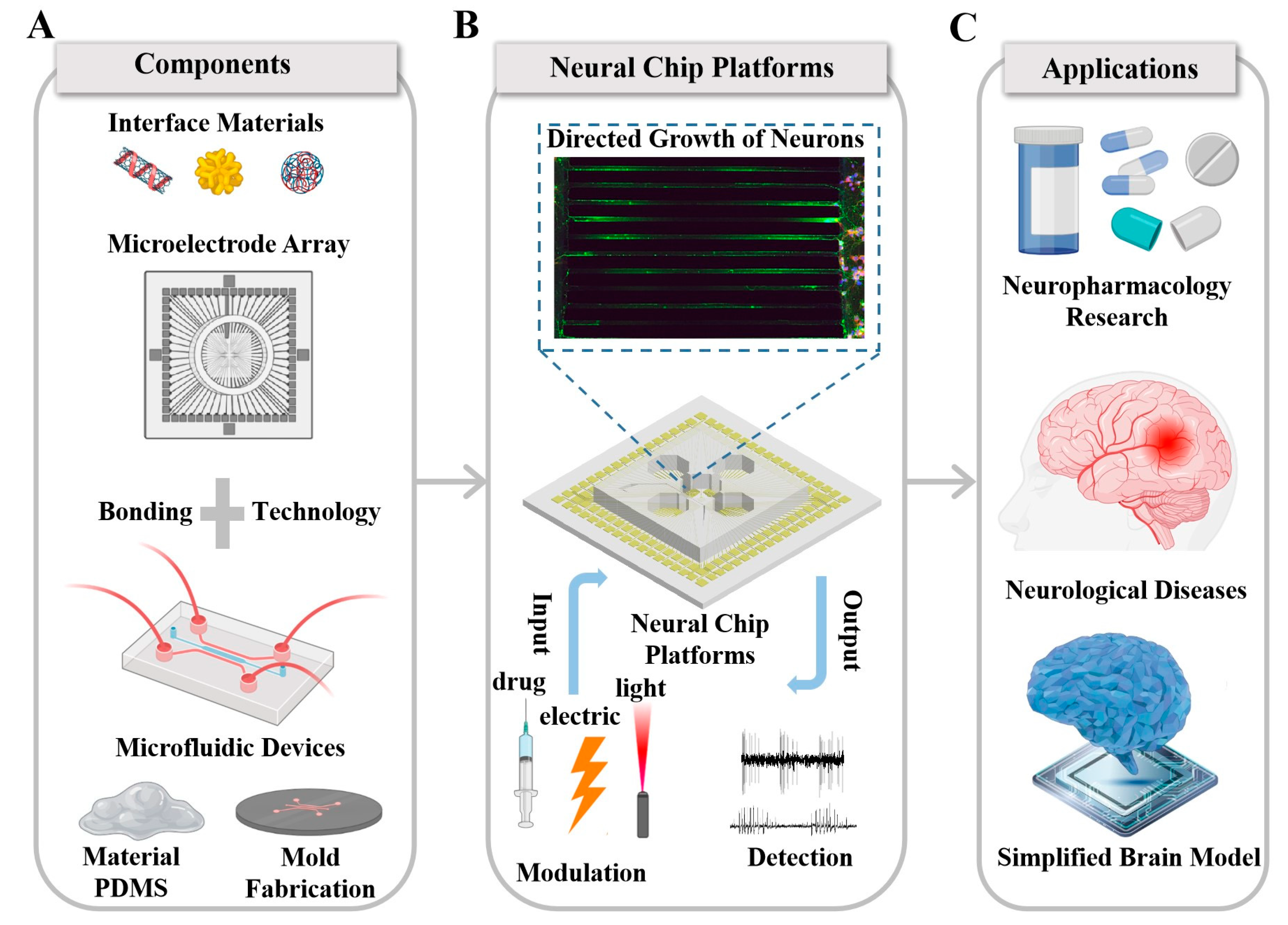
Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays
Abstract
1. Introduction
2. An Important Tool for the Modulation and Recording of Neural Information: Microelectrode Arrays
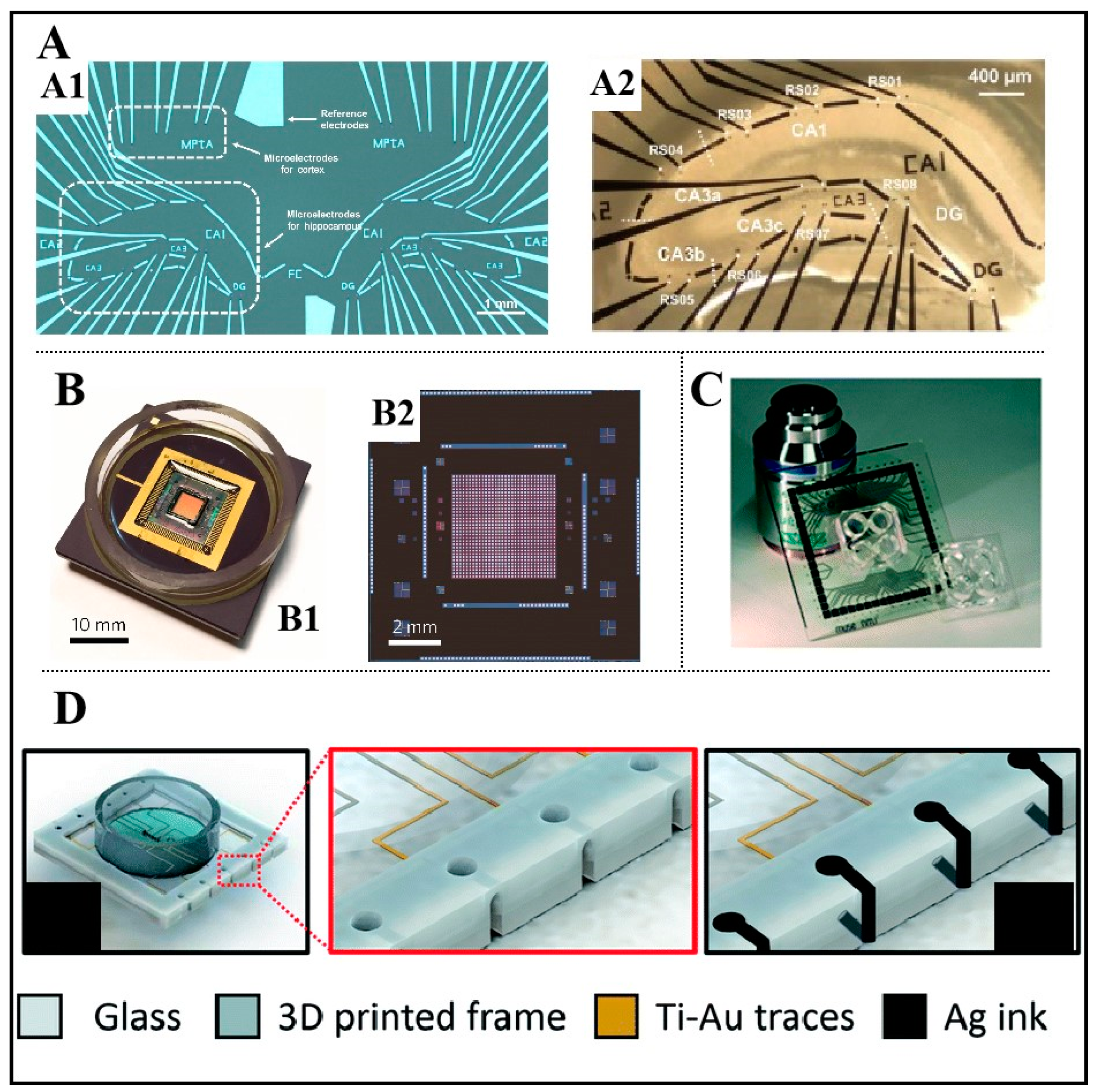
2.1. In Vitro MEAs
2.2. Methods to Improve the Performance of MEAs
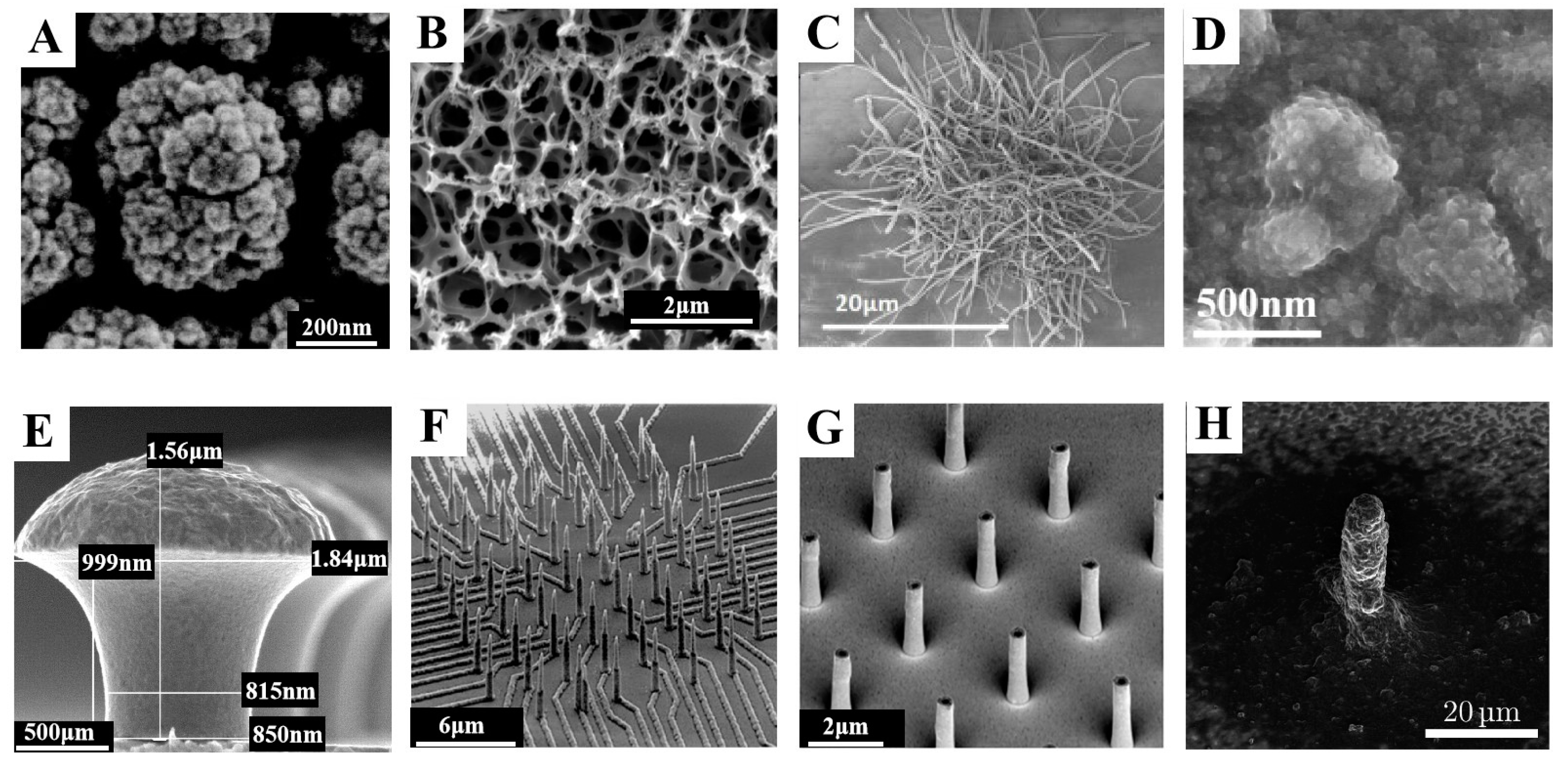
2.3. Development Direction of In Vitro MEAs
3. An Important Tool for Neural Network Customization: PDMS-Based Microfluidic Devices
3.1. PDMS-Based Microfluidic Devices
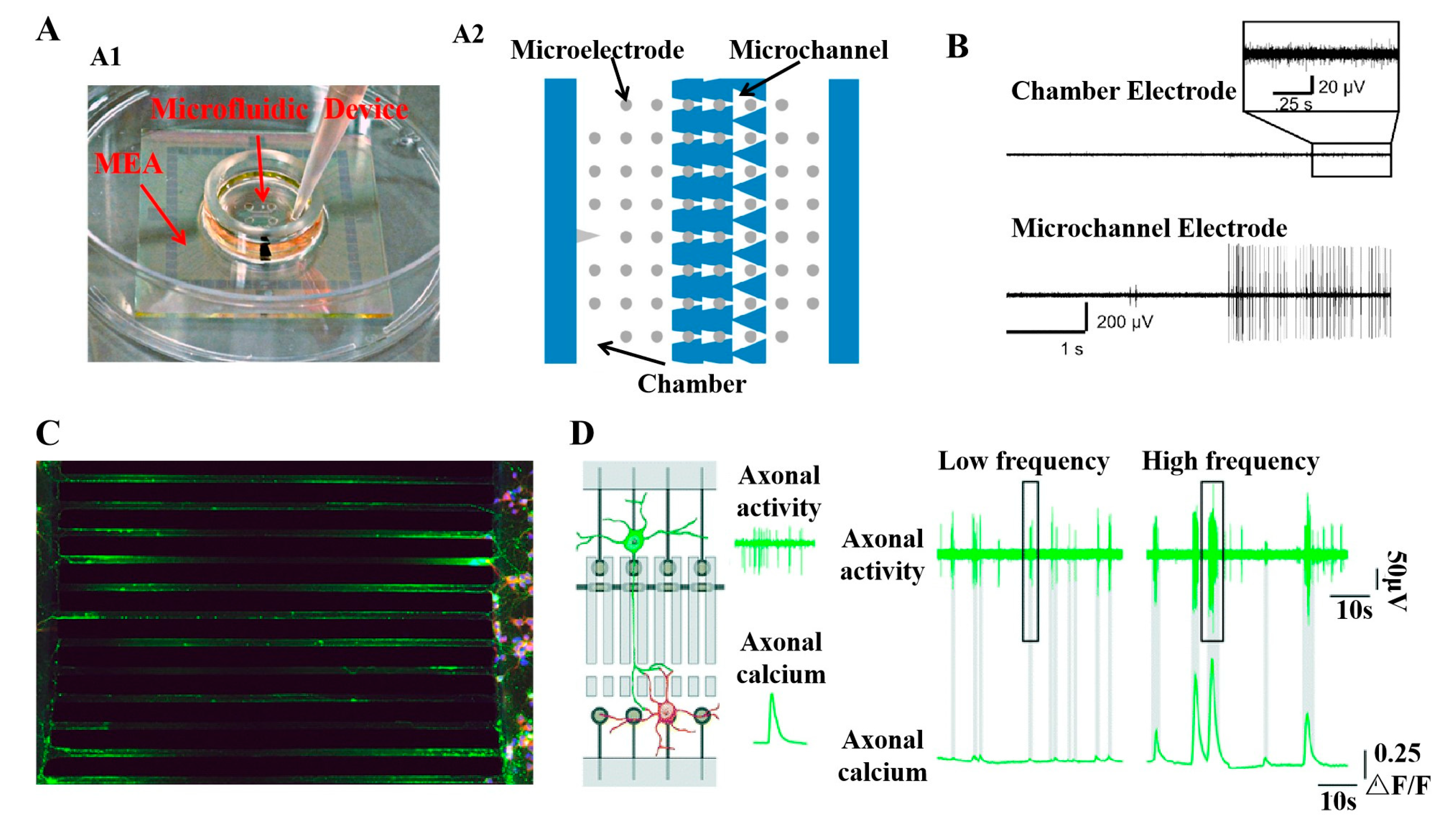
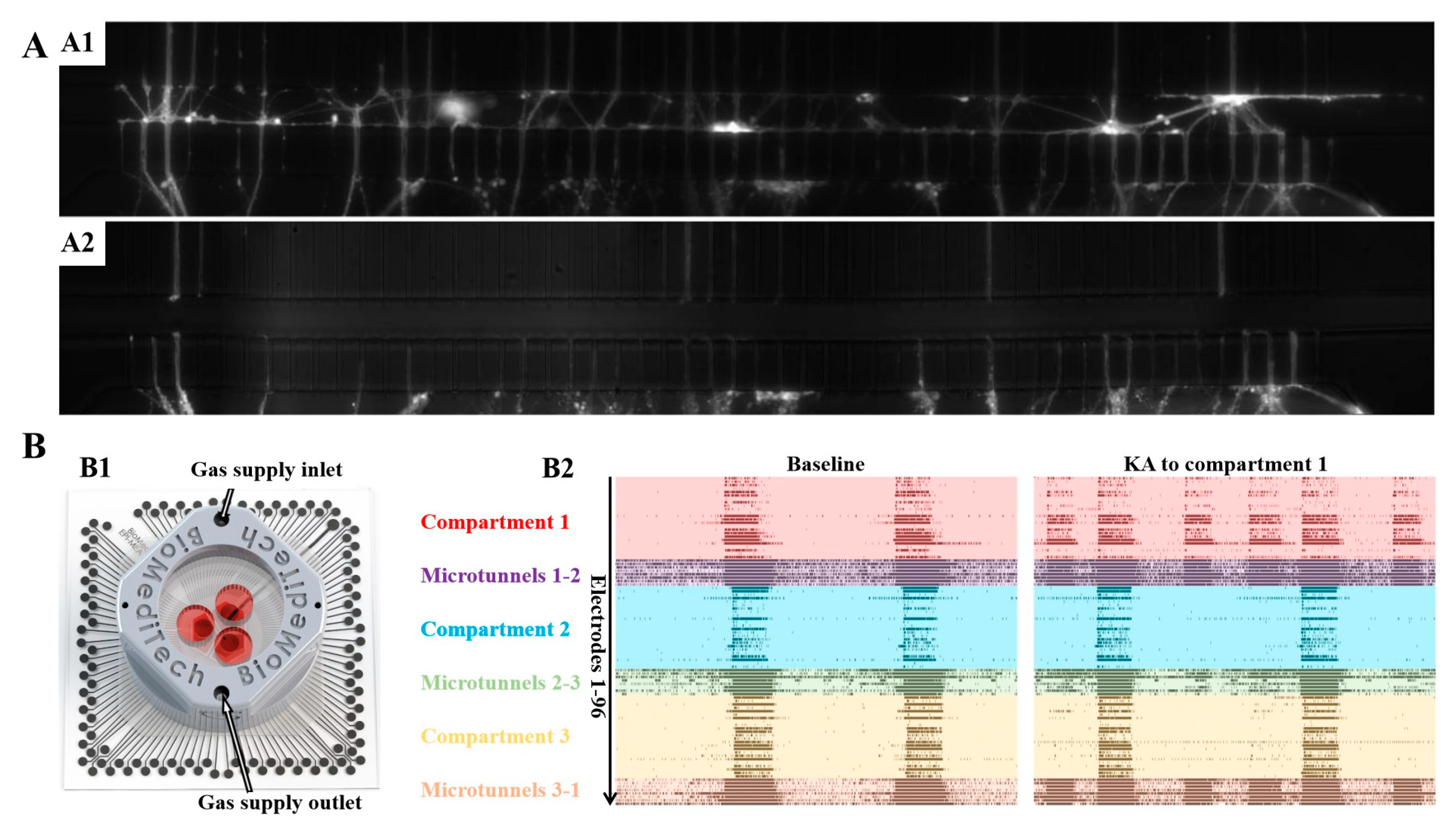
3.2. Development and Latest Design of PDMS Microfluidic Devices with Compartments of Controllable Neurite Growth
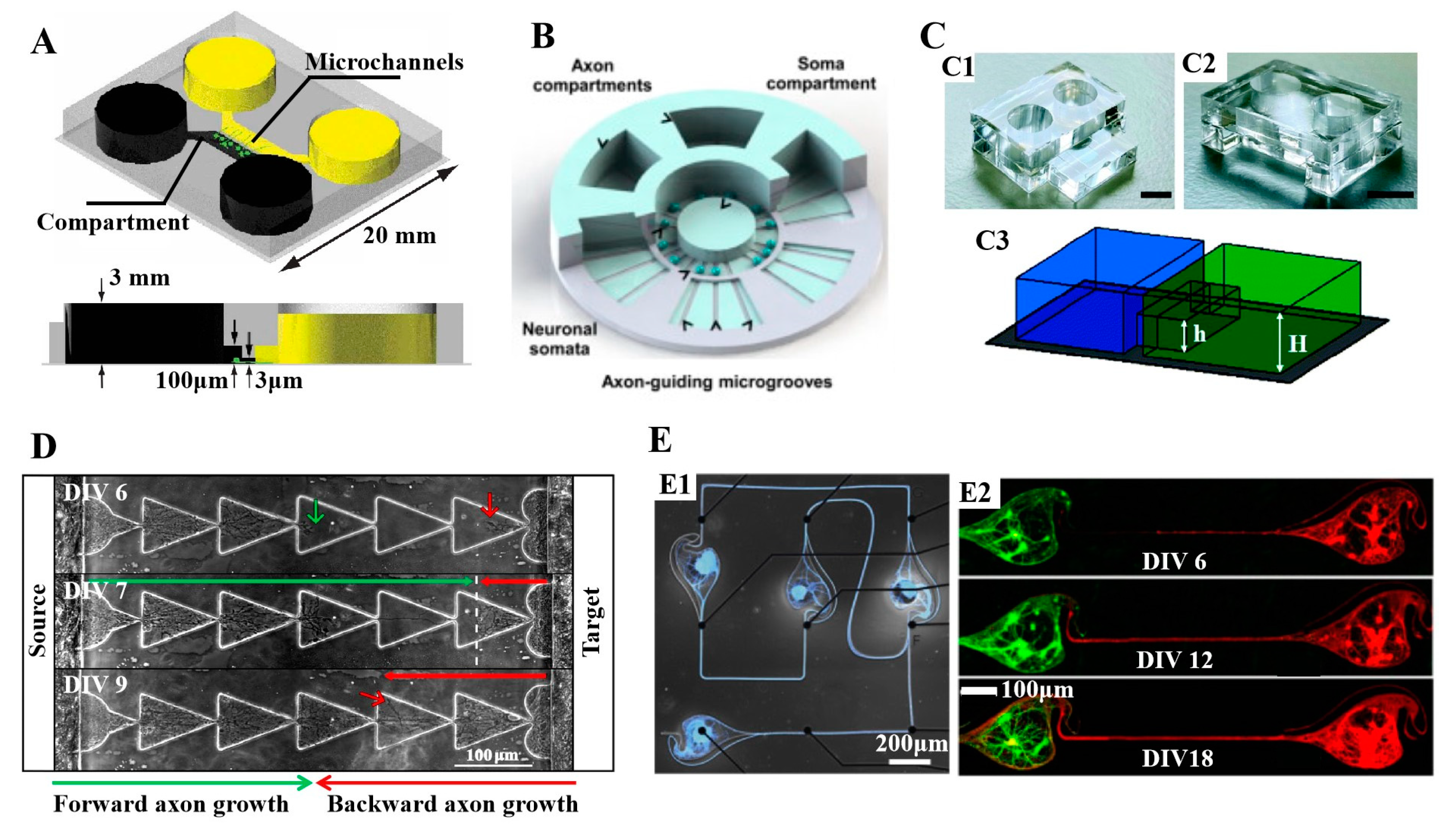
3.3. Important Applications of Compartmental PDMS Microfluidic Devices with Controlled Neurite Growth PDMS in Neuroscience
4. Fabrication of Neural Chips Integrating Microfluidics and Microelectrode Arrays
4.1. Fabrication of In Vitro MEAs
4.2. Fabrication of PDMS-Based Microfluidic Devices
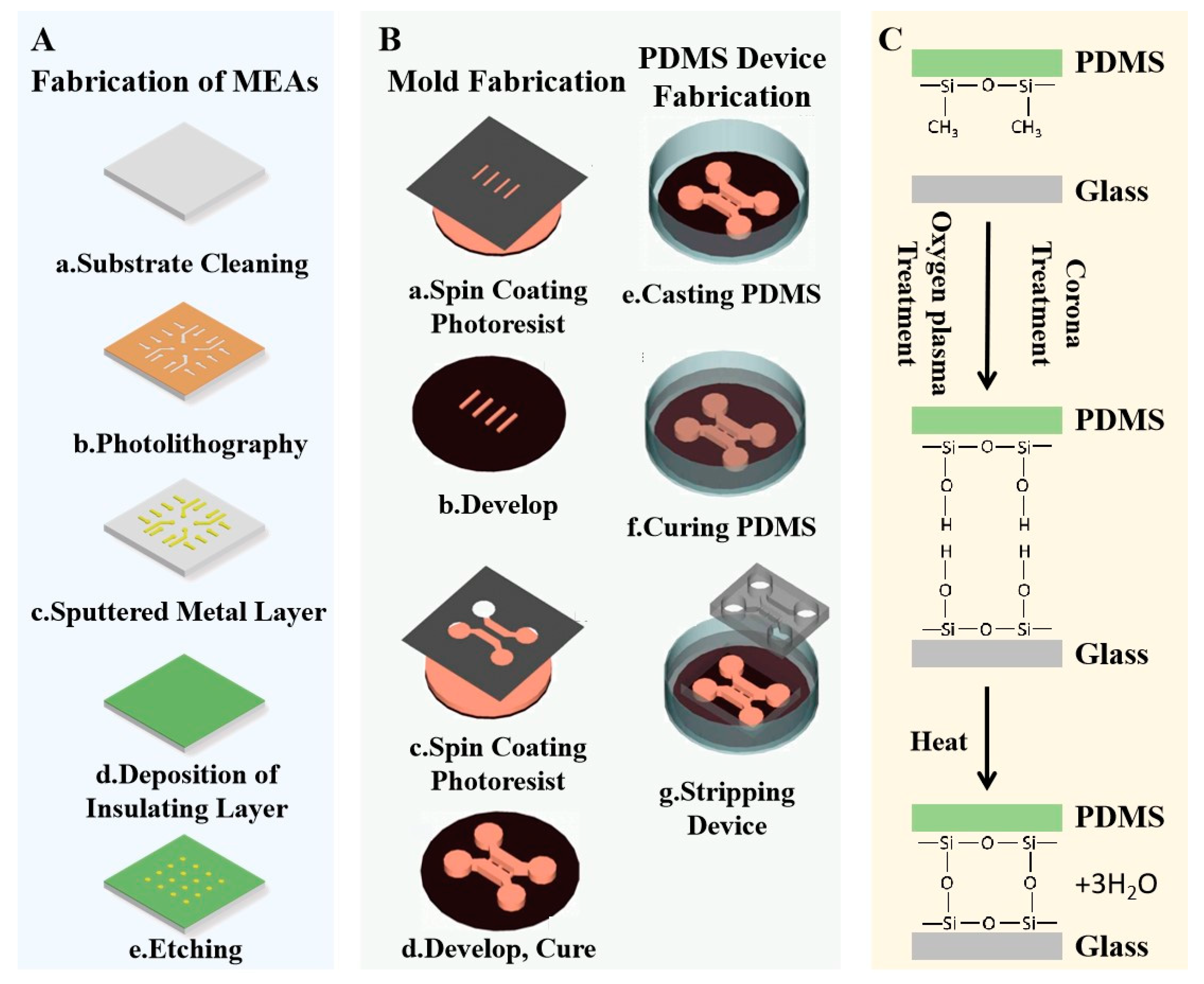
4.3. Bonding of In Vitro MEAs and Microfluidic Devices
5. Neural Chip Platforms Integrating Microfluidic Devices and Microelectrode Arrays Play a Key Role in the Application of the Neural Field
5.1. Recording and Modulation of Neural Signals
5.2. Neuropharmacology Research
5.3. Research of Neurological Diseases
5.4. The Study of the Brain by Means of Simplified Model
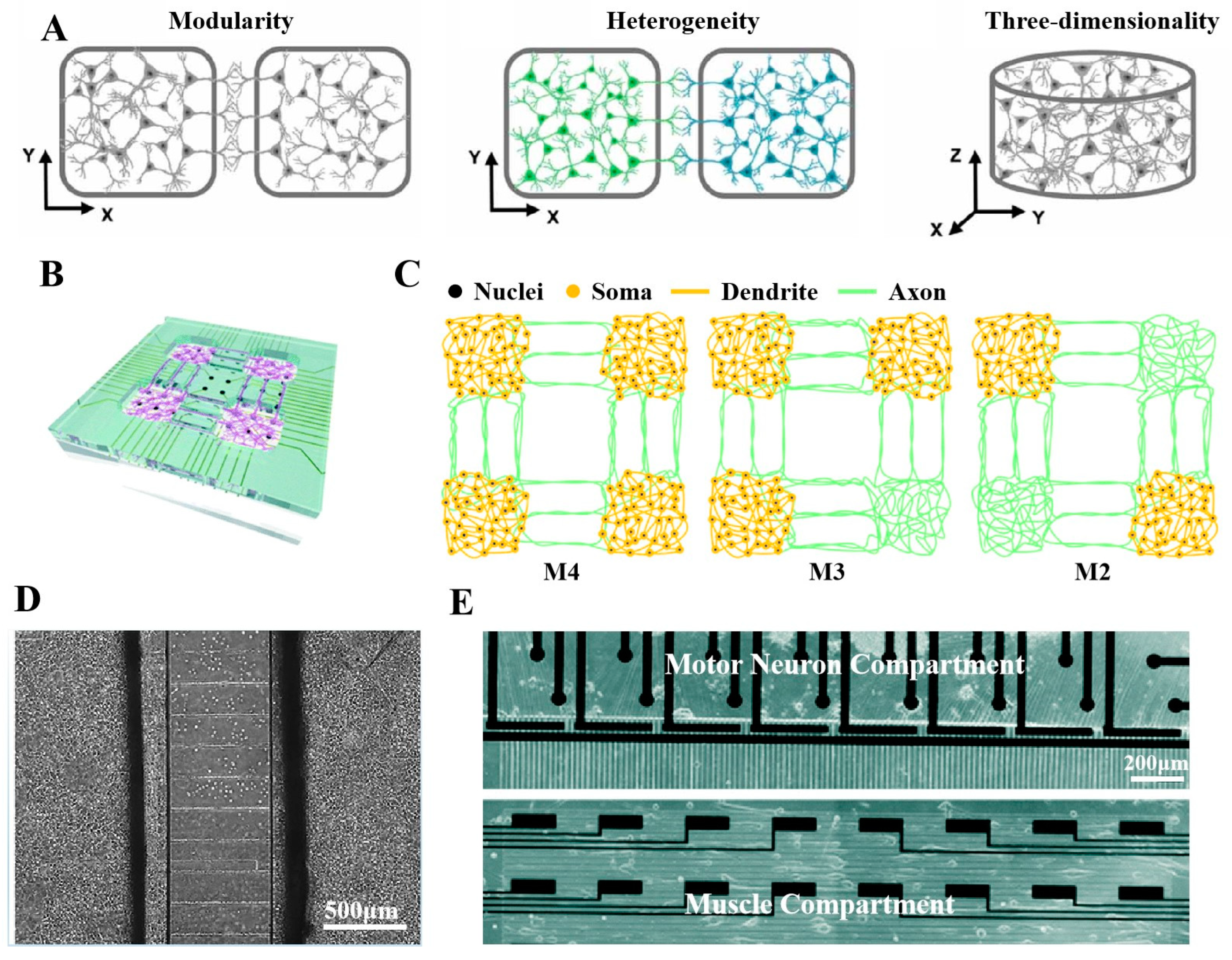
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azevedo, F.A.C.; Carvalho, L.R.B.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.L.; Leite, R.E.P.; Jacob, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Vazza, F.; Feletti, A. The quantitative comparison between the neuronal network and the cosmic web. Front. Phys. 2020, 8, 491. [Google Scholar] [CrossRef]
- Knowles, J.K.; Xu, H.; Soane, C.; Batra, A.; Saucedo, T.; Frost, E.; Tam, L.T.; Fraga, D.; Ni, L.; Villar, K.; et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci. 2022, 25, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gilron, R.; Little, S.; Perrone, R.; Wilt, R.; de Hemptinne, C.; Yaroshinsky, M.S.; Racine, C.A.; Wang, S.S.; Ostrem, J.L.; Larson, P.S.; et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 2021, 39, 1078–1085. [Google Scholar] [CrossRef]
- Dregni, A.J.; Duan, P.; Xu, H.; Changolkar, L.; El Mammeri, N.; Lee, V.M.; Hong, M. Fluent molecular mixing of Tau isoforms in Alzheimer’s disease neurofibrillary tangles. Nat. Commun. 2022, 13, 2967. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jacob, E.; Hanein, Y. Carbon nanotube micro-electrodes for neuronal interfacing. J. Mater. Chem. 2008, 18, 5181–5186. [Google Scholar] [CrossRef]
- Amirifar, L.; Shamloo, A.; Nasiri, R.; de Barros, N.R.; Wang, Z.Z.; Unluturk, B.D.; Libanori, A.; Ievglevskyi, O.; Diltemiz, S.E.; Sances, S.; et al. Brain-on-a-chip: Recent advances in design and techniques for microfluidic models of the brain in health and disease. Biomaterials 2022, 285, 121531. [Google Scholar] [CrossRef]
- Zhang, H.; Rong, G.; Bian, S.; Sawan, M. Lab-on-chip microsystems for ex vivo network of neurons studies: A review. Front. Bioeng. Biotechnol. 2022, 10, 841389. [Google Scholar] [CrossRef]
- Kapucu, F.E.; Vinogradov, A.; Hyvarinen, T.; Yla-Outinen, L.; Narkilahti, S. Comparative microelectrode array data of the functional development of hPSC-derived and rat neuronal networks. Sci. Data 2022, 9, 120. [Google Scholar] [CrossRef]
- Xu, L.; Hu, C.; Huang, Q.; Jin, K.; Zhao, P.; Wang, D.; Hou, W.; Dong, L.; Hu, S.; Ma, H. Trends and recent development of the microelectrode arrays (MEAs). Biosens. Bioelectron. 2021, 175, 112854. [Google Scholar] [CrossRef]
- Halliwell, R.F.; Salmanzadeh, H.; Coyne, L.; Cao, W.S. An electrophysiological and pharmacological study of the properties of human iPSC-derived neurons for drug discovery. Cells 2021, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Vato, A.; Berdondini, L.; Koudelka-Hep, M.; Martinoia, S. Network dynamics and synchronous activity in cultured cortical neurons. Int. J. Neural Syst. 2007, 17, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Gao, K.; Zhang, P.; Fu, Y.; Liu, X.; Bai, S.; Li, W.; Qian, Z. A biomimetic sensor using neurotransmitter detection to decode odor perception by an olfactory network. Biosens. Bioelectron. 2022, 211, 114391. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-based biosensors: Recent trends, challenges and future perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Cai, H.; Wu, Z.; Krzesniak, J.; Tian, C.; Lai, Y.Y.; Mackie, K.; Guo, F. Human spinal organoid-on-a-chip to model nociceptive circuitry for pain therapeutics discovery. Anal. Chem. 2022, 94, 1365–1372. [Google Scholar] [CrossRef]
- Odawara, A.; Shibata, M.; Ishibashi, Y.; Nagafuku, N.; Matsuda, N.; Suzuki, I. In vitro pain assay using human iPSC-derived sensory neurons and microelectrode array. Toxicol. Sci. 2022, 188, 131–141. [Google Scholar] [CrossRef]
- Bryson, A.; Mendis, D.; Morrisroe, E.; Reid, C.A.; Halgamuge, S.; Petrou, S. Classification of antiseizure drugs in cultured neuronal networks using multielectrode arrays and unsupervised learning. Epilepsia 2022, 63, 1693–1703. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Wang, Y.; Li, H.; Zheng, X. A novel robot system integrating biological and mechanical intelligence based on dissociated neural network-controlled closed-loop environment. PLoS ONE 2016, 11, e0165600. [Google Scholar] [CrossRef]
- Kagan, B.J.; Kitchen, A.C.; Tran, N.T.; Habibollahi, F.; Khajehnejad, M.; Parker, B.J.; Bhat, A.; Rollo, B.; Razi, A.; Friston, K.J. In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron 2022, 110, 3952–3969. [Google Scholar] [CrossRef]
- Hyvarinen, T.; Hyysalo, A.; Kapucu, F.E.; Aarnos, L.; Vinogradov, A.; Eglen, S.J.; Yla-Outinen, L.; Narkilahti, S. Functional characterization of human pluripotent stem cell-derived cortical networks differentiated on laminin-521 substrate: Comparison to rat cortical cultures. Sci. Rep. 2019, 9, 17125. [Google Scholar] [CrossRef]
- Muller, J.; Ballini, M.; Livi, P.; Chen, Y.; Radivojevic, M.; Shadmani, A.; Viswam, V.; Jones, I.L.; Fiscella, M.; Diggelmann, R.; et al. High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels. Lab Chip 2015, 15, 2767–2780. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, H.W.; Shin, M.; Lim, J.; Lee, J.Y.; Lee, S.N.; Choi, J.W. 3D neural network composed of neurospheroid and bionanohybrid on microelectrode array to realize the spatial input signal recognition in neurospheroid. Small Methods 2022, 6, 2200127. [Google Scholar] [CrossRef] [PubMed]
- Talebjedi, B.; Heydari, M.; Taatizadeh, E.; Tasnim, N.; Li, I.T.S.; Hoorfar, M. Neural network-based optimization of an acousto microfluidic system for submicron bioparticle separation. Front. Bioeng. Biotechnol. 2022, 10, 878398. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R.; Guedes, S.; Costa, J.P.D.; Kasicka, V. Microfluidics for peptidomics, proteomics, and cell analysis. Nanomaterials 2021, 11, 1118. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, L.; Mitra, K.; Bit, A. Fibroblast derived skin wound healing modeling on chip under the influence of micro-capillary shear stress. Micromachines 2022, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Bossink, E.; Vollertsen, A.R.; Loessberg-Zahl, J.T.; van der Meer, A.D.; Segerink, L.I.; Odijk, M. Systematic characterization of cleanroom-free fabricated macrovalves, demonstrating pumps and mixers for automated fluid handling tuned for organ-on-chip applications. Microsyst. Nanoeng. 2022, 8, 54. [Google Scholar] [CrossRef]
- Park, M.U.; Bae, Y.; Lee, K.-S.; Song, J.H.; Lee, S.-M.; Yoo, K.-H. Collective dynamics of neuronal activities in various modular networks. Lab Chip 2021, 21, 951–961. [Google Scholar] [CrossRef]
- Forro, C.; Thompson-Steckel, G.; Weaver, S.; Weydert, S.; Ihle, S.; Dermutz, H.; Aebersold, M.J.; Pilz, R.; Demko, L.; Voros, J. Modular microstructure design to build neuronal networks of defined functional connectivity. Biosens. Bioelectron. 2018, 122, 75–87. [Google Scholar] [CrossRef]
- Lewandowska, M.K.; Bakkum, D.J.; Rompani, S.B.; Hierlemann, A. Recording large extracellular spikes in microchannels along many axonal sites from individual neurons. PLoS ONE 2015, 10, e0118514. [Google Scholar] [CrossRef]
- Xiao, G.; Song, Y.; Zhang, Y.; Xing, Y.; Zhao, H.; Xie, J.; Xu, S.; Gao, F.; Wang, M.; Xing, G.; et al. Microelectrode arrays modified with nanocomposites for monitoring dopamine and Spike firings under deep brain stimulation in rat models of Parkinson’s disease. ACS Sens. 2019, 4, 1992–2000. [Google Scholar] [CrossRef]
- Xu, S.; Deng, Y.; Luo, J.; Liu, Y.; He, E.; Yang, Y.; Zhang, K.; Sha, L.; Dai, Y.; Ming, T.; et al. A neural sensor with a nanocomposite interface for the study of Spike characteristics of hippocampal neurons under learning training. Biosensors 2022, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mo, F.; Yang, G.; Fan, P.; Wang, Y.; Lu, B.; Xie, J.; Dai, Y.; Song, Y.; He, E.; et al. Grid cell remapping under three-dimensional object and social landmarks detected by implantable microelectrode arrays for the medial entorhinal cortex. Microsyst. Nanoeng. 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Luo, J.; Song, Y.; He, E.; Zhang, Y.; Xiao, G.; Cai, X. Microelectrode arrays for monitoring neural activity in neural stem cells with modulation by glutamate in vitro. Nanotechnol. Precis. Eng. 2020, 3, 69–74. [Google Scholar] [CrossRef]
- Negri, J.; Menon, V.; Young-Pearse, T.L. Assessment of spontaneous neuronal activity in vitro using multi-well multi-electrode arrays: Implications for assay development. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Matsuda, N.; Odawara, A.; Kinoshita, K.; Okamura, A.; Shirakawa, T.; Suzuki, I. Raster plots machine learning to predict the seizure liability of drugs and to identify drugs. Sci. Rep. 2022, 12, 2281. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Yang, Y.; Zhang, K.; He, E.; Liang, W.; Luo, J.; Wu, Y.; Cai, X. Nanomaterial-based microelectrode arrays for in vitro bidirectional brain–computer interfaces: A review. Microsyst. Nanoeng. 2023, 9, 36. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, Y.; Sun, H.; Lang, Y.; Wang, C.; Han, J.; Wang, Y.; Han, Y. Chronic electrical stimulation induces functional network changes in cortical neuron cultures. Sci. China Technol. Sci. 2020, 63, 637–647. [Google Scholar] [CrossRef]
- Chen, H.-l.; Tian, G.-z.; Yan, H.; Yang, S.-x.; Kim, D.-H. Fabrication of vertically aligned PEDOT nanotube arrays on microelectrodes to interface neurons. Electrochim. Acta 2022, 404, 139583. [Google Scholar] [CrossRef]
- Park, S.; Song, Y.J.; Boo, H.; Chung, T.D. Nanoporous Pt microelectrode for neural stimulation and recording: In vitro characterization. J. Phys. Chem. C 2010, 114, 8721–8726. [Google Scholar] [CrossRef]
- Tang, R.; Pei, W.; Chen, S.; Zhao, H.; Chen, Y.; Han, Y.; Wang, C.; Chen, H. Fabrication of strongly adherent platinum black coatings on microelectrodes array. Sci. China Inf. Sci. 2014, 57, 1–10. [Google Scholar] [CrossRef]
- Strickland, J.D.; Lefew, W.R.; Crooks, J.; Hall, D.; Ortenzio, J.N.R.; Dreher, K.; Shafer, T.J. In vitro screening of metal oxide nanoparticles for effects on neural function using cortical networks on microelectrode arrays. Nanotoxicology 2016, 10, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Frewin, C.L.; Tanjil, M.R.; Everly, R.; Bieber, J.; Kumar, A.; Wang, M.C.; Saddow, S.E. A flexible a-SiC-based neural interface utilizing pyrolyzed-photoresist film (C) active sites. Micromachines 2021, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible neural electrode array based-on porous graphene for cortical microstimulation and sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Travas-Sejdic, J. Fabrication of conducting polymer microelectrodes and microstructures for bioelectronics. J. Mater. Chem. C 2021, 9, 9730–9760. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Song, Y.; Luo, J.; Wei, W.; Xu, S.; Cai, X. Highly sensitive detection of quantal dopamine secretion from pheochromocytoma cells using neural microelectrode array electrodeposited with polypyrrole graphene. ACS Appl. Mater. Interfaces 2015, 7, 7619–7626. [Google Scholar] [CrossRef]
- Saunier, V.; Flahaut, E.; Blatche, M.C.; Bergaud, C.; Maziz, A. Carbon nanofiber-PEDOT composite films as novel microelectrode for neural interfaces and biosensing. Biosens. Bioelectron. 2020, 165, 112413. [Google Scholar] [CrossRef]
- He, E.; Zhou, Y.; Luo, J.; Xu, S.; Zhang, K.; Song, Y.; Wang, M.; Xu, S.; Dai, Y.; Yang, G.; et al. Sensitive detection of electrophysiology and dopamine vesicular exocytosis of hESC-derived dopaminergic neurons using multifunctional microelectrode array. Biosens. Bioelectron. 2022, 209, 114263. [Google Scholar] [CrossRef]
- Weidlich, S.; Krause, K.J.; Schnitker, J.; Wolfrum, B.; Offenhausser, A. MEAs and 3D nanoelectrodes: Electrodeposition as tool for a precisely controlled nanofabrication. Nanotechnology 2017, 28, 095302. [Google Scholar] [CrossRef]
- Hai, A.; Shappir, J.; Spira, M.E. In-cell recordings by extracellular microelectrodes. Nat. Methods 2010, 7, 200–202. [Google Scholar] [CrossRef]
- Hai, A.; Shappir, J.; Spira, M.E. Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 2010, 104, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Hai, A.; Dormann, A.; Shappir, J.; Yitzchaik, S.; Bartic, C.; Borghs, G.; Langedijk, J.P.; Spira, M.E. Spine-shaped gold protrusions improve the adherence and electrical coupling of neurons with the surface of micro-electronic devices. J. R. Soc. Interface 2009, 6, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, R.; Elthakeb, A.T.; Lee, S.H.; Hinckley, S.; Khraiche, M.L.; Scott, J.; Pre, D.; Hwang, Y.; Tanaka, A.; et al. High density individually addressable nanowire arrays record intracellular activity from primary rodent and human stem cell derived neurons. Nano Lett. 2017, 17, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Colistra, N.; Melle, G.; Cerea, A.; Hubarevich, A.; Deleye, L.; De Angelis, F.; Dipalo, M. Microfluidic multielectrode arrays for spatially localized drug delivery and electrical recordings of primary neuronal cultures. Front. Bioeng. Biotechnol. 2020, 8, 626. [Google Scholar] [CrossRef]
- Zips, S.; Grob, L.; Rinklin, P.; Terkan, K.; Adly, N.Y.; Weiss, L.J.K.; Mayer, D.; Wolfrum, B. Fully printed mu-needle electrode array from conductive polymer ink for bioelectronic applications. ACS Appl. Mater. Interfaces 2019, 11, 32778–32786. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.A.; Springer, P.A.; Okun, L.M.; Berwaldn, Y.; Loeb, G.E. A miniature microelectrodeI array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- He, E.; Xu, S.; Xiao, G.; Dai, Y.; Li, X.; Song, Y.; Gao, F.; Zhang, Y.; Xu, S.; Cai, X. MWCNTs/PEDOT:PSS nanocomposites-modified microelectrode array for spatial dynamics recording of epileptic discharges in multi-subregion of hippocampal slice. Sens. Actuators B Chem. 2021, 329, 129190. [Google Scholar] [CrossRef]
- Abbott, J.; Mukherjee, A.; Wu, W.; Ye, T.; Jung, H.S.; Cheung, K.M.; Gertner, R.S.; Basan, M.; Ham, D.; Park, H. Multi-parametric functional imaging of cell cultures and tissues with a CMOS microelectrode array. Lab Chip 2022, 22, 1286–1296. [Google Scholar] [CrossRef]
- Yuan, X.; Hierlemann, A.; Frey, U. Extracellular recording of entire neural networks using a dual-mode microelectrode array with 19584 electrodes and high SNR. IEEE J. Solid-State Circuits 2021, 56, 2466–2475. [Google Scholar] [CrossRef]
- Abbott, J.; Ye, T.; Qin, L.; Jorgolli, M.; Gertner, R.S.; Ham, D.; Park, H. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 2017, 12, 460–466. [Google Scholar] [CrossRef]
- Duc, P.; Vignes, M.; Hugon, G.; Sebban, A.; Carnac, G.; Malyshev, E.; Charlot, B.; Rage, F. Human neuromuscular junction on micro-structured microfluidic devices implemented with a custom micro electrode array (MEA). Lab Chip 2021, 21, 4223–4236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Wang, Y.; Chen, H.; Wang, Y.; Liu, Y.; Pei, W. Development of implantable optrode devices. Acta Phys.-Chim. Sin. 2020, 36, 1912054. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, L.; Wang, Y.; Chen, R.; Yang, X.; Wu, X.; Wang, Y.; Chen, H.; Xu, C.; Pei, W. Improve the spatial resolution of fiber photometry by μLED linear array for fluorescence detection. Sens. Actuators A Phys. 2021, 331, 112948. [Google Scholar] [CrossRef]
- Cabello, M.; Domínguez, I.; Macías, C.; Perdigones, F.; Aracil, C.; Quero, J.M. Low cost SU-8 lift-off process to fabricate a gold/glass microelectrodes array for culturing applications. Microsyst. Technol. 2021, 27, 3077–3081. [Google Scholar] [CrossRef]
- Morales-Carvajal, P.M.; Kundu, A.; Didier, C.M.; Hart, C.; Sommerhage, F.; Rajaraman, S. Makerspace microfabrication of a stainless steel 3D microneedle electrode array (3D MEA) on a glass substrate for simultaneous optical and electrical probing of electrogenic cells. RSC Adv. 2020, 10, 41577–41587. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro electromechanical systems (MEMS) based microfluidic devices for biomedical applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Bartlett, N.W.; Wood, R.J. Comparative analysis of fabrication methods for achieving rounded microchannels in PDMS. J. Micromech. Microeng. 2016, 26, 115013. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluidics 2021, 25, 99. [Google Scholar] [CrossRef]
- Jeon, H.; Kwon, T.; Yoon, J.; Han, J. Engineering a deformation-free plastic spiral inertial microfluidic system for CHO cell clarification in biomanufacturing. Lab Chip 2022, 22, 272–285. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Feng, Y.; Liang, F.; Wang, W. High content drug screening of primary cardiomyocytes based on microfluidics and real-time ultra-large-scale high-resolution imaging. Lab Chip 2022, 22, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Campenot, R.B. Local control of neurite development by nerve growth-factor. Proc. Natl. Acad. Sci. USA 1977, 74, 4516–4519. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Rhee, S.W.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Microfluidic multicompartment device for neuroscience research. Langmuir 2003, 19, 1551–1556. [Google Scholar] [CrossRef]
- Rhee, S.W.; Taylor, A.M.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Patterned cell culture inside microfluidic devices. Lab Chip 2005, 5, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Taylor, A.M.; Dieterich, D.C.; Ito, H.T.; Kim, S.A.; Schuman, E.M. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron 2010, 66, 57–68. [Google Scholar] [CrossRef]
- De Vitis, E.; La Pesa, V.; Gervaso, F.; Romano, A.; Quattrini, A.; Gigli, G.; Moroni, L.; Polini, A. A microfabricated multi-compartment device for neuron and Schwann cell differentiation. Sci. Rep. 2021, 11, 7019. [Google Scholar] [CrossRef]
- Coquinco, A.; Kojic, L.; Wen, W.; Wang, Y.T.; Jeon, N.L.; Milnerwood, A.J.; Cynader, M. A microfluidic based in vitro model of synaptic competition. Mol. Cell. Neurosci. 2014, 60, 43–52. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Park, S.I.; Choe, Y.; Li, J.; Han, A. A microchip for quantitative analysis of CNS axon growth under localized biomolecular treatments. J. Neurosci. Methods 2014, 221, 166–174. [Google Scholar] [CrossRef]
- Megarity, D.; Vroman, R.; Kriek, M.; Downey, P.; Bushell, T.J.; Zagnoni, M. A modular microfluidic platform to enable complex and customisable in vitro models for neuroscience. Lab Chip 2022, 22, 1989–2000. [Google Scholar] [CrossRef]
- Gladkov, A.; Pigareva, Y.; Kutyina, D.; Kolpakov, V.; Bukatin, A.; Mukhina, I.; Kazantsev, V.; Pimashkin, A. Design of cultured neuron networks in vitro with predefined connectivity using asymmetric microfluidic channels. Sci. Rep. 2017, 7, 15625. [Google Scholar] [CrossRef] [PubMed]
- Renault, R.; Durand, J.B.; Viovy, J.L.; Villard, C. Asymmetric axonal edge guidance: A new paradigm for building oriented neuronal networks. Lab Chip 2016, 16, 2188–2191. [Google Scholar] [CrossRef]
- Pirlo, R.K.; Sweeney, A.J.; Ringeisen, B.R.; Kindy, M.; Gao, B.Z. Biochip/laser cell deposition system to assess polarized axonal growth from single neurons and neuron/glia pairs in microchannels with novel asymmetrical geometries. Biomicrofluidics 2011, 5, 13408. [Google Scholar] [CrossRef] [PubMed]
- le Feber, J.; Postma, W.; de Weerd, E.; Weusthof, M.; Rutten, W.L. Barbed channels enhance unidirectional connectivity between neuronal networks cultured on multi electrode arrays. Front. Neurosci. 2015, 9, 412. [Google Scholar] [CrossRef]
- Peyrin, J.M.; Deleglise, B.; Saias, L.; Vignes, M.; Gougis, P.; Magnifico, S.; Betuing, S.; Pietri, M.; Caboche, J.; Vanhoutte, P.; et al. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip 2011, 11, 3663–3673. [Google Scholar] [CrossRef]
- Girardin, S.; Clement, B.; Ihle, S.J.; Weaver, S.; Petr, J.B.; Mateus, J.C.; Duru, J.; Krubner, M.; Forro, C.; Ruff, T.; et al. Topologically controlled circuits of human iPSC-derived neurons for electrophysiology recordings. Lab Chip 2022, 22, 1386–1403. [Google Scholar] [CrossRef]
- Ihle, S.J.; Girardin, S.; Felder, T.; Ruff, T.; Hengsteler, J.; Duru, J.; Weaver, S.; Forro, C.; Voros, J. An experimental paradigm to investigate stimulation dependent activity in topologically constrained neuronal networks. Biosens. Bioelectron. 2022, 201, 113896. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Hughes, C.; Deppmann, C. A microfluidic culture platform to assess axon degeneration. In Axon Degeneration: Methods and Protocols; Babetto, E., Ed.; Humana: New York, NY, USA, 2020; Volume 2143, pp. 83–96. [Google Scholar]
- Andrzejewska, A.; Janowski, M. Microfluidic systems in CNS studies. In Biomaterials- and Microfluidics-Based Tissue Engineered 3D Models; Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 87–95. [Google Scholar]
- Kunze, A.; Meissner, R.; Brando, S.; Renaud, P. Co-pathological connected primary neurons in a microfluidic device for Alzheimer studies. Biotechnol. Bioeng. 2011, 108, 2241–2245. [Google Scholar] [CrossRef]
- Kajtez, J.; Buchmann, S.; Vasudevan, S.; Birtele, M.; Rocchetti, S.; Pless, C.J.; Heiskanen, A.; Barker, R.A.; Martinez-Serrano, A.; Parmar, M.; et al. 3D-Printed soft lithography for complex compartmentalized microfluidic neural devices. Adv. Sci. 2020, 7, 2001150. [Google Scholar] [CrossRef]
- Virlogeux, A.; Moutaux, E.; Christaller, W.; Genoux, A.; Bruyere, J.; Fino, E.; Charlot, B.; Cazorla, M.; Saudou, F. Reconstituting corticostriatal network on-a-chip reveals the contribution of the presynaptic compartment to Huntington’s disease. Cell Rep. 2018, 22, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Gribaudo, S.; Tixador, P.; Bousset, L.; Fenyi, A.; Lino, P.; Melki, R.; Peyrin, J.M.; Perrier, A.L. Propagation of alpha-synuclein strains within human reconstructed neuronal network. Stem Cell Rep. 2019, 12, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.B.; Pluchon, P.; Harley, P.; Rigby, M.; Gonzalez Sabater, V.; Stevenson, D.C.; Hynes, S.; Lowe, A.; Burrone, J.; Viasnoff, V.; et al. In vitro modelling of nerve-muscle connectivity in a compartmentalised tissue culture device. Adv. Biosyst. 2019, 3, 1800307. [Google Scholar] [CrossRef]
- Kunze, A.; Lengacher, S.; Dirren, E.; Aebischer, P.; Magistretti, P.J.; Renaud, P. Astrocyte-neuron co-culture on microchips based on the model of SOD mutation to mimic ALS. Integr. Biol. 2013, 5, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Terrell-Hall, T.B.; Ammer, A.G.; Griffith, J.I.; Lockman, P.R. Permeability across a novel microfluidic blood-tumor barrier model. Fluids Barriers CNS 2017, 14, 3. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Raiteri, R.; Massobrio, P. On the road to the brain-on-a-chip: A review on strategies, methods, and applications. J. Neural Eng. 2021, 18, 041005. [Google Scholar] [CrossRef]
- Kim, W.; Kim, J.; Lee, S.-Y.; Kim, H.-M.; Jung, H.; Joo, K.M.; Nam, D.-H. Functional validation of the simplified in vitro 3D Co-culture based BBB model. Biochem. Biophys. Res. Commun. 2022, 625, 128–133. [Google Scholar] [CrossRef]
- Park, J.; Koito, H.; Li, J.; Han, A. Multi-compartment neuron-glia co-culture platform for localized CNS axon-glia interaction study. Lab Chip 2012, 12, 3296–3304. [Google Scholar] [CrossRef]
- Bellmann, J.; Goswami, R.Y.; Girardo, S.; Rein, N.; Hosseinzadeh, Z.; Hicks, M.R.; Busskamp, V.; Pyle, A.D.; Werner, C.; Sterneckert, J. A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits. Biomaterials 2019, 225, 119537. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, N.; Yang, X.; Hamada, Y.; Li, Q.; Zheng, W.; Jiang, X. An on-chip model for investigating the interaction between neurons and cancer cells. Integr. Biol. 2016, 8, 359–367. [Google Scholar] [CrossRef]
- Li, L.; Ren, L.; Liu, W.; Wang, J.C.; Wang, Y.; Tu, Q.; Xu, J.; Liu, R.; Zhang, Y.; Yuan, M.S.; et al. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal. Chem. 2012, 84, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, Y.; Staley, K.J.; Yarmush, M.L. Building and manipulating neural pathways with microfluidics. Lab Chip 2010, 10, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Ruther, P.; Paul, O. New approaches for CMOS-based devices for large-scale neural recording. Curr. Opin. Neurobiol. 2015, 32, 31–37. [Google Scholar] [CrossRef]
- Tanwar, A.; Gandhi, H.A.; Kushwaha, D.; Bhattacharya, J. A review on microelectrode array fabrication techniques and their applications. Mater. Today Chem. 2022, 26, 101153. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, H.J.; Rajaraman, S.; Kim, D.-H. Recent advances in three-dimensional microelectrode array technologies for in vitro and in vivo cardiac and neuronal interfaces. Biosens. Bioelectron. 2021, 171, 112687. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.H.D.; Robbins, J.; Bowen, C.R.; Taylor, J. Commercialisation of CMOS integrated circuit technology in multi-electrode arrays for neuroscience and cell-based biosensors. Sensors 2011, 11, 4943–4971. [Google Scholar] [CrossRef]
- Horiuchi, T.; Niwa, O.; Morita, M. Fabrication and photoelectrochemical properties of interdigitated array microelectrodes consisting of optically transparent and nontransparent band electrodes. J. Electrochem. Soc. 1995, 142, L146. [Google Scholar] [CrossRef]
- Donaldson, P.D.; Swisher, S.L. Transparent, low-impedance inkjet-printed PEDOT:PSS microelectrodes for multimodal neuroscience. Phys. Status Solidi (a) 2022, 219, 2100683. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.Y.; Zong, C.; Liu, B.; Zhang, D.X.; Sun, W.; Wu, Y.F.; Lu, M.; Tian, Z.Q. Design and fabrication of an MEA microchip for cell culture study. Integr. Ferroelectr. 2012, 135, 71–76. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, Y.-G.; Kim, S.; Park, J.-U. 3D electrodes for bioelectronics. Adv. Mater. 2021, 33, 2005805. [Google Scholar] [CrossRef]
- Teixeira, H.; Dias, C.; Aguiar, P.; Ventura, J. Gold-mushroom microelectrode arrays and the quest for intracellular-like recordings: Perspectives and outlooks. Adv. Mater. Technol. 2021, 6, 2000770. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Guo, L. Opportunities and dilemmas of in vitro nano neural electrodes. RSC Adv. 2019, 10, 187–200. [Google Scholar] [CrossRef]
- Banik, S.; Uchil, A.; Kalsang, T.; Chakrabarty, S.; Ali, M.A.; Srisungsitthisunti, P.; Mahato, K.K.; Surdo, S.; Mazumder, N. The revolution of PDMS microfluidics in cellular biology. Crit. Rev. Biotechnol. 2022, 1–19. [Google Scholar] [CrossRef]
- Park, J.W.; Vahidi, B.; Taylor, A.M.; Rhee, S.W.; Jeon, N.L. Microfluidic culture platform for neuroscience research. Nat. Protoc. 2006, 1, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Kasi, D.G.; de Graaf, M.N.S.; Motreuil-Ragot, P.A.; Frimat, J.M.S.; Ferrari, M.D.; Sarro, P.M.; Mastrangeli, M.; van den Maagdenberg, A.; Mummery, C.L.; Orlova, V.V. Rapid prototyping of organ-on-a-chip devices using maskless photolithography. Micromachines 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Wang, M.-H.; Jang, L.-S. Microfluidics-based hairpin resonator biosensor for biological cell detection. Sens. Actuators B Chem. 2018, 263, 129–136. [Google Scholar] [CrossRef]
- Chen, W.; Lam, R.H.; Fu, J. Photolithographic surface micromachining of polydimethylsiloxane (PDMS). Lab Chip 2012, 12, 391–395. [Google Scholar] [CrossRef]
- Gao, S.; Tung, W.-T.; Wong, D.S.-H.; Bian, L.; Zhang, A.P. Direct optical micropatterning of poly(dimethylsiloxane) for microfluidic devices. J. Micromech. Microeng. 2018, 28, 095011. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip 2007, 7, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Wu, J.; Wen, W.; Li, W.; Alici, G. A simple and cost-effective method for fabrication of integrated electronic-microfluidic devices using a laser-patterned PDMS layer. Microfluid. Nanofluidics 2011, 12, 751–760. [Google Scholar] [CrossRef]
- Oyama, T.G.; Barba, B.J.D.; Hosaka, Y.; Taguchi, M. Single-step fabrication of polydimethylsiloxane microwell arrays with long-lasting hydrophilic inner surfaces. Appl. Phys. Lett. 2018, 112, 213704. [Google Scholar] [CrossRef]
- Clancy, A.; Chen, D.; Bruns, J.; Nadella, J.; Stealey, S.; Zhang, Y.; Timperman, A.; Zustiak, S.P. Hydrogel-based microfluidic device with multiplexed 3D in vitro cell culture. Sci. Rep. 2022, 12, 17781. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.C.; Randhawa, A.; Grist, S.M.; Bennet, T.; Hua, J.; Alde, L.G.; Caffrey, T.M.; Wellington, C.L.; Cheung, K.C. PDMS organ-on-chip eesign and fabrication: Strategies for improving fluidic integration and chip robustness of rapidly prototyped microfluidic in vitro models. Micromachines 2022, 13, 1573. [Google Scholar] [CrossRef]
- Hong, N.; Nam, Y. Neurons-on-a-Chip: In Vitro NeuroTools. Mol. Cells 2022, 45, 76–83. [Google Scholar] [CrossRef]
- Waters, L.J.; Finch, C.V.; Bhuiyan, A.; Hemming, K.; Mitchell, J.C. Effect of plasma surface treatment of poly(dimethylsiloxane) on the permeation of pharmaceutical compounds. J. Pharm. Anal. 2017, 7, 338–342. [Google Scholar] [CrossRef]
- Bakouche, M.T.; Ganesan, S.; Guérin, D.; Hourlier, D.; Bouazaoui, M.; Vilcot, J.P.; Maricot, S. Leak-free integrated microfluidic channel fabrication for surface plasmon resonance applications. J. Micromech. Microeng. 2020, 30, 125003. [Google Scholar] [CrossRef]
- Hoang, M.V.; Chung, H.-J.; Elias, A.L. Irreversible bonding of polyimide and polydimethylsiloxane (PDMS) based on a thiol-epoxy click reaction. J. Micromech. Microeng. 2016, 26, 105019. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Polydimethylsiloxane (PDMS) irreversible bonding to untreated plastics and metals for microfluidics applications. APL Mater. 2019, 7, 081108. [Google Scholar] [CrossRef]
- Thompson, C.S.; Abate, A.R. Adhesive-based bonding technique for PDMS microfluidic devices. Lab Chip 2013, 13, 632–635. [Google Scholar] [CrossRef]
- Borok, A.; Laboda, K.; Bonyar, A. PDMS bonding technologies for microfluidic applications: A review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Pigareva, Y.; Gladkov, A.; Kolpakov, V.; Mukhina, I.; Bukatin, A.; Kazantsev, V.B.; Pimashkin, A. Experimental platform to study spiking pattern propagation in modular networks in vitro. Brain Sci. 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.F.; Mateus, J.C.; Aguiar, P. Interfacing microfluidics with microelectrode arrays for studying neuronal communication and axonal signal propagation. Jove-J. Vis. Exp. 2018, 142, e58878. [Google Scholar] [CrossRef]
- Shimba, K.; Sakai, K.; Isomura, T.; Kotani, K.; Jimbo, Y. Axonal conduction slowing induced by spontaneous bursting activity in cortical neurons cultured in a microtunnel device. Integr. Biol. 2015, 7, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Joo, S.; Nam, Y. Characterization of axonal spikes in cultured neuronal networks using microelectrode arrays and microchannel devices. IEEE Trans. Biomed. Eng. 2017, 64, 492–498. [Google Scholar] [CrossRef]
- Goshi, N.; Girardi, G.; Souza, F.D.; Gardner, A.; Lein, P.J.; Seker, E. Influence of microchannel geometry on device performance and electrophysiological recording fidelity during long-term studies of connected neural populations. Lab Chip 2022, 22, 3961–3975. [Google Scholar] [CrossRef]
- Heiney, K.; Mateus, J.C.; Lopes, C.D.F.; Neto, E.; Lamghari, M.; Aguiar, P. microSpikeHunter: An advanced computational tool for the analysis of neuronal communication and action potential propagation in microfluidic platforms. Sci. Rep. 2019, 9, 5777. [Google Scholar] [CrossRef]
- Xu, S.; Deng, Y.; Luo, J.; He, E.; Liu, Y.; Zhang, K.; Yang, Y.; Xu, S.; Sha, L.; Song, Y.; et al. High-throughput PEDOT:PSS/PtNPs-modified microelectrode array for simultaneous recording and stimulation of hippocampal neuronal networks in gradual learning process. ACS Appl. Mater. Interfaces 2022, 14, 15736–15746. [Google Scholar] [CrossRef]
- Gladkov, A.A.; Kolpakov, V.N.; Pigareva, Y.I.; Bukatin, A.S.; Kazantsev, V.B.; Mukhina, I.V.; Pimashkin, A.S. Study of stimulus-induced plasticity in neural networks cultured in microfluidic chips. Sovrem. Tehnol. V Med. 2017, 9, 15–23. [Google Scholar] [CrossRef][Green Version]
- Kim, J.W.; Choi, Y.Y.; Park, S.H.; Ha, J.H.; Lee, H.U.; Kang, T.; Sun, W.; Chung, B.G. Microfluidic electrode array chip for electrical stimulation-mediated axonal regeneration. Lab Chip 2022, 22, 2122–2130. [Google Scholar] [CrossRef]
- Toivanen, M.; Pelkonen, A.; Makinen, M.; Yla-Outinen, L.; Sukki, L.; Kallio, P.; Ristola, M.; Narkilahti, S. Optimised PDMS tunnel devices on MEAs increase the probability of detecting electrical activity from human stem cell-derived neuronal networks. Front. Neurosci. 2017, 11, 606. [Google Scholar] [CrossRef]
- Moutaux, E.; Charlot, B.; Genoux, A.; Saudou, F.; Cazorla, M. An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 2018, 18, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.R.; Kim, C.Y.; Kim, J.; Ryu, B.; Lee, S.G.; Baek, J.; Kim, Y.J.; Lee, J.M.; Lee, Y.; Choi, S.O.; et al. Establishment of neurotoxicity assessment using microelectrode array (MEA) with hiPSC-derived neurons and evaluation of new psychoactive substances (NPS). Int. J. Stem Cells 2022, 15, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kaswan, K.; Barman, S.R.; Lin, Y.Z.; Chung, J.H.; Sharma, M.K.; Liu, K.L.; Chen, B.H.; Wu, C.C.; Lee, S.; et al. Microfluidic nanodevices for drug sensing and screening applications. Biosens. Bioelectron. 2022, 219, 114783. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Verpoorte, E.; Linder, V.; Franks, W.; Hierlemann, A.; Heer, F.; Hafizovic, S.; Fujii, T.; de Rooij, N.F.; Koster, S. Characterization of a microfluidic dispensing system for localised stimulation of cellular networks. Lab Chip 2006, 6, 218–229. [Google Scholar] [CrossRef]
- Biffi, E.; Piraino, F.; Pedrocchi, A.; Fiore, G.B.; Ferrigno, G.; Redaelli, A.; Menegon, A.; Rasponi, M. A microfluidic platform for controlled biochemical stimulation of twin neuronal networks. Biomicrofluidics 2012, 6, 24106–2410610. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathi, T.T.; Ciliberti, D.; Martinoia, S.; Wadman, W.J.; Decre, M.M. Dual-compartment neurofluidic system for electrophysiological measurements in physically segregated and functionally connected neuronal cell culture. Front. Neuroeng. 2011, 4, 13. [Google Scholar] [CrossRef]
- Enright, H.A.; Felix, S.H.; Fischer, N.O.; Mukerjee, E.V.; Soscia, D.; McNerney, M.; Kulp, K.; Zhang, J.; Page, G.; Miller, P.; et al. Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform. Analyst 2016, 141, 5346–5357. [Google Scholar] [CrossRef]
- Miny, L.; Maisonneuve, B.G.C.; Quadrio, I.; Honegger, T. Modeling neurodegenerative diseases using in vitro compartmentalized microfluidic devices. Front. Bioeng. Biotechnol. 2022, 10, 919646. [Google Scholar] [CrossRef]
- van de Wijdeven, R.; Ramstad, O.H.; Valderhaug, V.D.; Kollensperger, P.; Sandvig, A.; Sandvig, I.; Halaas, O. A novel lab-on-chip platform enabling axotomy and neuromodulation in a multi-nodal network. Biosens. Bioelectron. 2019, 140, 111329. [Google Scholar] [CrossRef]
- Tong, Z.; Segura-Feliu, M.; Seira, O.; Homs-Corbera, A.; Del Río, J.A.; Samitier, J. A microfluidic neuronal platform for neuron axotomy and controlled regenerative studies. RSC Adv. 2015, 5, 73457–73466. [Google Scholar] [CrossRef]
- Habibey, R.; Golabchi, A.; Latifi, S.; Difato, F.; Blau, A. A microchannel device tailored to laser axotomy and long-term microelectrode array electrophysiology of functional regeneration. Lab Chip 2015, 15, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, A.; Mzezewa, R.; Sukki, L.; Ryynanen, T.; Kreutzer, J.; Hyvarinen, T.; Vinogradov, A.; Aarnos, L.; Lekkala, J.; Kallio, P.; et al. A modular brain-on-a-chip for modelling epileptic seizures with functionally connected human neuronal networks. Biosens. Bioelectron. 2020, 168, 112553. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Wheeler, B.C.; DeMarse, T.B.; Brewer, G.J. Pattern separation and completion of distinct axonal inputs transmitted via micro-tunnels between co-cultured hippocampal dentate, CA3, CA1 and entorhinal cortex networks. J. Neural Eng. 2018, 15, 046009. [Google Scholar] [CrossRef]
- Vakilna, Y.S.; Tang, W.C.; Wheeler, B.C.; Brewer, G.J. The flow of axonal information among hippocampal subregions: 1. feed-forward and feedback network spatial dynamics underpinning emergent information processing. Front. Neural Circuits 2021, 15, 660837. [Google Scholar] [CrossRef]
- Callegari, F.; Brofiga, M.; Poggio, F.; Massobrio, P. Stimulus-evoked activity modulation of in vitro engineered cortical and hippocampal networks. Micromachines 2022, 13, 1212. [Google Scholar] [CrossRef]
- Kanagasabapathi, T.T.; Massobrio, P.; Barone, R.A.; Tedesco, M.; Martinoia, S.; Wadman, W.J.; Decre, M.M.J. Functional connectivity and dynamics of cortical-thalamic networks co-cultured in a dual compartment device. J. Neural Eng. 2012, 9, 036010. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.Q.; Kwak, E.; Aguiar, A.; Peng, B.; Pouton, C.W.; Voelcker, N.H.; Haynes, J.M. Compartmentalized microfluidic chambers enable long-term maintenance and communication between human pluripotent stem cell-derived forebrain and midbrain neurons. Lab Chip 2021, 21, 4016–4030. [Google Scholar] [CrossRef]
- Chang, C.; Furukawa, T.; Asahina, T.; Shimba, K.; Kotani, K.; Jimbo, Y. Coupling of in vitro neocortical-hippocampal coculture bursts induces different spike rhythms in individual networks. Front. Neurosci. 2022, 16, 873664. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Tedesco, M.; Boccaccio, A.; Massobrio, P. Functional inhibitory connections modulate the electrophysiological activity patterns of cortical-hippocampal ensembles. Cereb. Cortex 2022, 32, 1866–1881. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Tedesco, M.; Raiteri, R.; Massobrio, P. Three-dimensionality shapes the dynamics of cortical interconnected to hippocampal networks. J. Neural Eng. 2020, 17, 056044. [Google Scholar] [CrossRef]
- Cojocaru, A.E.; Corna, A.; Reh, M.; Zeck, G. High spatial resolution artificial vision inferred from the spiking output of retinal ganglion cells stimulated by optogenetic and electrical means. Front. Cell. Neurosci. 2022, 16, 605. [Google Scholar] [CrossRef] [PubMed]
- Duru, J.; Küchler, J.; Ihle, S.J.; Forró, C.; Bernardi, A.; Girardin, S.; Hengsteler, J.; Wheeler, S.; Vörös, J.; Ruff, T. Engineered biological neural networks on high density CMOS microelectrode arrays. Front. Neurosci. 2022, 16, 829884. [Google Scholar] [CrossRef]
- Huang, Y.; Williams, J.C.; Johnson, S.M. Brain slice on a chip: Opportunities and challenges of applying microfluidic technology to intact tissues. Lab Chip 2012, 12, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Shrirao, A.B.; Kung, F.H.; Omelchenko, A.; Schloss, R.S.; Boustany, N.N.; Zahn, J.D.; Yarmush, M.L.; Firestein, B.L. Microfluidic platforms for the study of neuronal injury in vitro. Biotechnol. Bioeng. 2018, 115, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Susloparova, A.; Halliez, S.; Begard, S.; Colin, M.; Buee, L.; Pecqueur, S.; Alibart, F.; Thomy, V.; Arscott, S.; Pallecchi, E.; et al. Low impedance and highly transparent microelectrode arrays (MEA) for in vitro neuron electrical activity probing. Sens. Actuators B Chem. 2021, 327, 128895. [Google Scholar] [CrossRef]
- Bentil, S.A.; Dupaix, R.B. Simulations of hydrogel-coated neural microelectrodes to assess biocompatibility improvement using strain as a metric for micromotion. Biomed. Phys. Eng. Express 2018, 4, 035036. [Google Scholar] [CrossRef]
| Neural Chip Components | Arrangement of MEA | Detection Object | Application | Reference |
|---|---|---|---|---|
| MEA | Microelectrodes fitting the shape of the dentate gyrus of the hippocampal slices | Hippocampal slices | Research on epilepsy circuit | He et al. (2021) [57] |
| MEA | 128 microelectrodes are distributed in the center of the MEA | Hippocampal neurons | Research on the learning function of neural networks in vitro | Xu et al. (2022) [139] |
| CMOS MEA | 4225 recording electrodes and 1024 stimulation electrodes | Retina | Research on visual restoration | Cojocaru et al. (2022) [163] |
| MEA and PDMS-based microfluidic device | Microelectrodes are in the microchannel | Neural stem cells | Detection of neurite signals | Kim et al. (2022) [141] |
| MEA and PDMS-based microfluidic device | Microelectrodes are arranged at the edges of three chambers | Human stem cell-derived neurons | Research on epileptic seizures | Pelkonen et al. (2020) [154] |
| MEA and PDMS-based microfluidic device | Microelectrodes are arranged on both sides of the microchannel | Co-culture of motor neurons and muscle cells | Construction of the neuron–muscle model in vitro | Duc et al. (2021) [61] |
| CMOS MEA and PDMS-based microfluidic device | 26,400 electrodes located in an area of 3.85 × 2.10 mm2 | Cortical neurons | High-density detection of neural signals | Duru et al. (2022) [164] |
| MEA, PDMS-based microfluidic device, and magnetic bead | 60 microelectrodes are evenly distributed in two chambers of microfluidic devices | Cortical and hippocampal neurons | Cultivate three-dimensional brain network | Brofiga et al. (2020) [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Liu, Y.; Yang, Y.; Zhang, K.; Liang, W.; Xu, Z.; Wu, Y.; Luo, J.; Zhuang, C.; Cai, X. Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays. Micromachines 2023, 14, 709. https://doi.org/10.3390/mi14040709
Xu S, Liu Y, Yang Y, Zhang K, Liang W, Xu Z, Wu Y, Luo J, Zhuang C, Cai X. Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays. Micromachines. 2023; 14(4):709. https://doi.org/10.3390/mi14040709
Chicago/Turabian StyleXu, Shihong, Yaoyao Liu, Yan Yang, Kui Zhang, Wei Liang, Zhaojie Xu, Yirong Wu, Jinping Luo, Chengyu Zhuang, and Xinxia Cai. 2023. "Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays" Micromachines 14, no. 4: 709. https://doi.org/10.3390/mi14040709
APA StyleXu, S., Liu, Y., Yang, Y., Zhang, K., Liang, W., Xu, Z., Wu, Y., Luo, J., Zhuang, C., & Cai, X. (2023). Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays. Micromachines, 14(4), 709. https://doi.org/10.3390/mi14040709







