TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and EGTA Treatment
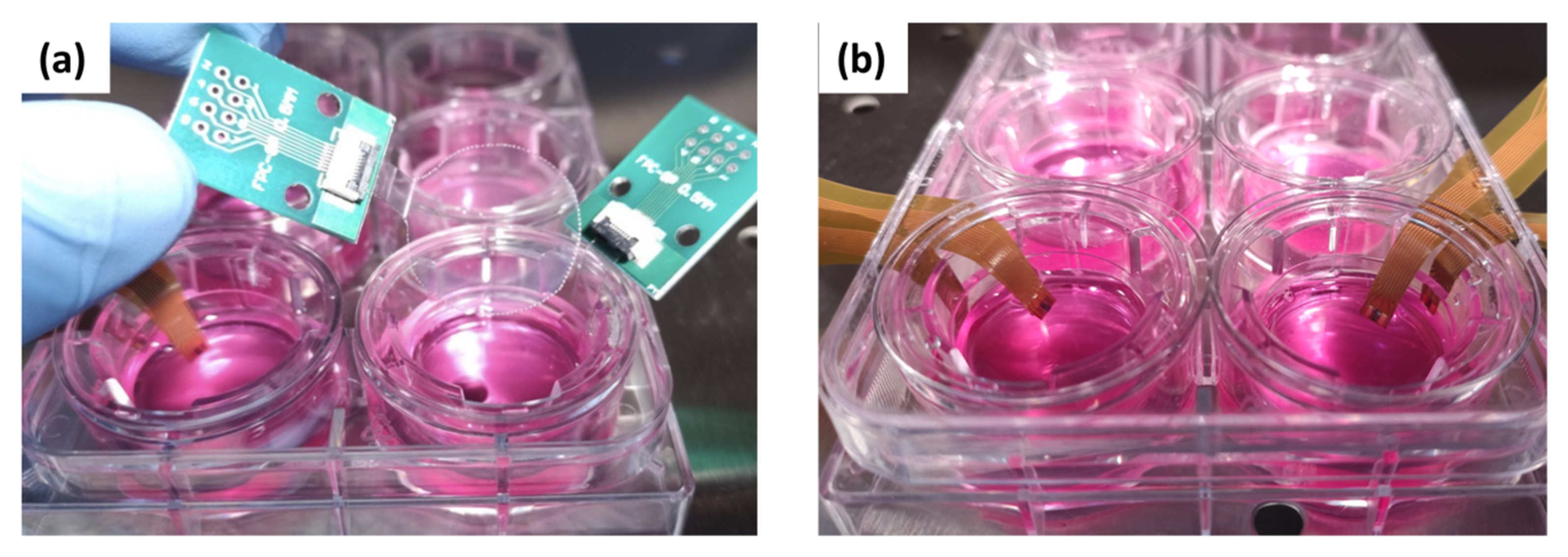
2.3. Sensors Fabrication
2.4. Membrane Blend Fabrication
2.5. TEER Measurements
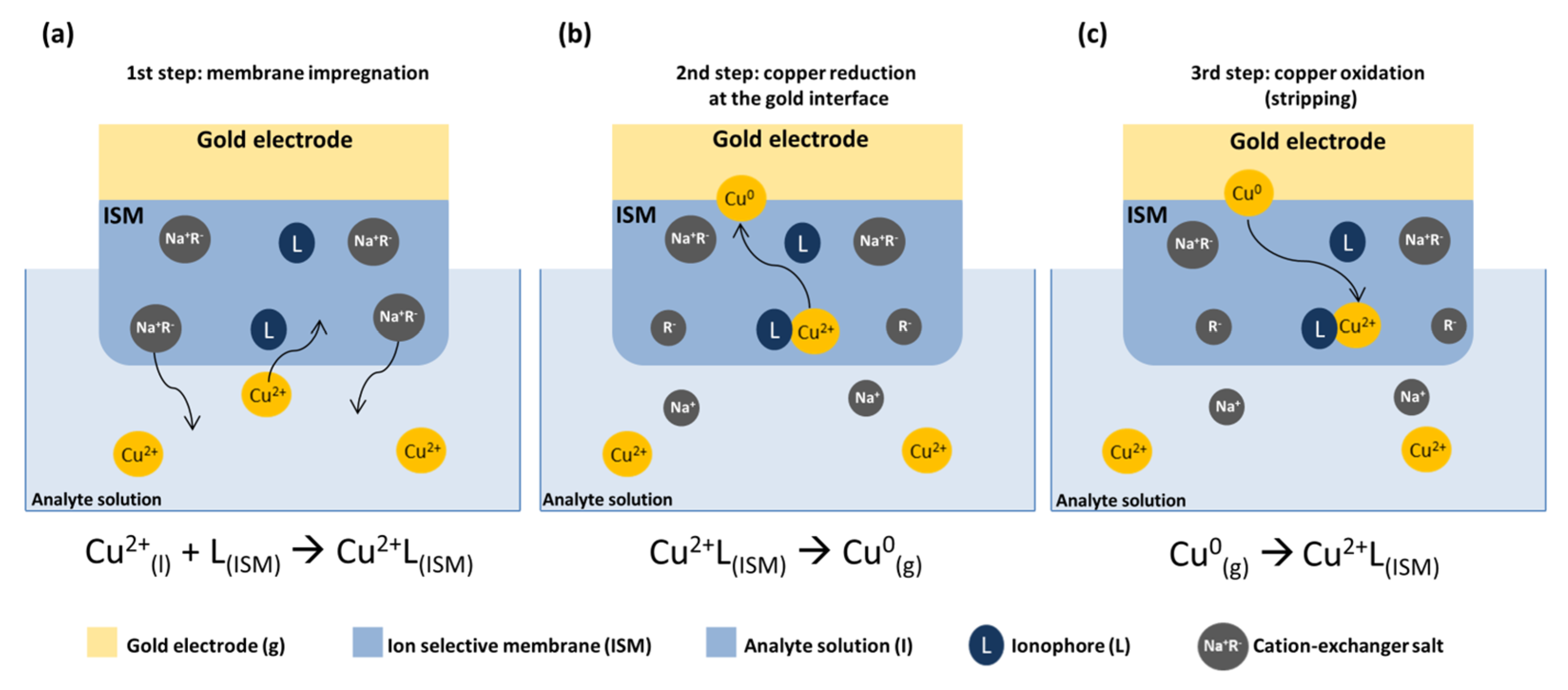
2.6. Measurements of Ion Sensing Capabilities
2.7. Immunofluorescent Staining
3. Results and Discussion
3.1. Experimental Design
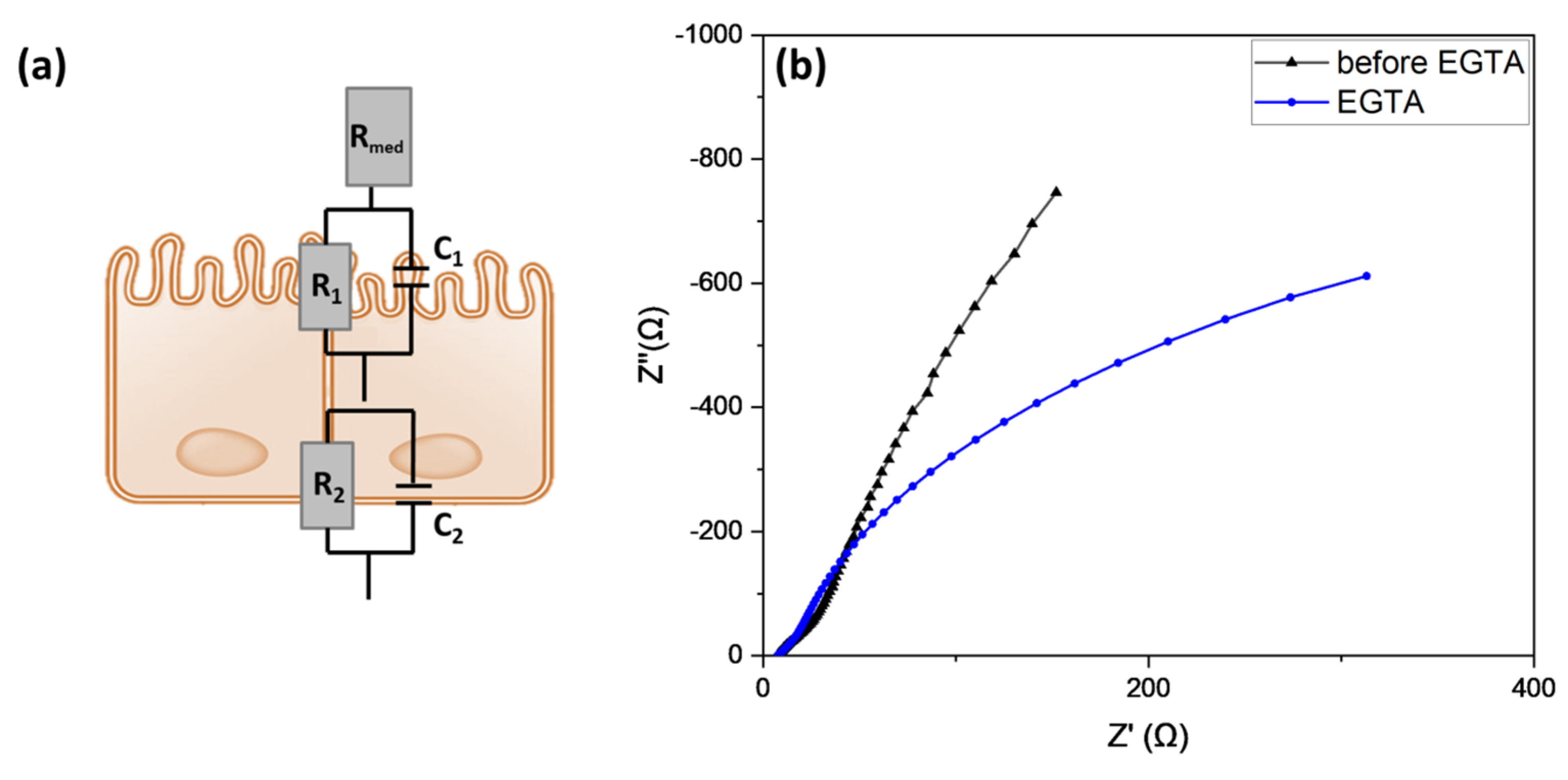
3.2. TEER Measurements and Equivalent Electrical Circuit Fitting
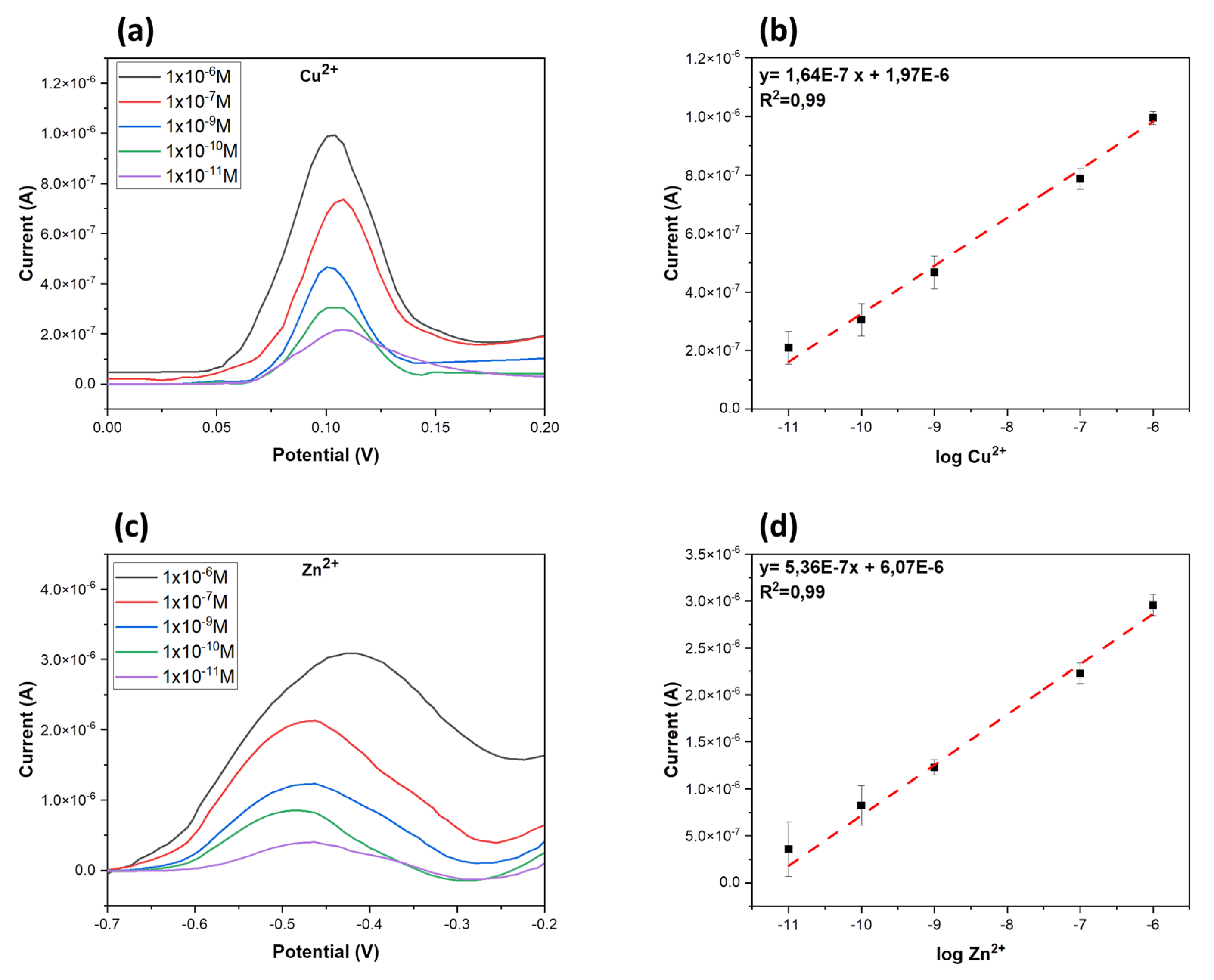
3.3. Ion-Sensing
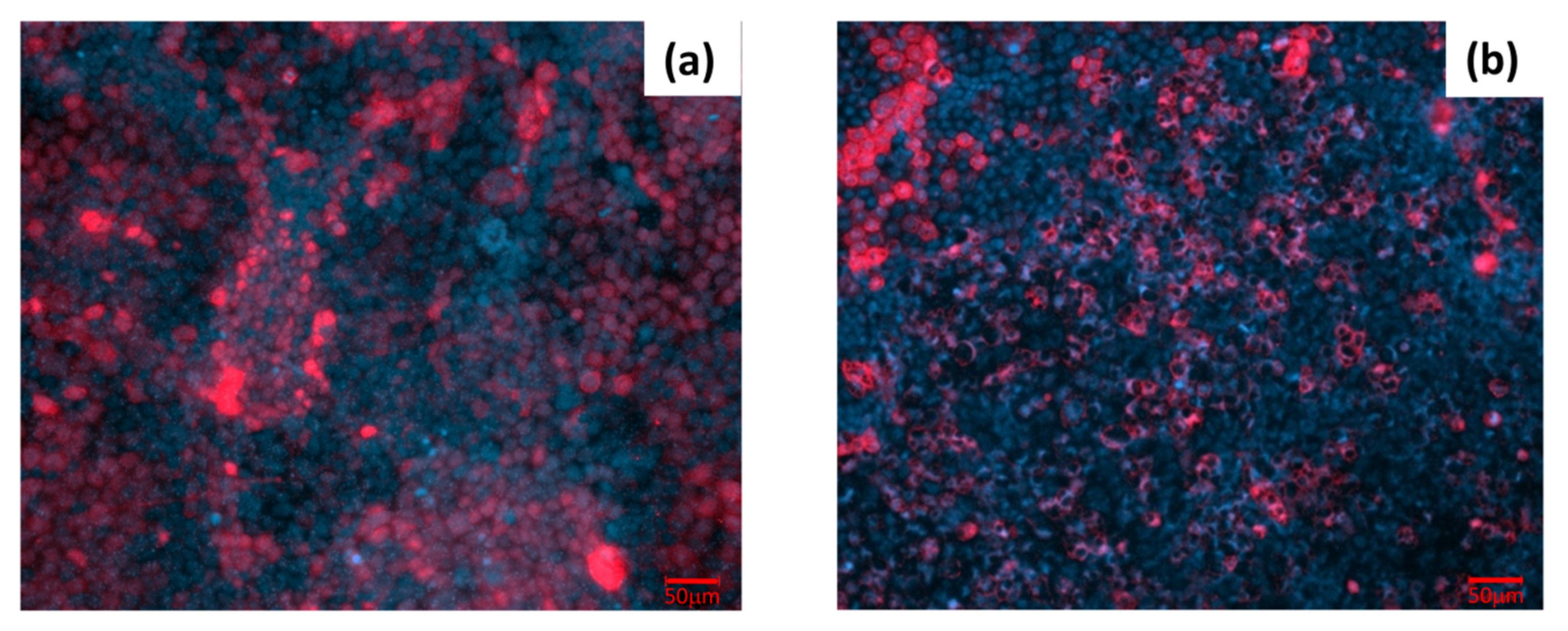
3.4. Immunofluorescent Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastasova, S.; Kassanos, P.; Yang, G.Z. Multi-Parametric Rigid and Flexible, Low-Cost, Disposable Sensing Platforms for Biomedical Applications. Biosens. Bioelectron. 2018, 102, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Kassanos, P.; Seichepine, F.; Wales, D.; Yang, G.-Z. Towards a Flexible/Stretchable Multiparametric Sensing Device for Surgical and Wearable Applications. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; ISBN 9781509006175. [Google Scholar]
- Chmayssem, A.; Verplanck, N.; Tanase, C.E.; Costa, G.; Monsalve-Grijalba, K.; Amigues, S.; Alias, M.; Gougis, M.; Mourier, V.; Vignoud, S.; et al. Development of a Multiparametric (Bio)Sensing Platform for Continuous Monitoring of Stress Metabolites. Talanta 2021, 229, 122275. [Google Scholar] [CrossRef] [PubMed]
- Otero, F.; Magner, E. Biosensors—Recent Advances and Future Challenges. Sensors 2020, 20, 6645. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent Advances in Potentiometric Biosensors. TrAC—Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Giampetruzzi, L.; Barca, A.; Casino, F.; Capone, S.; Verri, T.; Siciliano, P.; Francioso, L. Multi-Sensors Integration in a Human Gut-On-Chip Platform. Proceedings 2018, 2, 1022. [Google Scholar] [CrossRef]
- Clarke, G.A.; Hartse, B.X.; Ehsan, A.; Asli, N.; Taghavimehr, M.; Hashemi, N.; Shirsavar, M.A.; Montazami, R.; Alimoradi, N.; Nasirian, V.; et al. Advancement of Sensor Integrated Organ-on-Chip Devices. Sensors 2021, 21, 1367. [Google Scholar] [CrossRef]
- Ferrari, E.; Palma, C.; Vesentini, S.; Occhetta, P.; Rasponi, M. Integrating Biosensors in Organs-on-Chip Devices: A Perspective on Current Strategies to Monitor Microphysiological Systems. Biosensors 2020, 10, 110. [Google Scholar] [CrossRef]
- Signore, M.A.; De Pascali, C.; Giampetruzzi, L.; Siciliano, P.A.; Francioso, L. Gut-on-Chip Microphysiological Systems: Latest Advances in the Integration of Sensing Strategies and Adoption of Mature Detection Mechanisms. Sens. Bio-Sens. Res. 2021, 33, 100443. [Google Scholar] [CrossRef]
- Wu, C.; Selberg, J.; Nguyen, B.; Pansodtee, P.; Jia, M.; Dechiraju, H.; Teodorescu, M.; Rolandi, M. A Microfluidic Ion Sensor Array. Small 2020, 16, 1906436. [Google Scholar] [CrossRef]
- Eylem, C.C.; Taştekin, M.; Kenar, A. Simultaneous Determination of Copper and Zinc in Brass Samples by PCR and PLS1 Methods Using a Multiple Ion-Selective Electrode Array. Talanta 2018, 183, 184–191. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X.; Wang, P. Rapid and Wide-Range Determination of Cd(II), Pb(II), Cu(II) and Hg(II) in Fish Tissues Using Light Addressable Potentiometric Sensor. Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Connolly, J.; Khlystov, A.; Fair, R.B. Digital Microfluidics for the Detection of Selected Inorganic Ions in Aerosols. Sensors 2020, 20, 1281. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Moltedo, O.; Verde, C.; Capasso, A.; Parisi, E.; Remondelli, P.; Bonatti, S.; Alvarez-Hernandez, X.; Glass, J.; Alvino, C.G.; Leone, A. Zinc Transport and Metallothionein Secretion in the Intestinal Human Cell Line Caco-2. J. Biol. Chem. 2000, 275, 31819–31825. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc Supplementation Modifies Tight Junctions and Alters Barrier Function of CACO-2 Human Intestinal Epithelial Layers. Dig. Dis. Sci. 2013, 58, 77–87. [Google Scholar] [CrossRef]
- Ferruzza, S.; Sambuy, Y.; Ciriolo, M.R.; De Martino, A.; Santaroni, P.; Rotilio, G.; Scarino, M.L. Copper Uptake and Intracellular Distribution in the Human Intestinal Caco-2 Cell Line. BioMetals 2000, 13, 179–185. [Google Scholar] [CrossRef]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, s3, 1–18. [Google Scholar] [CrossRef]
- Reznik, N.; Gallo, A.D.; Rush, K.W.; Javitt, G.; Fridmann-Sirkis, Y.; Ilani, T.; Nairner, N.A.; Fishilevich, S.; Gokhman, D.; Chacón, K.N.; et al. Intestinal Mucin Is a Chaperone of Multivalent Copper. Cell 2022, 185, 4206–4215.e11. [Google Scholar] [CrossRef]
- You, R.; Li, P.; Jing, G.; Cui, T. Ultrasensitive Micro Ion Selective Sensor Arrays for Multiplex Heavy Metal Ions Detection. Microsyst. Technol. 2019, 25, 845–849. [Google Scholar] [CrossRef]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Barón-Jaimez, J.; Joya, M.R.; Barba-Ortega, J. Anodic Stripping Voltammetry—ASV for Determination of Heavy Metals. J. Phys. Conf. Ser. 2013, 466, 012023. [Google Scholar] [CrossRef]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the Practicalities of Anodic Stripping Voltammetry for Heavy Metal Detection: A Tutorial Review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Guzinski, M.; Pendley, B.; Chaum, E. Plasticized PVC Membrane Modified Electrodes: Voltammetry of Highly Hydrophobic Compounds. Membranes 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Zdrachek, E.; Bakker, E. From Molecular and Emulsified Ion Sensors to Membrane Electrodes: Molecular and Mechanistic Sensor Design. Acc. Chem. Res. 2019, 52, 1400–1408. [Google Scholar] [CrossRef]
- Bodor, S.; Zook, J.M.; Lindner, E.; Tóth, K.; Gyurcsányi, R.E. Electrochemical Methods for the Determination of the Diffusion Coefficient of Ionophores and Ionophore-Ion Complexes in Plasticized PVC Membranes. Analyst 2008, 133, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Jing, G.; Cui, T. Trace Determination of Arsenite with an Ionophore-Coated Selective Micro Sensor. IEEE Sens. J. 2018, 18, 4364–4371. [Google Scholar] [CrossRef]
- Park, S.; Maier, C.S.; Koley, D. Anodic Stripping Voltammetry on a Carbon-Based Ion-Selective Electrode. Electrochim. Acta 2021, 390, 138855. [Google Scholar] [CrossRef]
- Kabagambe, B.; Garada, M.B.; Ishimatsu, R.; Amemiya, S. Subnanomolar Detection Limit of Stripping Voltammetric Ca2+-Selective Electrode: Effects of Analyte Charge and Sample Contamination. Anal. Chem. 2014, 86, 7939–7946. [Google Scholar] [CrossRef]
- Marziano, M.; Tonello, S.; Cantù, E.; Abate, G.; Vezzoli, M.; Rungratanawanich, W.; Serpelloni, M.; Lopomo, N.F.; Memo, M.; Sardini, E.; et al. Monitoring Caco-2 to Enterocyte-like Cells Differentiation by Means of Electric Impedance Analysis on Printed Sensors. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 893–902. [Google Scholar] [CrossRef]
- Tan, H.Y.; Trier, S.; Rahbek, U.L.; Dufva, M.; Kutter, J.P.; Andresen, T.L. A Multi-Chamber Microfluidic Intestinal Barrier Model Using Caco-2 Cells for Drug Transport Studies. PLoS ONE 2018, 13, e0197101. [Google Scholar] [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicol. Vitr. 2012, 26, 1243–1246. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; ISBN 9783319161044. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Ramadan, Q.; Jing, L. Characterization of Tight Junction Disruption and Immune Response Modulation in a Miniaturized Caco-2/U937 Coculture-Based in Vitro Model of the Human Intestinal Barrier. Biomed. Microdevices 2016, 18, 11. [Google Scholar] [CrossRef]
- Gerasimenko, T.; Nikulin, S.; Zakharova, G.; Poloznikov, A.; Petrov, V.; Baranova, A.; Tonevitsky, A. Impedance Spectroscopy as a Tool for Monitoring Performance in 3D Models of Epithelial Tissues. Front. Bioeng. Biotechnol. 2020, 7, 474. [Google Scholar] [CrossRef]
- Kulthong, K.; Duivenvoorde, L.; Mizera, B.Z.; Rijkers, D.; Ten Dam, G.; Oegema, G.; Puzyn, T.; Bouwmeester, H.; Van Der Zande, M. Implementation of a Dynamic Intestinal Gut-on-a-Chip Barrier Model for Transport Studies of Lipophilic Dioxin Congeners. RSC Adv. 2018, 8, 32440–32453. [Google Scholar] [CrossRef]
- Santbergen, M.J.C.; Van Der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W.F. Dynamic in Vitro Intestinal Barrier Model Coupled to Chip-Based Liquid Chromatography Mass Spectrometry for Oral Bioavailability Studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122. [Google Scholar] [CrossRef]
- Orchardt, R.O.T.B. Paracellular Diffusion in Caco-2 Cell Monolayers: Effect of Perturbation on the Transport of Hydrophilic Compounds That Vary in Charge and Size. J. Pharm. Sci. 1997, 86, 1105–1110. [Google Scholar]
- Kamata, S. Zinc-Selective Membrane Electrode Using Tetrabutyl Thiuram Disulfide Neutral Carrier. Anal. Sci. 1994, 10, 409–412. [Google Scholar] [CrossRef]
- Selvakumaran, J.; Hughes, M.P.; Keddie, J.L.; Ewins, D.J. Assessing Biocompatibility of Materials for Implantable Microelectrodes Using Cytotoxicity and Protein Adsorption Studies. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Madison, WI, USA, 2–4 May 2002; pp. 261–264. [Google Scholar] [CrossRef]
- Vázquez, M.; Devesa, V.; Vélez, D. Toxicology in Vitro Characterization of the Intestinal Absorption of Inorganic Mercury in Caco-2 Cells. Toxicol. In Vitro 2015, 29, 93–102. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Linz, G.; Djeljadini, S.; Steinbeck, L.; Köse, G.; Kiessling, F.; Wessling, M. Cell Barrier Characterization in Transwell Inserts by Electrical Impedance Spectroscopy. Biosens. Bioelectron. 2020, 165, 112345. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, S.V.; Gerasimenko, T.N.; Shilin, S.A.; Zakharova, G.S.; Gazizov, I.N.; Poloznikov, A.A.; Sakharov, D.A. 512 Application of Impedance Spectroscopy for the Control of the Integrity of In Vitro Models of Barrier Tissues. Eksp. Biol. Meditsiny 2018, 166, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Erlij, D.; De Smet, P.; Van Driessche, W. Effect of Insulin on Area and Na+ Channel Density of Apical Membrane of Cultured Toad Kidney Cells. J. Physiol. 1994, 481, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.; Jimison, L.H.; Hama, A.; Bongo, M.; Owens, R.M. Sensing of EGTA Mediated Barrier Tissue Disruption with an Organic Transistor. Biosensors 2013, 3, 44–57. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciurti, E.; Blasi, L.; Prontera, C.T.; Barca, A.; Giampetruzzi, L.; Verri, T.; Siciliano, P.A.; Francioso, L. TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model. Micromachines 2023, 14, 496. https://doi.org/10.3390/mi14030496
Sciurti E, Blasi L, Prontera CT, Barca A, Giampetruzzi L, Verri T, Siciliano PA, Francioso L. TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model. Micromachines. 2023; 14(3):496. https://doi.org/10.3390/mi14030496
Chicago/Turabian StyleSciurti, Elisa, Laura Blasi, Carmela Tania Prontera, Amilcare Barca, Lucia Giampetruzzi, Tiziano Verri, Pietro Aleardo Siciliano, and Luca Francioso. 2023. "TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model" Micromachines 14, no. 3: 496. https://doi.org/10.3390/mi14030496
APA StyleSciurti, E., Blasi, L., Prontera, C. T., Barca, A., Giampetruzzi, L., Verri, T., Siciliano, P. A., & Francioso, L. (2023). TEER and Ion Selective Transwell-Integrated Sensors System for Caco-2 Cell Model. Micromachines, 14(3), 496. https://doi.org/10.3390/mi14030496









