments and Perspectives. Crystals 2020, 10, 824. [Google Scholar] [CrossRef]
- Hagelüken, L.; Sasikumar, P.V.W.; Lee, H.-Y.; Di Stadio, D.; Chandorkar, Y.; Rottmar, M.; Maniura-Weber, K.; Blugan, G.; Brugger, J. Multiscale 2D/3D microshaping and property tuning of polymer-derived SiCN ceramics. J. Eur. Ceram. Soc. 2022, 42, 1963–1970. [Google Scholar] [CrossRef]
- Andronenko, S.I.; Stiharu, I.; Misra, S.K. Synthesis and characterization of polyureasilazane derived SiCN ceramics. J. Appl. Phys. 2006, 99, 113907. [Google Scholar] [CrossRef]
- Leo, A.; Andronenko, S.; Stiharu, I.; Bhat, R.B. Thick and thin film SiCN for pressure sensor at high temperature and influence of temperature on mechanical properties. Sensors 2010, 10, 1338–1354. [Google Scholar] [CrossRef]
- Leo, A.; Andronenko, S.I.; Stiharu, I. Fabrication and machining of SiCN for pressure sensor at high temperature for aero-engines applications. Int. J. Mater. Sci. Res. 2018, 1, 36–42. [Google Scholar] [CrossRef]
- Fujimori, H.; Ohnuma, S.; Kobayashi, N.; Masumoto, T. Spintronics in metal-insulator nanogranular magnetic thin films. J. Magn. Magn. Mater. 2006, 304, 32–35. [Google Scholar] [CrossRef]
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; von Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A spin-based electronics vision for the future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-H.; Li, C.-H.; Chang, Y.-C.; Hsiao, M. Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery. Pharmaceutics 2022, 14, 2584. [Google Scholar] [CrossRef]
- Bao, Y.P.; Wen, T.L.; Samia, A.C.S.; Khandhar, A.; Krishnan, K.M. Magnetic nanoparticles: Material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 2016, 51, 513–553. [Google Scholar] [CrossRef]
- Ren, Z.; Mujib, S.B.; Singh, G. High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review. Materials 2021, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shao, G.; Cao, Y.; An, L.; Xu, C. Temperature sensor made of polymer-derived ceramics for high-temperature applications. Sens. Actuators A Phys. 2014, 219, 58–64. [Google Scholar] [CrossRef]
- Saha, A.; Shah, S.R.; Raj, R.; Russek, S.E. Polymer derived SiCN composite with magnetic properties. J. Mater. Res. 2003, 18, 2549–2551. [Google Scholar] [CrossRef]
- Dumitru, A.; Ciupina, V.; Stamatin, I.; Prodan, G.; Morozan, A.; Mirea, C. Plasma polymerization of ferrocene with silane and silazane monomers for design of nanostructured magnetic ceramics. J. Optoelectron. Adv. Mater. 2006, 8, 50–54. [Google Scholar]
- Dumitru, A.; Stamatin, I.; Morozan, A.; Mirea, C.; Ciupina, V. Si-C-N-Fe nanostructured ceramics from inorganic polymer precursors obtained by plasma polymerization. Mater. Sci. Eng. C 2007, 27, 1331–1337. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Z.; Reng, C.; Zhang, Z.; Gao, W.; Yang, S.; Xie, Z. Preparation of Si-C-N-Fe magnetic ceramics from iron-containing polysilazane. Appl. Organomet. Chem. 2003, 17, 120–126. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Prasad, R.M.; Fasel, C.; Riedel, R.; Ionescu, E. Single-source-precursor synthesis of soft magnetic Fe3Si- and Fe5Si3-containing SiOC ceramic nanocomposites. Eur. J. Ceram. Soc. 2013, 33, 2465–2472. [Google Scholar] [CrossRef]
- Zaheer, M.; Schmalz, T.; Motz, G.; Kempe, R. Polymer derived non-oxide ceramics modified with late transition metals. Chem. Soc. Rev. 2012, 41, 5102–5116. [Google Scholar] [CrossRef] [PubMed]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic nanocomposites from tailor-made preceramics polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cheng, X.; Han, G.; Hauser, R.; Riedel, R. Synthesis and magnetic properties of polymer derived metal/SiCN ceramic composites. Key Eng. Mater. 2007, 353–358, 1485–1488. [Google Scholar] [CrossRef]
- Andronenko, S.I.; Stiharu, I.; Misra, S.K.; Lacroix, C.; Menard, D. EPR/FMR, FTIR, X-ray, and Raman investigations of Fe-doped SiCN ceramics. Appl. Magn. Reson. 2010, 38, 385–402. [Google Scholar] [CrossRef]
- Misra, S.K.; Andronenko, S.; Gilmutdinov, I.; Yusupov, R. EPR and magnetization studies of polymer-derived Fe-doped SiCN nanoceramics annealed at various temperatures: Blocking temperatures, superparamagnetism and size distribution. Appl. Magn. Reson. 2018, 49, 1397–1415. [Google Scholar] [CrossRef]
- Andronenko, S.I.; Rodionov, A.A.; Misra, S.K. A variable temperature X- and W-band EPR study of Fe-doped SiCN ceramics annealed at 1000 °C, 1100 °C, and 1285 °C: Dangling bonds, ferromagnetism and superparamagnetism. Appl. Magn. Reson. 2018, 49, 335–344. [Google Scholar] [CrossRef]
- Pushkarev, R.V.; Fainer, N.I.; Maurya, K.K. Structural and magnetic properties of nanocomposite iron-containing SiCxNy films. Superlattices Microstruct. 2017, 102, 119–126. [Google Scholar] [CrossRef]
- Pushkarev, R.; Fainer, N.; Kirienko, V.; Matsynin, A.; Nadolinnyy, V.; Merenkov, I.; Trubina, S.; Ehrenburg, S.; Kvashnina, K. SiCxNy:Fe films as a tunable ferromagnetic material with tailored conductivity. J. Mater. Chem. C 2019, 7, 4250–4258. [Google Scholar] [CrossRef]
- Stepina, N.P.; Pushkarev, R.V.; Zinovieva, A.F.; Kirienko, V.V.; Bogomyakov, A.S.; Gutakovskii, A.K.; Fainer, N.I.; Dvurechenskii, A.V. Magnetic properties of granulated SiCxNy:Fe films with different structure of α-Fe nanoclusters. J. Magn. Magn. Mater. 2020, 499, 166242. [Google Scholar] [CrossRef]
- Fainer, N.I.; Plekhanov, A.G.; Pushkarev, R.V.; Shayapov, V.R.; Maksimovskiy, E.A.; Nadolinny, V.A.; Korotaev, E.V.; Kaichev, V.V. Synthesis of magnetic nanocomposite films SiCxNyFez by plazma-enhanced chemical decomposition of a gaseous mixture of 1,1,1,3,3,3-hexamethyldisilazane, ferrocene, and helium. J. Struct. Chem. 2020, 61, 1865–1875. [Google Scholar] [CrossRef]
- Lomayeva, S.F.; Maratkanova, A.N. Structure, phase composition and magnetic properties of Fe-Si-C system due to mechanical activation of Fe-Si alloy in organic liquids. Intermetallics 2009, 17, 714–721. [Google Scholar] [CrossRef]
- Yelsukov, E.P.; Maratkanova, A.N.; Lomayeva, S.F.; Konyagin, G.N.; Nemtsova, O.M.; Ul’yanov, A.I.; Chulkina, A.A. Structure, phase composition and magnetic properties of mechanically alloyed and annealing quasibinary Fe(70)Si(x)C(30-x) alloys. J. Alloys Compd. 2006, 407, 98–105. [Google Scholar] [CrossRef]
- Viard, A.; Kurz, H.; Lale, A.; Heymann, L.; Weber, B.; Bernard, S.; Knauer, M.; Motz, G. Superparamagnetic silicon carbonitride ceramic fibers through in situ generation of iron silicide nanoparticles during pyrolysis of an iron-modified polysilazane. ACS Appl. Mater. Interfaces 2021, 13, 8745–8753. [Google Scholar] [CrossRef] [PubMed]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar] [CrossRef]
- Mekata, M.; Yoshimura, H.; Takaki, H. Magnetic study on hexagonal nitrides of 3d transition metals. J. Phys. Soc. Jpn. 1972, 33, 62–69. [Google Scholar] [CrossRef]
- Sawatzky, E. Magnetic and magnetooptical properties of sputtered Fe5Si3 films. IEEE Trans. Magn. 1971, 7, 374–376. [Google Scholar] [CrossRef]
- Murad, E.; Cashion, J. Mossbauer Spectroscopy of Environmental Materials and Their Industrial Utilization; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Oosterhuis, W.T.; Lang, G.; Debenedetti, S. Mössbauer spectra of K3Fe(CN)6 at low temperatures. Phys. Lett. A 1967, 24, 346–347. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, W.; Zhang, Y.; Zhang, P.; Liu, J. Effects of Fe2O3 content on microstructure and mechanical properties of CaO-Al2O3-SiO2 system. Trans. Nonferrous Met. Soc. China 2015, 25, 137–145. [Google Scholar] [CrossRef]
- Afanas’ev, A.M.; Chuev, M.A. New relaxation model for superparamagnetic particles in Mössbauer spectroscopy. J. Exp. Theor. Phys. Lett. 2001, 74, 107–110. [Google Scholar] [CrossRef]



| Component | CS | QS | HF | W | A | Assigned Phase |
|---|---|---|---|---|---|---|
| I | 0.26(4) | 0.52(8) | 288(3) | 0.4(1) | 9(3) | Fe rich FexSiyCz [34,35] |
| II | 0.60(3) | 0.56(4) | - | 0.42(8) | 14(3) | [Fe3+O6] |
| III | 0.5(1) | - | - | 4.2(8) | 63(4) | FeNx with distribution x < 0.5 [38] |
| IV | 0.00(3) | - | - | 0.55(8) | 15(2) | Superparamagnetic α-Fe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiharu, I.; Andronenko, S.; Zinnatullin, A.; Vagizov, F. SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study. Micromachines 2023, 14, 925. https://doi.org/10.3390/mi14050925
Stiharu I, Andronenko S, Zinnatullin A, Vagizov F. SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study. Micromachines. 2023; 14(5):925. https://doi.org/10.3390/mi14050925
Chicago/Turabian StyleStiharu, Ion, Sergey Andronenko, Almaz Zinnatullin, and Farit Vagizov. 2023. "SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study" Micromachines 14, no. 5: 925. https://doi.org/10.3390/mi14050925
APA StyleStiharu, I., Andronenko, S., Zinnatullin, A., & Vagizov, F. (2023). SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study. Micromachines, 14(5), 925. https://doi.org/10.3390/mi14050925







