Research Progress on Bonding Wire for Microelectronic Packaging
Abstract
1. Introduction
2. Au Bonding Wire
2.1. Au Wire
2.2. Au Alloy Wire
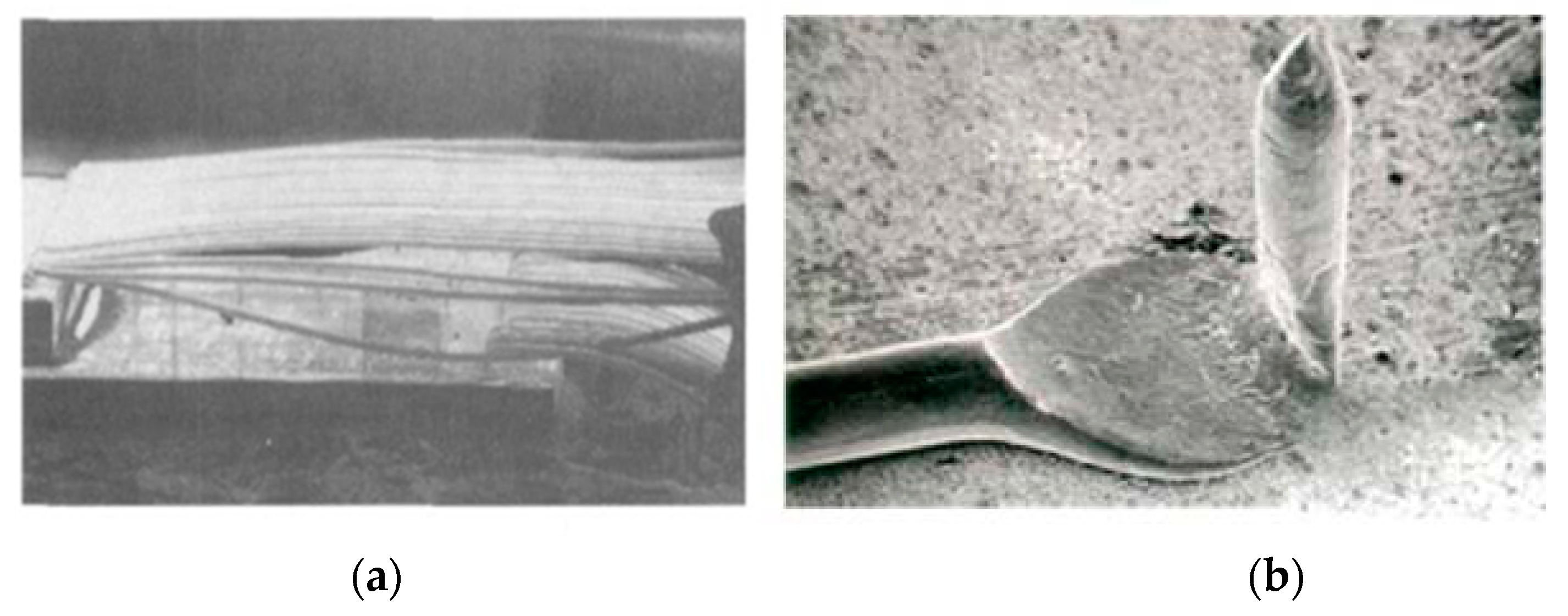
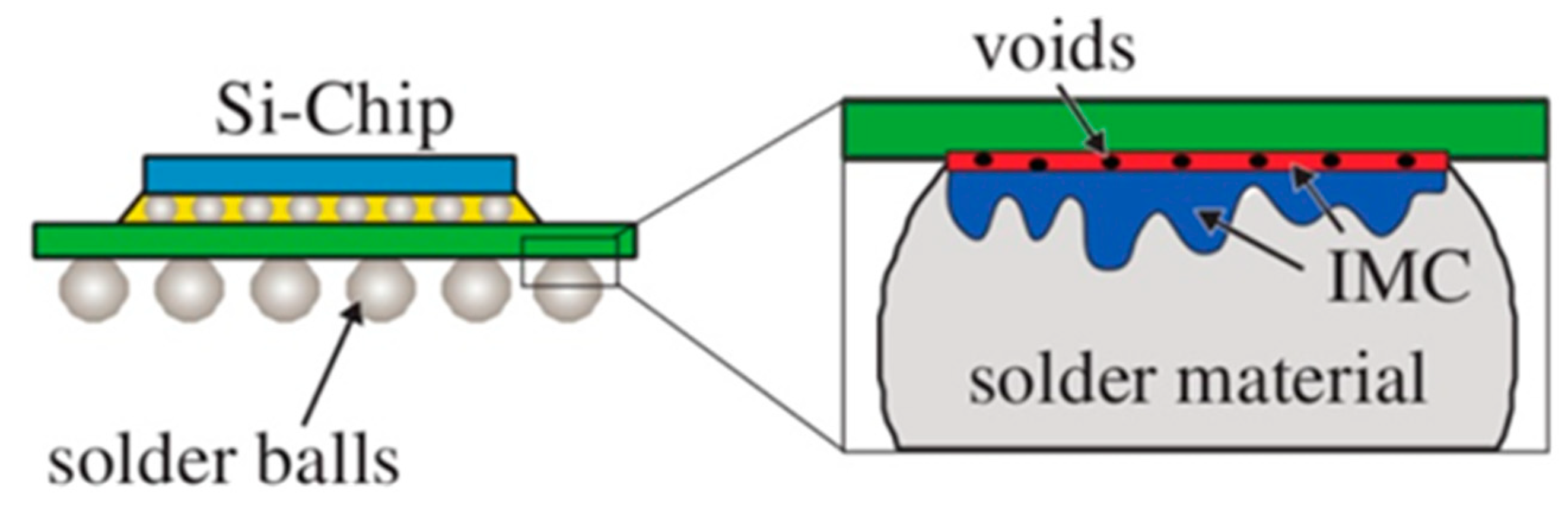
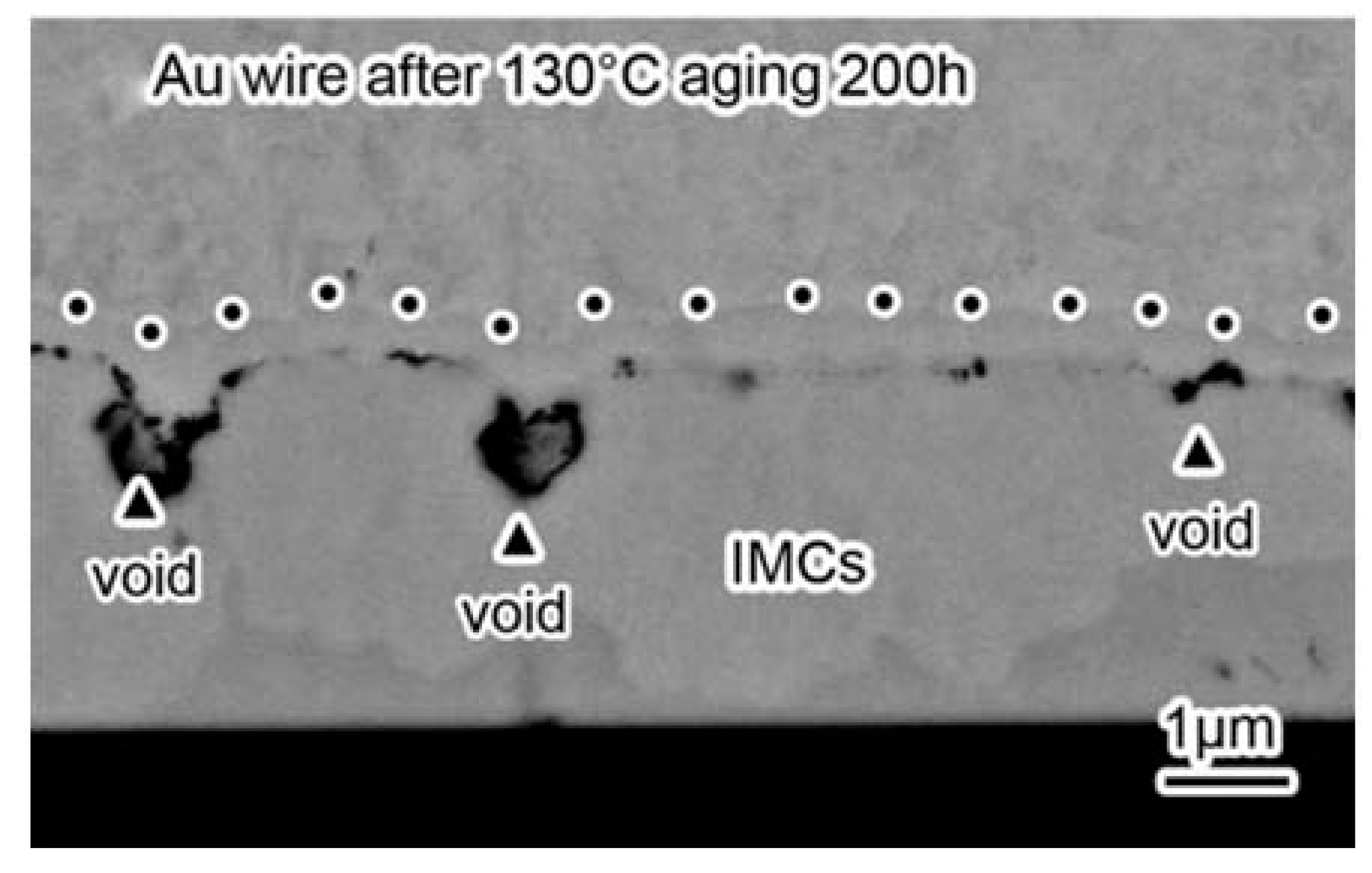
2.3. Reliability of Au Bonding Wire
3. Cu Bonding Wire
3.1. Bare Cu Wire
3.2. Cu Alloy Wire
3.3. Coated Cu Wire
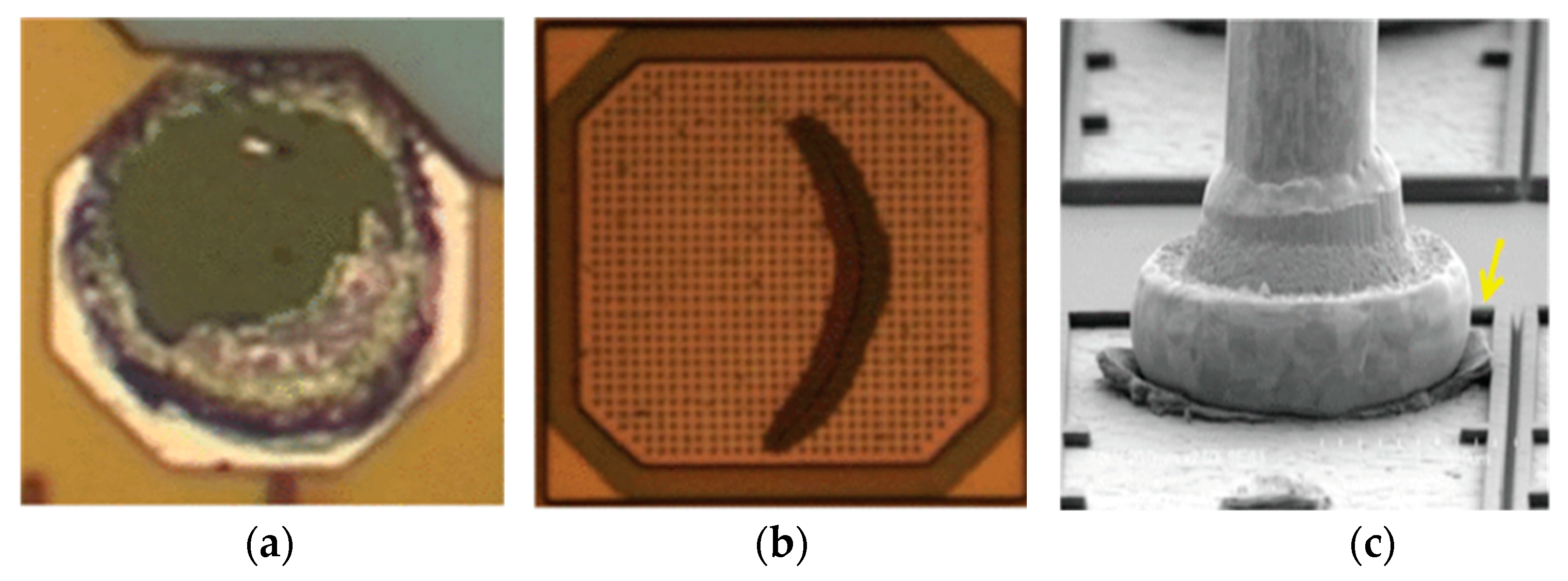
3.4. Reliability of Cu Bonding Wire
4. Ag Bonding Wire
4.1. Coated Ag Wire
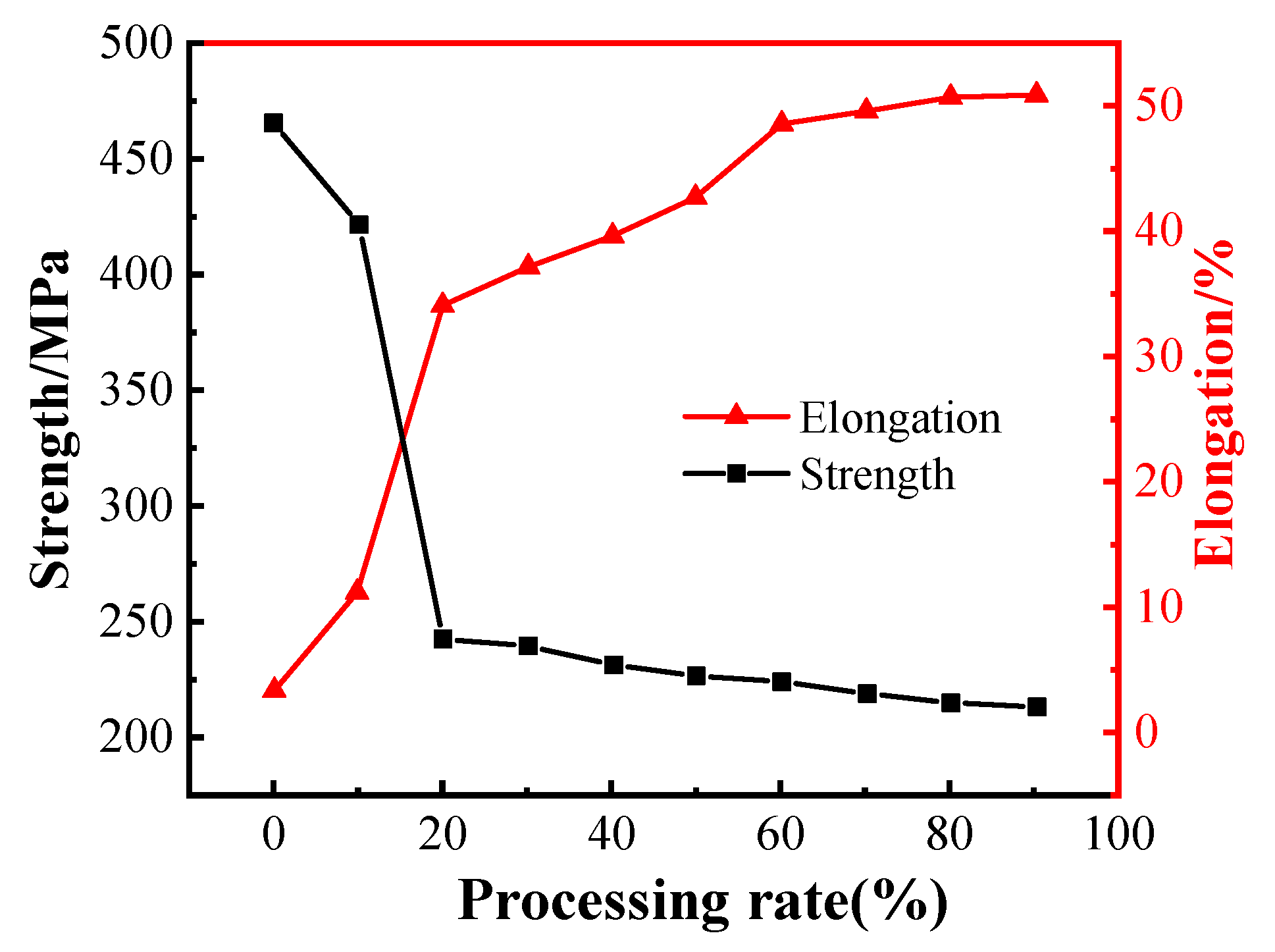
4.2. Ag Alloy Wire
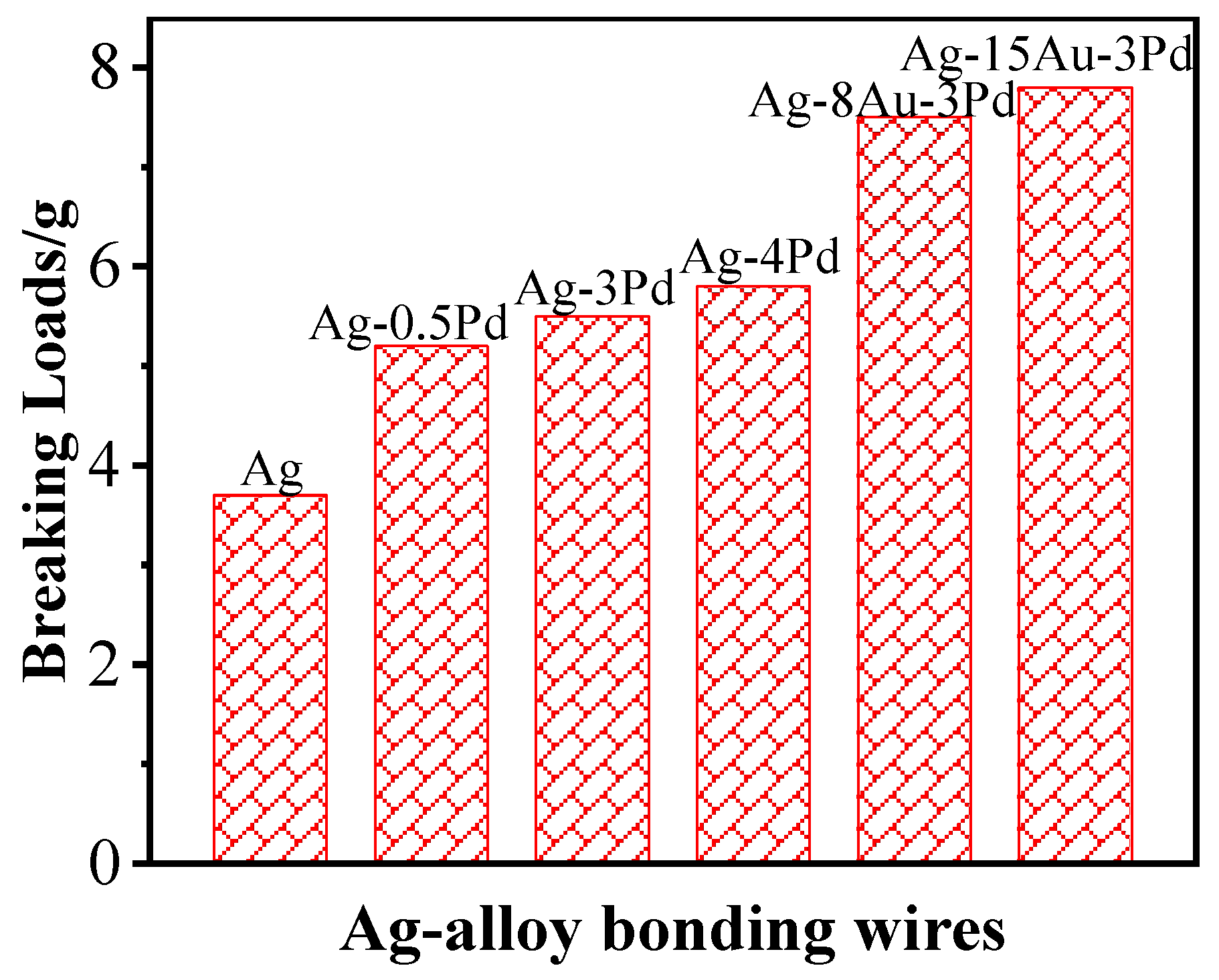
4.3. Other Ag Alloy Wires
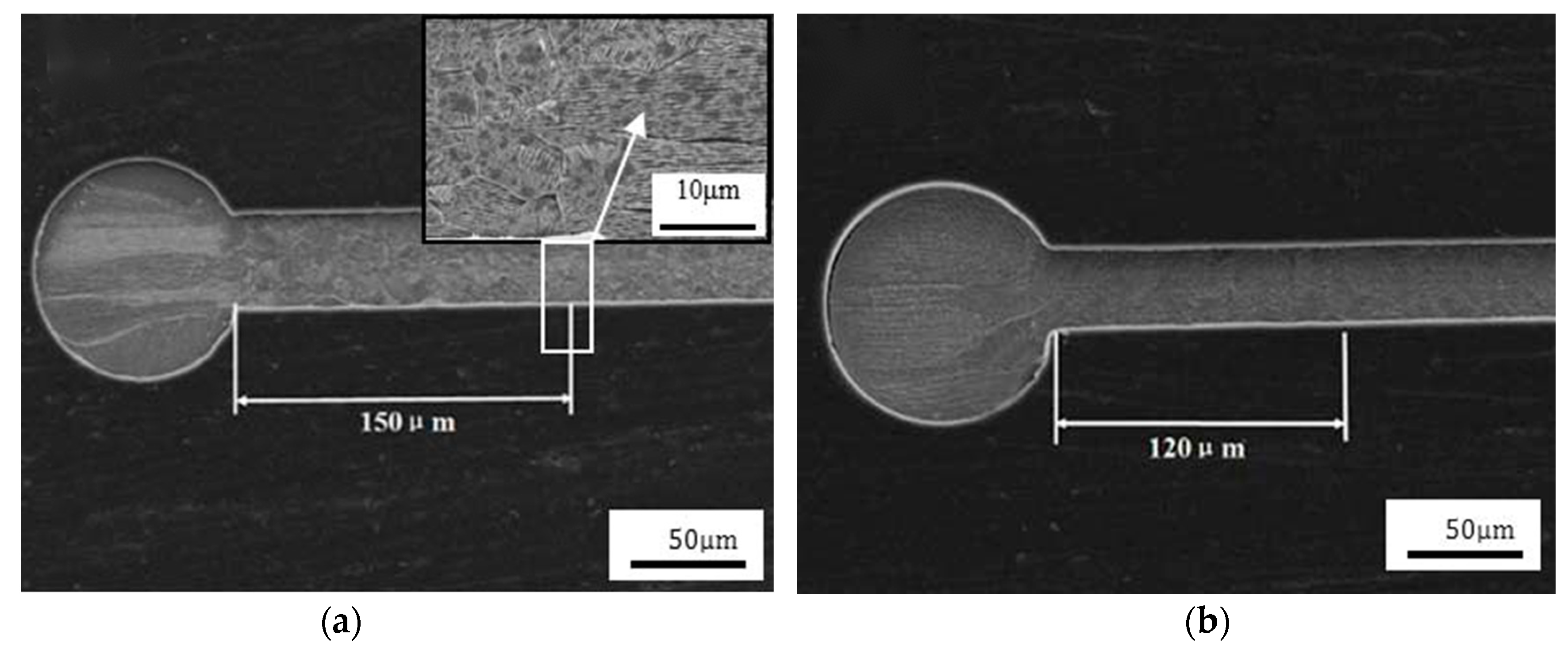
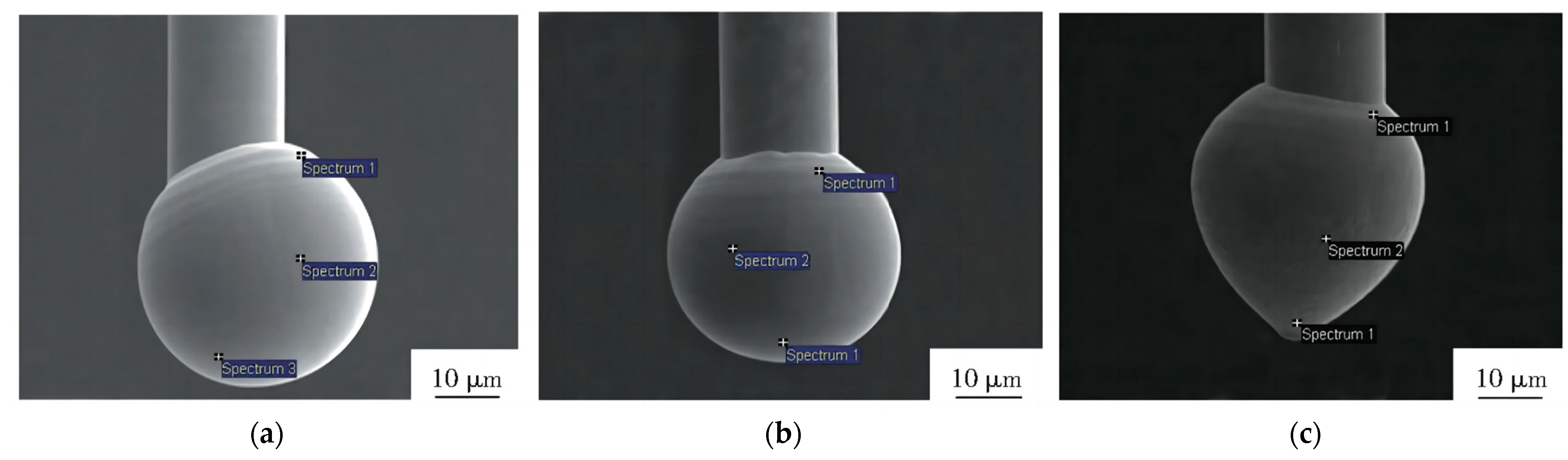
4.4. The Reliability of Ag Bonding Wire
5. Comparisons
6. Summaries
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alim, M.A.; Abdullah, M.Z.; Aziz, M.S.A.; Kamarudin, R. Die attachment, wire bonding, and encapsulation process in LED packaging: A review. Sens. Actuators A Phys. 2021, 329, 112817. [Google Scholar] [CrossRef]
- Hamid, K.A.; Badarisman, A.H.; Jalar, A.; Bakar, M.A. Investigation of integrated factors in the occurrence of copper wire bonding corrosion of semiconductor packages. J. Phys. Conf. Ser. 2022, 2169, 12016. [Google Scholar] [CrossRef]
- Su, L.; Yu, X.N.; Li, K.; Pecht, M. Defect inspection of flip chip solder joints based on non-destructive methods: A review. Microelectron. Reliab. 2020, 110, 113657. [Google Scholar] [CrossRef]
- Ershova, N.Y.; Reginya, S.A.; Lunkov, P.V.; Kirienko, D.A. Multi-chip assemblies combining wire bond and flip-chip package technologies. IOP Conf. Ser. Mater. Sci. Eng. 2020, 862, 52059. [Google Scholar] [CrossRef]
- Liu, D.S.; Chen, C.Y.; Chao, Y.C. A Thermomechanical Study of the Electrical Resistance of Cu Lead Interconnections. J. Electron. Mater. 2006, 35, 958–965. [Google Scholar] [CrossRef]
- Jaafar, N.B.; Choong, C.S. Establishment of Highly Dense Wire Bonding with Insulated Au Wire. In Proceedings of the 2022 IEEE 24th Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2022. [Google Scholar]
- Gomes, J.; Mayer, M.; Lin, B. Development of a fast method for optimization of Au ball bond process. Microelectron. Reliab. 2015, 55, 602–607. [Google Scholar] [CrossRef]
- Yu, C.M.; Lai, K.K.; Chen, K.S.; Chang, T.C. Process-Quality Evaluation for Wire Bonding with Multiple Gold Wires. IEEE Access 2020, 8, 106075–106082. [Google Scholar] [CrossRef]
- Kumar, G.I.A.; Lambert, A.; Caperton, J.; Asokan, M.; Yi, W.; Chyan, O. Comparative Study of Chloride and Fluoride Induced Aluminum Pad Corrosion in Wire-Bonded Device Packaging Assembly. Corros. Mater. Degrad. 2021, 2, 447–460. [Google Scholar] [CrossRef]
- Ding, K.W. Research on Bonding Reliability of Semiconductor Gold Wire. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2014. [Google Scholar]
- Schneider-Ramelow, M.; Ehrhardt, C. The reliability of wire bonding using Ag and Al. Microelectron. Reliab. 2016, 63, 336–341. [Google Scholar] [CrossRef]
- Chuang, T.H.; Lin, H.J.; Chuang, C.H.; Shiue, Y.Y.; Shieu, F.S.; Huang, Y.L.; Hsu, P.C.; Lee, J.D.; Tsai, H.H. Thermal stability of grain structure and material properties in an annealing twinned Ag–4Pd alloy wire. J. Alloys Compd. 2014, 615, 891–898. [Google Scholar] [CrossRef]
- Gu, B.K.; Shen, S.N.; Li, H. Mechanism of microweld formation and breakage during Cu–Cu wire bonding investigated by molecular dynamics simulation. Chin. Phys. B 2022, 31, 016101. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.N.; Li, Y.B.; Yuan, C.; Chen, Y.J. Research progress of fine copper wire bonding technology. In Proceedings of the 14th National Special Processing Academic Conference, Suzhou, China, 22–25 October 2011. [Google Scholar]
- He, X.K.; Guo, L.B.; Gong, G.S.; Su, F.L.; Zhu, D.C. Effects of different inhibitor on antioxidation of copper bonding wire at room temperature. J. Mater. Sci. Mater. Electron. 2022, 33, 10561–10571. [Google Scholar] [CrossRef]
- Motoki, E.; Noritoshi, A.; Takashi, Y.; Masaaki, S.; Shinji, F. Microstructural characterization of alloyed palladium coated copper wire under high temperature. Microelectron. Reliab. 2021, 120, 114125. [Google Scholar]
- Manoharan, S.; Patel, C.; Mccluskey, P. Advancements in Silver Wire Bonding. In Proceedings of the ASME 2017 International Technical Conference and Exhibition on Packaging and Integration of Electronic and Photonic Microsystems, San Francisco, CA, USA, 29 August 2017. [Google Scholar]
- Zhao, J. Discussion the characteristics of coating Pd copper wire bonding. Electron. Packag. 2012, 12, 36–41. [Google Scholar]
- Li, X.W.; Cai, J.L.; Chen, L.; Lin, J.T. Analysis and comparison of performance of bonding wire in LED packaging. China Light Light. 2019, 49, 18–22. [Google Scholar]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H. Microstructure, electric flame-off characteristics and tensile properties of silver bonding wires. Microelectron. Reliab. 2011, 51, 2243–2249. [Google Scholar] [CrossRef]
- Zhong, M.J.; Huang, F.X.; Yuan, H.G. Research progress on the copper and silver bonding wire materials. Mater. Rep. 2017, 31, 99–102. [Google Scholar]
- Xie, Q.; Long, K.; Lu, D.N.; Li, D.W.; Zhang, Y.; Wang, J. Integrated Circuit Gold Wire Bonding Measurement via 3-D Point Cloud Deep Learning. IEEE Trans. Ind. Electron. 2022, 69, 11807–11815. [Google Scholar] [CrossRef]
- Cao, J.; Wu, W.X. Research and Development Prospect of Ag Alloy Bonding Wire in Microelectronic Packaging. Precious Met. 2017, 38, 7–11. [Google Scholar]
- Papadopoulos, C.; Villiger, T.; Steiner, S.; Prinz, J.; Morabito, C.; Cammarata, M.; Rodriguez, A.; Kwon, J.; Geilenkeuser, R.; Breuer, D.; et al. Gold wire bond study for automotive application. Microelectron. Reliab. 2020, 114, 113899. [Google Scholar] [CrossRef]
- Xie, S.; Lin, P.R.; Yao, Q.B. Interface mechanical behavior of gold alloy wire bonding. J. Phys. Conf. Ser. 2021, 1907, 12021. [Google Scholar] [CrossRef]
- März, B.; Graff, A.; Klengel, R.; Petzold, M. Interface microstructure effects in Au thermosonic ball bonding contacts by high reliability wire materials. Microelectron. Reliab. 2014, 54, 2000–2005. [Google Scholar] [CrossRef]
- Humpston, G.; Jacobson, D.M. A new high strength gold bond wire. Gold Bull. 1992, 25, 132–145. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhao, J.Y.; Xu, B.Q.; Kong, L.X.; Jiang, W.L.; Yang, B. Theoretical research on vacuum separation of Au-Ag alloy. T. Nonferr. Metal. Soc. 2020, 32, 2719–2726. [Google Scholar] [CrossRef]
- Zhu, J.G. Trend of Alloying Investigation for Gold Bonding Wires. Rare Met. 2002, 23, 57–61. [Google Scholar]
- Ning, Y.T. Properties and applications of some gold alloys modified by rare earth additions. Gold Bull. 2005, 38, 3–8. [Google Scholar] [CrossRef]
- Simons, C.; Schräpler, L.; Herklotz, G. Doped and low-alloyed gold bonding wires. Gold Bull. 2000, 33, 89–96. [Google Scholar] [CrossRef]
- Hong, H.L.; Yang, C.H.; Wang, K.C.; Gao, H.; Yan, H.X. Composition analysis of AU–AG and AU–CU solid solution alloys by Friedel’s periodic spherical oscillation model. Mod. Phys. Lett. B 2021, 35, 2141015. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, T.W.; Jeong, E.K.; Kim, W.Y.; Lim, S.H. Effects of alloying elements on microstructure and thermal aging properties of Au bonding wire. Microelectron. Reliab. 2011, 51, 2250–2256. [Google Scholar] [CrossRef]
- Lin, L.; Zang, X.D. Introduction of a Corrosion-resistant High-reliability Silver AlloyBonding Wire in Package. Electron. Packag. 2014, 14, 9–13. [Google Scholar]
- Tang, R.; Yang, X.L.; Wu, B.A.; Tang, H.Y.; Zhang, C. Analysis and prospect on alloy system and compositions of gold–silver alloy bonding wire from the perspective of patent citations. Electron. Compon. Mater. 2019, 38, 8–13. [Google Scholar]
- Goh, C.; Chong, W.; Lee, T.; Breach, C. Corrosion Study and Intermetallics Formation in Gold and Copper Wire Bonding in Microelectronics Packaging. Crystals 2013, 3, 391–404. [Google Scholar] [CrossRef]
- Liu, P.; Cong, S.; Tan, X.W.; Lin, X.; Wu, P. The reliability assessment of Au–Al bonds using parallel gap resistance microwelding. J. Mater. Sci. Mater. Electron. 2020, 31, 6313–6320. [Google Scholar] [CrossRef]
- Noolu, N.J.; Murdeshwar, N.M.; Ely, K.J.; Lippold, J.C.; Baeslack, W.A. Degradation and failure mechanisms in thermally exposed Au–Al ball bonds. J. Mater. Res. 2004, 19, 1374–1386. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, M.; Paik, K.; Moon, J.; Song, J. Investigation of interfacial phenomena of alloyed Au wire bonding. In Proceedings of the 2013 IEEE 15th Electronics Packaging Technology Conference (EPTC 2013), Singapore, 1 December 2013. [Google Scholar]
- Gam, S.A.; Kim, H.J.; Cho, J.S.; Park, Y.J.; Moon, J.T.; Paik, K.W. Effects of Cu and Pd addition on Au bonding wire/Al pad interfacial reactions and bond reliability. J. Electron. Mater. 2006, 35, 2048–2055. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, B.Y.; Chen, X.P.; Zhang, X.H.; Zhu, X.K.; Du, H.S. Computational simulation of voids formation and evolution in Kirkendall effect. Physical A 2020, 554, 124285. [Google Scholar] [CrossRef]
- Schneider-Ramelow, M.; Schmitz, S.; Schuch, B.; Grübl, W. Kirkendall voiding in au ball bond interconnects on al chip metallization in the temperature range from 100–200 °C after optimized intermetallic coverage. In Proceedings of the 2009 European Microelectronics and Packaging Conference (EMPC), Rimini, Italy, 15–18 June 2009. [Google Scholar]
- Ahmad, S.S.; Smith, S.C. Au/Al Wire Bond Interface Resistance Degradation Rate Simulations. IEEE Trans. Device Mater. Reliab. 2019, 19, 774–781. [Google Scholar] [CrossRef]
- Du, Y.H.; Gao, L.Y.; Yu, D.Q.; Liu, Z.Q. Comparison and mechanism of electromigration reliability between Cu wire and Au wire bonding in molding state. J. Mater. Sci. Mater. Electron. 2020, 31, 2967–2975. [Google Scholar] [CrossRef]
- Murali, S.; Srikanth, N.; Vath, C.J. Effect of wire size on the formation of intermetallics and Kirkendall voids on thermal aging of thermosonic wire bonds. Mater. Lett. 2004, 58, 3096–3101. [Google Scholar] [CrossRef]
- Tang, J.Y.; Li, L.T.; Zhang, G.Q.; Zhang, J.; Liu, P. Finite element modeling and analysis of ultrasonic bonding process of thick aluminum wires for power electronic packaging. Microelectron. Reliab. 2022, 139, 114859. [Google Scholar] [CrossRef]
- Pan, Y.B.; Zhu, F.L.; Lin, X.X.; Fan, J.J.; Liu, S. Comparison of ultrasonic wire bonding process between gold and copper by nonlinear structure analysis. J. Adhes. Sci. Technol. 2018, 32, 2007–2018. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, S.Q.; Long, J.Y.; Hou, M.X.; Chen, X.; Gao, J.; He, Y.B.; Wong, C.P. Rationally Designing the Trace of Wire Bonder Head for Large-Span-Ratio Wire Bonding in 3D Stacked Packaging. IEEE Access 2020, 8, 206571–206580. [Google Scholar] [CrossRef]
- Huang, W.J.; Samanta, A.; Chen, Y.; Baek, S.; Shaw, S.K.; Ding, H.T. Machine learning model for understanding laser superhydrophobic surface functionalization. J. Manuf. Process. 2021, 69, 491–502. [Google Scholar] [CrossRef]
- Nazir, Q.; Shao, C.H. Online tool condition monitoring for ultrasonic metal welding via sensor fusion and machine learning. J. Manuf. Process. 2021, 62, 806–816. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.R.; Moghaddam, N.S. Process modeling in laser powder bed fusion towards defect detection and quality control via machine learning: The state-of-the-art and research challenges. J. Manuf. Process. 2022, 73, 961–984. [Google Scholar] [CrossRef]
- Hou, M.X.; Ou, Z.P.; Long, J.Y.; Ding, S.Q.; Wen, G.H.; Chen, Y.; Chen, X. Machine learning enables accurate wire loop profile prediction for advanced microelectronics packaging. J. Manuf. Process. 2022, 84, 394–402. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.C.; Wu, B.A.; Tang, H.Y.; Lv, C.C.; Song, K.X.; Yang, G.N.; Cui, C.Q.; Gao, Y.G. Study on Manufacturing Technology of Ag-8.5Au-3.5Pd Fine Alloy Wire. Micromachines 2021, 12, 938. [Google Scholar] [CrossRef]
- Ding, U.T.; Cao, J.; Xu, G.J.; Kou, S.Z.; Hu, R. Research and Application of Copper Bonding Wire in Electronic Packaging. Foundry Technol. 2006, 27, 971–974. [Google Scholar]
- Yuan, F.S. Current Situation and Development Trend of Bonding Wire Market. In Proceedings of the 2021 China Copper Processing Industry Annual Conference and China (Sanmenxia) Copper Industry High Quality Development Conference, Sanmenxia, China, 10 June 2021. [Google Scholar]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z.; Acoff, V.L. Intermetallic phase transformations in Au–Al wire bonds. Intermetallics 2011, 19, 1808–1816. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, Z.; Jiang, Y.B.; Lei, Q.; Xie, J.X. Composition design, phase transition and fabrication of copper alloys with high strength and electrical conductivity. Chin. J. Nonferrous Met. 2019, 29, 2009–2049. [Google Scholar]
- Song, K.X.; Zhou, Y.J.; Mi, X.J.; Xi, Z.; Cao, J.; Ding, Y.T.; Wu, B.A.; Feng, C.L.; Li, Z.; Chen, D.B.; et al. Preparation, microstructure and properties of copper based wire. Chin. J. Nonferrous Met. 2020, 30, 2845–2874. [Google Scholar]
- Hamid, K.A.; Badarisman, A.H.; Jalar, A.; Bakar, M.A. Effects of electrolyte towards copper wire metallurgical interconnection in semiconductor. J. Phys. Conf. Ser. 2022, 2169, 012013. [Google Scholar] [CrossRef]
- Lu, K.; Ren, C.L.; Gao, N.Y.; Ding, R.Z. The Process and Reliability Researches of Copper Wire Bonding. Electron. Packag. 2010, 10, 1–6. [Google Scholar]
- Gan, C.L.; Ng, E.K.; Chan, B.L.; Classe, F.C.; Kwuanjai, T.; Hashim, U. Wearout Reliability and Intermetallic Compound Diffusion Kinetics of Au and PdCu Wires Used in Nanoscale Device Packaging. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Zhong, Z.W. Wire bonding using copper wire. Microelectron. Int. 2009, 26, 10–16. [Google Scholar] [CrossRef]
- Kam, J.; Meng, H.; Calipito, D.; Dittmer, K. Materials characteristics of soft copper wires designed for advance application. In Proceedings of the SemiCon Singapore 2007, Singapore, 15 May 2007. [Google Scholar]
- Wulff, F.W.; Breach, C.D.; Stephan, D.; Dittmer, K.; Garnier, M. Further Characterisation of Intermetallic Growth in Copper and Gold Ball Bonds on Aluminium Metallisation. In Proceedings of the Proc SEMICON® Singapore 2005, Singapore, 6 May 2005. [Google Scholar]
- Kim, H.; Lee, J.Y.; Paik, K.; Koh, K.; Won, J.; Choe, S.; Lee, J.; Moon, J.; Park, Y. Effects of Cu/Al intermetallic compound (IMC) on copper wire and aluminum pad bondability. IEEE Trans. Compon. Packag. Technol. 2003, 26, 367–374. [Google Scholar]
- Uno, T. Bond reliability under humid environment for coated copper wire and bare copper wire. Microelectron. Reliab. 2010, 51, 148–156. [Google Scholar] [CrossRef]
- Kenji, T.; Kazuya, F.; Syozo, M.; Takamichi, M. Development of Copper Wire Bonding Application Technology. IEEE Trans. Comp. Hybrids Manufact. Technol. 1990, 13, 667–672. [Google Scholar]
- Murali, S.; Srikanth, N.; Vath, C.J. An analysis of intermetallics formation of gold and copper ball bonding on thermal aging. Mater. Res. Bull. 2003, 38, 637–646. [Google Scholar] [CrossRef]
- Yuan, D.W.; Zeng, H.; Xiao, X.P.; Wang, H.; Han, B.J.; Liu, B.X.; Yang, B. Effect of Mg addition on Fe phase morphology, distribution and aging kinetics of Cu-6.5 Fe alloy. Mater. Sci. Eng. A 2021, 812, 141064. [Google Scholar] [CrossRef]
- Goh, K.S.; Zhong, Z.W. A new bonding-tool solution to improve stitch bondability. Microelectron. Eng. 2007, 84, 173–179. [Google Scholar] [CrossRef]
- Breach, C.D.; Wulff, F.W. A brief review of selected aspects of the materials science of ball bonding. Microelectron. Reliab. 2010, 50, 1–20. [Google Scholar] [CrossRef]
- Shah, A.; Rockey, T.; Xu, H.; Qin, I.; Jie, W.; Yauw, O.; Chylak, B. Advanced wire bonding technology for Ag wire. In Proceedings of the 2015 17th Electronics Packaging Technology Conference (EPTC), Singapore, 2 December 2015. [Google Scholar]
- Wu, X.Q.; Ye, D.H.; Zhang, H.M.; Song, L.; Guo, L.P. Improvement of inter layer dielectric crack for LQFP C90FG wafer technology devices in copper wire bonding process. Microelectron. Int. 2022, 39, 14–21. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, L.G.; Ma, B.K.; Deng, L.H.; Han, L. Dynamics Features of Cu-Wire Bonding during Overhang Bonding Process. IEEE Electron. Device Lett. 2011, 32, 1731–1733. [Google Scholar]
- Chauhan, P.; Zhong, Z.W.; Pecht, M. Copper Wire Bonding Concerns and Best Practices. J. Electron. Mater. 2013, 42, 2415–2434. [Google Scholar] [CrossRef]
- Uno, T. Enhancing bondability with coated copper bonding wire. Microelectron. Reliab. 2011, 51, 88–96. [Google Scholar] [CrossRef]
- Zhang, G. Cu bonding wire technology for semiconductor packaging. New Technol. New Prod. China 2021, 29, 70–72. [Google Scholar]
- Cao, J.; Fan, J.L.; Li, Y.M.; Wu, X.F. Corrosion-Resistant Bonding Copper Wire and Preparation Method Thereof. China Patent CN104278169A, 7 December 2013. [Google Scholar]
- Fang, Y.B.; Zheng, Z.F.; Zheng, K.D. Bonded Copper Wire and Preparing Method Thereof. China Patent CN1949492A, 3 November 2006. [Google Scholar]
- Yang, F.; Zhang, X.D.; Fang, F. Microstructure and properties of cold-drawn Cu and Cu-Fe alloy wires. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1249, 012057. [Google Scholar] [CrossRef]
- Lv, C.K.; Lv, C.C. Copper linking wire and production method thereof. China Patent CN101487090A, 17 January 2008. [Google Scholar]
- Sato, M.; Suzuki, I.; Yamamori, K. Bonding Wire. Japan Patent JPH06168975A, 15 July 1993. [Google Scholar]
- Sengul, S.; Guder, V. Key factors of deformation mechanism of Cu-Ag alloy. J. Non-Cryst. Solids 2022, 576, 121270. [Google Scholar] [CrossRef]
- Zhu, X.F.; Xiao, Z.; An, J.H.; Jiang, H.Y.; Jiang, Y.B.; Li, Z. Microstructure and properties of Cu-Ag alloy prepared by continuously directional solidification. J. Alloys Compd. 2021, 883, 160769. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Xiao, Z.; Li, Z.; Qiu, W.T.; Jiang, H.Y.; Lei, Q.; Liu, Z.; Jiang, Y.B.; Zhang, S.J. Microstructure and properties of a Cu-Ni-Si-Co-Cr alloy with high strength and high conductivity. Mater. Sci. Eng. A. 2019, 759, 396–403. [Google Scholar] [CrossRef]
- Zhu, X.F.; Xiao, Z.; Song, K.X.; Sheng, X.Y.; Dai, J.; Jiang, H.B.; Zhou, S.Q. Microstructure and properties evolution of Cu-Ag alloy prepared by downward continuous casting. Chin. J. Nonferrous Met. 2021, 31, 1176–1187. [Google Scholar]
- Kong, L.B.; Zhou, Y.J.; Song, K.X.; Cao, J.; Lv, C.C.; Li, K.; Liu, Q.L.; Wu, B.A.; Tang, H.Y.; Zhang, X.B.; et al. Microstructure and Properties of Cu-Ag Alloy after Annealing at Different Temperatures. Mater. Mech. Eng. 2020, 44, 29–32. [Google Scholar]
- Zhao, H.M.; Fu, H.D.; Xie, M.; Xie, J.X. Effect of Ag content and drawing strain on microstructure and properties of directionally solidified Cu-Ag alloy. Vacuum 2018, 154, 190–199. [Google Scholar] [CrossRef]
- Xie, M.W.; Huang, W.; Chen, H.M.; Gong, L.K.; Xie, W.B.; Wang, H.; Yang, B. Microstructural evolution and strengthening mechanisms in cold-rolled Cu–Ag alloys. J. Alloys Compd. 2021, 851, 156893. [Google Scholar] [CrossRef]
- Chen, M.H.; Wu, Y.; Zhang, W.H. Analysis on Quality Problem and Preventive Measure of Copper Rod Drawing. Nonferrous Met. Process. 2011, 40, 31–34. [Google Scholar]
- Application of New Technologies in the Drawing of Coated Wire. Available online: https://www.wirecable.in/2016/02/application-of-new-technologies-in-the-drawing-of-coated-wire/ (accessed on 10 February 2016).
- Du, Y.H.; Liu, Z.Q.; Ji, H.J.; Li, M.Y.; Wen, M. The mechanism of Pd distribution in the process of FAB formation during Pd-coated Cu wire bonding. J. Mater. Sci. Mater. Electron. 2018, 29, 13774–13781. [Google Scholar] [CrossRef]
- Clauberg, H.; Chylak, B.; Wong, N.; Yeung, J.; Milke, E. Wire bonding with Pd-coated copper wire. In Proceedings of the IEEE 2010 IEEE CPMT Symposium Japan, Kyoto, Japan, 24 August 2010. [Google Scholar]
- Qin, W.T.; Anderson, T.; Chang, G. Mechanism to improve the reliability of copper wire bonding with palladium-coating of the wire. Microelectron. Reliab. 2019, 99, 239–244. [Google Scholar] [CrossRef]
- Singh, I.; Qin, I.; Xu, H.; Huynh, C.; Low, S.; Clauberg, H.; Chylak, B.; Acoff, V.L. Pd-coated Cu wire bonding technology: Chip design, process optimization, production qualification and reliability test for high reliability semiconductor devices. In Proceedings of the IEEE 2012 IEEE 62nd Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 29 May 2012. [Google Scholar]
- Chen, K.J.; Hung, F.Y.; Chang, C.Y. A Study of the Sulfidation Behavior on Palladium-Coated Copper Wire with a Flash-Gold Layer (PCA) after Wire Bonding. Electronics 2019, 8, 792. [Google Scholar] [CrossRef]
- Lau, K.T.; Cha, C.L. A Review of Palladium Coated Copper Wire Bonding for Automotive Device. Int. J. Mech. Prod. Eng. Res. Dev. 2020, 10, 4479–4492. [Google Scholar]
- Zheng, K.D.; Feng, X.L.; Li, C.L. Palladium-plated bonded copper wire and production method thereof. China Patent CN101707194A, 11 November 2009. [Google Scholar]
- Fang, Y.B. Surface Palladium-Plated Bonding Brass Wire. China Patent CN102130067B, 31 December 2010. [Google Scholar]
- Fan, J.L.; Cao, J.; Gao, W.B.; Liu, Z.Q. Effect of coating speed and annealing on properties of direct Pd-coated copper bonding wire. Heat Treat. Met. 2016, 41, 89–93. [Google Scholar]
- Liu, X.J.; Zhang, B.H.; Wang, D.J.; Cong, Y.Q.; Wang, J.Y. Properties of Cu-Al Intermetallic Compounds in Copper Wire Bonding. Semicond. Technol. 2011, 36, 880–884. [Google Scholar]
- Mokhtari, O.; Nishikawa, H. Effect of surface potential distribution on corrosion behavior of Cu/Al interface in Cu wire bonding applications. Microelectron. Reliab. 2020, 113, 113942. [Google Scholar] [CrossRef]
- Gan, C.L.; Hashim, U. Reliability Assessment and Activation Energy Study of Au and Pd-Coated Cu Wires Post High Temperature Aging in Nanoscale Semiconductor Packaging. J. Electron. Packag. 2013, 135, 210101–210107. [Google Scholar] [CrossRef]
- Xu, H.; Hang, C.J.; Wang, C.Q.; Tian, Y.H. Contrast Research on the Reliability of Gold and Copper Wire/Ball Bonding. Equip. Electron. Prod. Manuf. 2006, 5, 23–27. [Google Scholar]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z.; Acoff, V.L. Behavior of aluminum oxide, intermetallics and voids in Cu–Al wire bonds. Acta Mater. 2011, 59, 5661–5673. [Google Scholar] [CrossRef]
- Jin, O.; Koizumi, M.; Araki, I. Investigation of the Reliability of Copper Ball bonds to Aluminum Electrodes. IEEE Trans. Comp. Hybrids Manufact. Technol. 1987, 12, 550–555. [Google Scholar]
- Guo, Y.J.; Liu, G.W.; Jin, H.Y. Intermetallic phase formation in diffusion-bonded Cu/Al laminates. J. Mater. Sci. 2011, 46, 2467–2473. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z. A re-examination of the mechanism of thermosonic copper ball bonding on aluminium metallization pads. Scr. Mater. 2009, 61, 165–168. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hwang, W.S. Effect of Annealing on the Interfacial Structure of Aluminum-Copper Joints. Mater. Trans. 2007, 48, 1938–1947. [Google Scholar] [CrossRef]
- Wu, S.P.; Cai, X.L.; Zhou, L.; Yang, C.J.; Li, W.H.; Cheng, Y.C. Contribution of Mechanical Activation on the Growth of Intermetallic Compound Layers at the Cu/Al Interface during Vacuum Hot Pressing. Trans. Indian Inst. Met. 2022, 75, 2129–2137. [Google Scholar] [CrossRef]
- Lim, A.B.Y.; Boothroyd, C.B.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Interfacial evolution and bond reliability in thermosonic Pd coated Cu wire bonding on aluminum metallization: Effect of palladium distribution. Microelectron. Reliab. 2016, 4, 3. [Google Scholar] [CrossRef]
- Chen, H.; Ricky Lee, S.W.; Ding, Y.T. Evaluation of Bondability and Reliability of Single Crystal Copper Wire Bonding. In Proceedings of the IEEE 2005 Conference on High Density Microsystem Design and Packaging and Component Failure Analysis, Shanghai, China, 27 June 2005. [Google Scholar]
- Yuan, B.; Luo, Z.; Zhu, M.; Xu, Y.G.; Peng, S.Y. High-Reliability Copper Alloy Bonding Wire for Electronic Packaging and Preparation Method Therefor. U.S. Patent US20200373272A1, 26 November 2020. [Google Scholar]
- Murali, S.; Yeung, J.; Perez, R. Alloyed Copper Bonding Wire with Homogeneous Microstructure. In Proceedings of the IEEE 2012 35th IEEE/CPMT International Electronics Manufacturing Technology Conference (IEMT), Ipoh, Malaysia, 6–8 November 2012. [Google Scholar]
- Kaimori, S.; Nonaka, T.; Mizoguchi, A. The Development of Cu Bonding Wire with Oxidation-Resistant Metal Coating. IEEE Trans. Adv. Packag. 2006, 29, 227–231. [Google Scholar] [CrossRef]
- Tajedini, M.; Osmanson, A.T.; Kim, Y.R.; Madanipour, H.; Kim, C.U.; Glasscock, B.; Khan, M. Electromigration Effect on the Pd Coated Cu Wirebond. In Proceedings of the IEEE 71st Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 1 June–4 July 2021. [Google Scholar]
- Lim, A.B.Y.; Chang, A.C.K.; Lee, C.X.; Yauw, O.; Chylak, B.; Chen, Z. Palladium-coated and Bare Copper Wire Study for Ultra-Fine Pitch Wire Bonding. ECS Trans. Adv. 2013, 52, 717–730. [Google Scholar] [CrossRef]
- Qin, I.; Xu, H.; Milton, B.; Clauberg, H.; Chylak, B. Molded Reliability Study for Different Cu Wire Bonding Configurations. In Proceedings of the IEEE 2013 IEEE 63th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2013. [Google Scholar]
- Lim, A.; Neo, W.J.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Evaluation of the corrosion performance of Cu-Al intermetallic compounds and the effect of Pd addition. Microelectron. Reliab. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- Eto, M.; Araki, N.; Yamada, T.; Sugiyama, M.; Fujimoto, S. Influence of post-bonding heating process on the long-term reliability of Cu/Al contact. Microelectron. Reliab. 2021, 118, 114058. [Google Scholar] [CrossRef]
- Jeon, J.; Na, S.H.; Jeon, S.H.; Mo, M.; Kang, D.B.; Lim, K.; Kim, J.Y. High Reliability Challenges with Cu Wire Bonding for Automotive Devices in the AEC-Q006. In Proceedings of the IEEE 2017 IEEE 67th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2017. [Google Scholar]
- Eto, M.; Araki, N.; Yamada, T.; Klengel, R.; Klengel, S.; Petzold, M.; Sugiyama, M.; Fujimoto, S. Effects of alloying elements in high reliability copper wire bond material for high temperature applications. Microelectron. Reliab. 2020, 114, 113819. [Google Scholar] [CrossRef]
- Sun, X.T.; Chen, W.J.; Xiong, X.Y.; Chen, W.H.; Jin, Y. A Variable Configuration Force Sensor with Adjustable Resolution for Robotic Applications. IEEE Trans. Ind. Electron. 2023, 70, 2066–2075. [Google Scholar] [CrossRef]
- Hu, G.J. Comparison of copper, silver and gold wire bonding on interconnect metallization. In Proceedings of the IEEE 2012 13th International Conference on Electronic Packaging Technology & High Density Packaging (ICEPT-HDP), Guilin, China, 13–16 August 2012. [Google Scholar]
- Yang, G.N.; Zhou, Z.Q.; Zhang, H.D.; Zhang, Y.; Peng, Z.; Gong, P.; Wang, X.; Cui, C.Q. Improved Anti-Vulcanization and Bonding Performance of a Silver Alloy Bonding Wire by a Cathodic Passivation Treatment with Palladium. Materials 2022, 15, 2355. [Google Scholar] [CrossRef]
- Lu, C. Review on silver wire bonding. In Proceedings of the IEEE 2013 8th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taiwan, China, 22–25 December 2013. [Google Scholar]
- Ren, Z. Silver bonding wire for BSOB(Bond-Stitch-on-Ball)/BBOS(Bonding-Ball-on-Stitch). In Proceedings of the IEEE 2016 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 13–14 March 2016. [Google Scholar]
- Du, Y.H.; Liu, Z.Q.; Xiong, T.; Yuan, Z.B.; Leng, F.; Lv, H.L.; Yu, D.Q. Bond reliability under humid environment for Pd coated Cu and Ag alloy wire bonding. In Proceedings of the IEEE 2015 16th International Conference on Electronic Packaging Technology (ICEPT), Changsha, China, 11–14 August 2015. [Google Scholar]
- Tanna, S.; Pisigan, J.L.; Song, W.H.; Halmo, C.; Persic, J.; Mayer, M. Low cost Pd coated Ag bonding wire for high quality FAB in air. In Proceedings of the IEEE 2012 IEEE 62nd Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 29 May–1 June 2012. [Google Scholar]
- Xi, J.Q.; Mendoza, N.; Chen, K.; Yang, T.; Reyes, E.; Bezuk, S.; Lin, J.; Ke, S.G.; Chen, E. Evaluation of Ag Wire Reliability on Fine Pitch Wire Bonding. In Proceedings of the IEEE 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015. [Google Scholar]
- Chuang, T.H.; Lee, P.I.; Lin, Y.C. An Optimized Ag–5Pd–3.5Au Bonding Wire for the Resistance of Ag Ion Migration in LED Packages. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 1989–1995. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Hung, F.Y.; Lui, T.S. Microstructure, tensile and electrical properties of gold-coated silver bonding wire. Microelectron. Reliab. 2015, 55, 608–612. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hung, F.Y.; Lui, T.S.; Chen, K.J. Study of wire bonding reliability of Ag-Pd-Au alloy wire with flash-gold after chlorination and sulfidation. Microelectron. Reliab. 2019, 99, 183–196. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Hung, F.Y.; Lui, T.S. Microstructure, Mechanical and High-Temperature Electrical Properties of Cyanide-Free Au-Coated Ag Wire (ACA). Mater. Trans. 2015, 56, 441–444. [Google Scholar] [CrossRef]
- Kang, F.F.; Zhou, W.Y.; Wu, Y.J. Microstructure and Properties of Gold-Coated Silver Composite Bonding Wire for Microelectronic Package. Semicond. Technol. 2018, 43, 702–707. [Google Scholar]
- Liang, Z.; Xie, Y.H.; Yan, R.; Xiang, C.H.; Zhou, X.G.; Su, H.F. A Bonding Wire. China Patent CN105428335A, 23 March 2016. [Google Scholar]
- Gao, M.Y.; Xie, H.B.; Fang, Y.T.; Wang, H.T.; Liu, J.B. Progress in surface treatment techniques of copper and copper alloys. Chin. J. Nonferrous Met. 2021, 31, 1121–1133. [Google Scholar]
- Song, K.X.; Ding, Y.T.; Wu, B.A.; Hu, R.; Chen, D.B.; Lu, W.W.; Hu, H.; Zhou, Y.J. Controlling the wettability of Pd coating solution and its effect on the uniformity of coating layer. J. Funct. Mater. 2021, 52, 7060–7063. [Google Scholar]
- Fan, J.L.; Zhu, L.X.; Cao, J.; Hua, H. Effect of Au component in Ag alloy bonding wire on bonded strength and bonded reliability. Funct. Mater. 2019, 50, 10145–10148. [Google Scholar]
- Kuo, B.H.; Tsai, D.C.; Huang, Y.L.; Hsu, P.C.; Chuang, T.H.; Lee, J.D.; Tsai, H.H.; Shieu, F.S. Effect of alloying Au on the microstructural, mechanical and electrical properties of Ag-based alloy wires. J. Mater. Sci. Mater. Electron. 2019, 30, 9396–9409. [Google Scholar] [CrossRef]
- Rosalbino, F.; Delsante, S.; Borzone, G.; Scavino, G. Influence of noble metals alloying additions on the corrosion behaviour of tita-nium in a fluoride-containing environment. J. Mater. Sci. Mater. Med. 2012, 23, 1129–1137. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lee, J.D.; Tsai, C.H.; Wang, H.C.; Chang, C.C.; Chuang, T.H. An innovative annealing-twinned Ag-Au-Pd bonding wire for IC and LED packaging. In Proceedings of the IEEE 2012 7th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, China, 24–26 October 2012. [Google Scholar]
- Tsai, C.H.; Chuang, C.H.; Tsai, H.H.; Lee, J.D.; Chang, D.; Lin, H.J.; Chuang, T.H. Materials Characteristics of Ag-Alloy Wires and Their Applications in Advanced Packages. IEEE Trans. Compon. Packag. Manuf. Technol. 2016, 6, 298–305. [Google Scholar] [CrossRef]
- Cao, J.; Wu, W.X.; Zhang, C.; Hua, H.; Xu, X. Effects of heat treatment temperature on properties and microstructure of Ag-4Pd alloy bonding wire. Trans. Mater. Heat Treat. 2018, 39, 44–48. [Google Scholar]
- Cao, J.; Fan, J.L.; Gao, W.B. Effects of Drawing and Annealing on Properties of Ag-4Pd Alloy Bonding Wire. Chin. J. Mech. 2016, 52, 92–97. [Google Scholar] [CrossRef]
- Chuang, T.H.; Wang, H.C.; Chuang, C.H.; Lee, J.D.; Tsai, H.H. Effect of Annealing Twins on Electromigration in Ag-8Au-3Pd Bonding Wires. J. Electron. Mater. 2013, 42, 545–551. [Google Scholar] [CrossRef]
- Chuang, T.H.; Tsai, C.H.; Wang, H.C.; Chang, C.C.; Chuang, C.H.; Lee, J.D.; Tsai, H.H. Effects of Annealing Twins on the Grain Growth and Mechanical Properties of Ag-8Au-3Pd Bonding Wires. J. Electron. Mater. 2012, 41, 3215–3222. [Google Scholar] [CrossRef]
- Chuang, T.H.; Wang, H.C.; Tsai, C.H.; Chang, C.C.; Chuang, C.H.; Lee, J.D.; Tsai, H.H. Thermal stability of grain structure and material properties in an annealing-twinned Ag–8Au–3Pd alloy wire. Scr. Mater. 2012, 67, 605–608. [Google Scholar] [CrossRef]
- Luechinger, C.; Chen, R.; Fu, J.; Poncelet, B.; Valentin, O.; Walker, T.J.; Xu, T. Aluminum–copper ribbon interconnects for power devices. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 7, 1567–1577. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, Y.C.; Groth, A.; Lai, Y.C.; Lin, C.Y.; Chang, H.M.; Chuang, T.H. Ultrasonic Bonding of Ag and Ag-Alloy Ribbon—An Innovative Alternative for High Power IC Packages. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 1061–1068. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, Y.C.; Chuang, T.H. Grain growth and twin formation in a Ag-4Pd alloy ribbon after annealing treatments. J. Alloys Compd. 2021, 863, 158619. [Google Scholar] [CrossRef]
- Chen, C.H.; Chuang, T.H. Optimization of Ag-alloy ribbon bonding—An approach to reliable interconnection for high power IC packaging. Microelectron. Reliab. 2022, 137, 114786. [Google Scholar] [CrossRef]
- Hung, F.Y.; Lui, T.S.; Chen, L.H.; Lan, K.A. Microstructures and Fusing Electrical Current of Microelectronic Sn-9Zn-(0.25RE) Solders. Mater. Trans. 2008, 49, 1491–1495. [Google Scholar] [CrossRef]
- Hsueh, H.W.; Hung, F.Y.; Lui, T.S.; Chen, L.H.; Chang, J.K. Microstructure, electric flame-off (EFO) characteristics and tensile properties of silver–lanthanum alloy wire. Microelectron. Reliab. 2014, 54, 2564–2569. [Google Scholar] [CrossRef]
- Cao, J.; Fan, J.L.; Liu, Z.Q.; Zhang, Y.M. Effect of silver alloy bonding wire properties on bond strengths and reliability. In Proceedings of the ICEP-IAAC, Kyoto, Japan, 14 April 2015. [Google Scholar]
- Cao, J.; Wu, W.X. Effects of Au coated Ag bonding wire properties on bonded quality. Mater. Sci. Technol. 2018, 26, 30–35. [Google Scholar]
- Cao, J.; Zhang, J.C.; Persic, J.; Song, K.X. Effects of Bonding Parameters on Free Air Ball Properties and Bonded Strength of Ag-10Au-3.6Pd Alloy Bonding Wire. Micromachines 2020, 11, 777. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wu, Y.J.; Chen, J.L.; Yang, G.X.; Kong, J.W.; Kang, F.F. Effect of Electronic Flame off Parameters on the Bonding Using Silve Wire. Precious Met. 2017, 38, 34–39. [Google Scholar]
- Krumbein, S.J. Metallic Electromigration Phenomena. IEEE Trans. Compon. Hybrids Manuf. Technol. 1988, 11, 5–15. [Google Scholar] [CrossRef]
- Chuang, C.J.; Lin, Y.C.; He, Y.Z.; Tsai, C.H.; Lee, J.D.; Wang, A.C.; Tsai, H.H. Inhibition of Silver Electrolytic Migration in Ag-alloy Bonding Wires. Mater. Sci. Forum 2016, 863, 95–101. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lee, P.L.; Wu, P.C.; Chen, C.H.; Chuang, T.H. Effects of Grain Size on the Ag Dissolution and Ion Migration of Ag-4Pd Alloy Wires. J. Electron. Mater. 2021, 50, 5955–5964. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Tang, H.Y.; Zhang, H.H.; Sun, L.; Xie, L.; Wu, B.A.; Xie, W.D.; Cai, X.N.; Li, F. Effect of Au Coating on the Electrolytic Migration of Ag Bonding Wires for Electronic Packaging Applications. J. Phys. Conf. Ser. 2022, 2393, 012013. [Google Scholar] [CrossRef]
- Cho, J.S.; YOO, K.A.; Moon, J.T.; Son, S.B.; Lee, S.H.; Oh, K.H. Pd effect on reliability of Ag bonding wires in microelectronic devices in high-humidity environments. Met. Mater. Int. 2012, 18, 881–1885. [Google Scholar] [CrossRef]
- Cai, X.H.; Li, J.D.; Lin, X.R. Study on Electromigration Failure of High Performance Ag-4Pd Alloy Wire. In Proceedings of the 2014 Cross-Strait Destruction Science and Materials Testing Academic Conference and the 12th Destruction Science Symposium/The 10th National MTS Materials Testing Academic Conference, Taiwan, China, 22 October 2014. [Google Scholar]
- Guo, R.; Gao, L.M.; Li, M.; Mao, D.L.; Qian, K.Y.; Chiu, H. Microstructure evolution of Ag-8Au-3Pd alloy wire during electromigration. Mater. Charact. 2015, 110, 44–51. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, P.I.; Chuang, T.H. Microstructure evolution and failure mechanism of electromigration in Ag-alloy bonding wire. J. Alloys Compd. 2022, 913, 165266. [Google Scholar] [CrossRef]
- Peng, C.; Liang, S.; Huang, F.X.; Zhong, M.J.; Ran, X.J. Research Progress on Bonding Interface of Bonded Wire. Mater. Rep. 2019, 33, 501–504. [Google Scholar]
- Hsu, T.Y.; Chang, J.Y.; Leu, F.J.; Chang, H.M.; Ouyang, F.Y. Ag alloy wire bonding under electromigration test. In Proceedings of the ICEP-IAAC, Kyoto, Japan, 14 April 2015. [Google Scholar]
- Fu, S.W.; Lee, C.C. Solid-state reactions of silver and aluminum associated with silver wire bonds. In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference, Las Vegas, NV, USA, 31 May–3 June 2016. [Google Scholar]
- Ho, C.C.; Chen, K.J.; Hung, F.Y.; Lui, T.S. Study of electrical fatigue test in gold-coated siliver-4 wt.% palladium bonding wire. Microelectron. Reliab. 2019, 103, 113502. [Google Scholar] [CrossRef]
- Chuang, T.H.; Chang, C.C.; Chuang, C.H.; Lee, J.D.; Tsai, H.H. Formation and Growth of Intermetallics in an Annealing-Twinned Ag-8Au-3Pd Wire Bonding Package during Reliability Tests. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 3, 3–9. [Google Scholar] [CrossRef]
- Long, Y.Y.; Arndt, M.; Dencker, F.; Wurz, M.; Twiefel, J.; Wallaschek, J. Impact of surface texture on ultrasonic wire bonding process. J. Mater. Res. Technol. 2022, 20, 1828–1838. [Google Scholar] [CrossRef]
- Zou, Y.S.; Gan, C.L.; Chung, M.; Takiar, H. A review of interconnect materials used in emerging memory device packaging: First- and second-level interconnect materials. J. Mater. Sci. Mater. Electron. 2021, 32, 27133–27147. [Google Scholar] [CrossRef]
- Liang, S.; Huang, F.X.; Peng, C.; Zhong, M.J.; Wu, B.A.; Tang, H.Y. Research progress on copper and siliver bonding wires for microelectronic packaging technology. J. Funct. Mater. 2019, 50, 5048–5053. [Google Scholar]
- Chen, X. Comparative study on bonding of palladium-coated copper wire and bare copper wire in IC package. Silicon Val. 2014, 7, 152–153. [Google Scholar]
- Yu, C.F.; Chan, C.M.; Chan, L.C.; Hsieh, K.C. Cu wire bond microstructure analysis and failure mechanism. Microelectron. Reliab. 2011, 51, 119–124. [Google Scholar] [CrossRef]
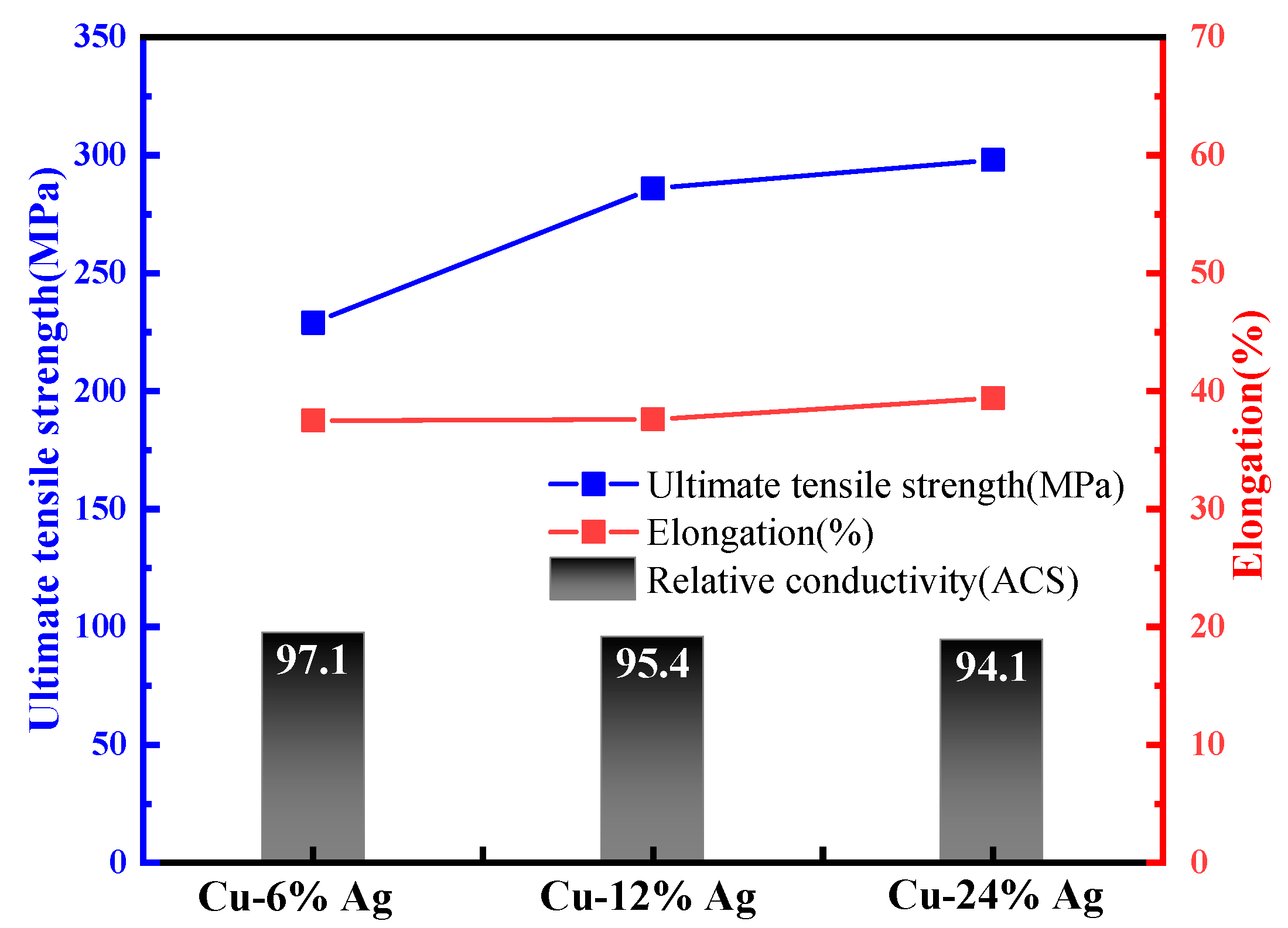
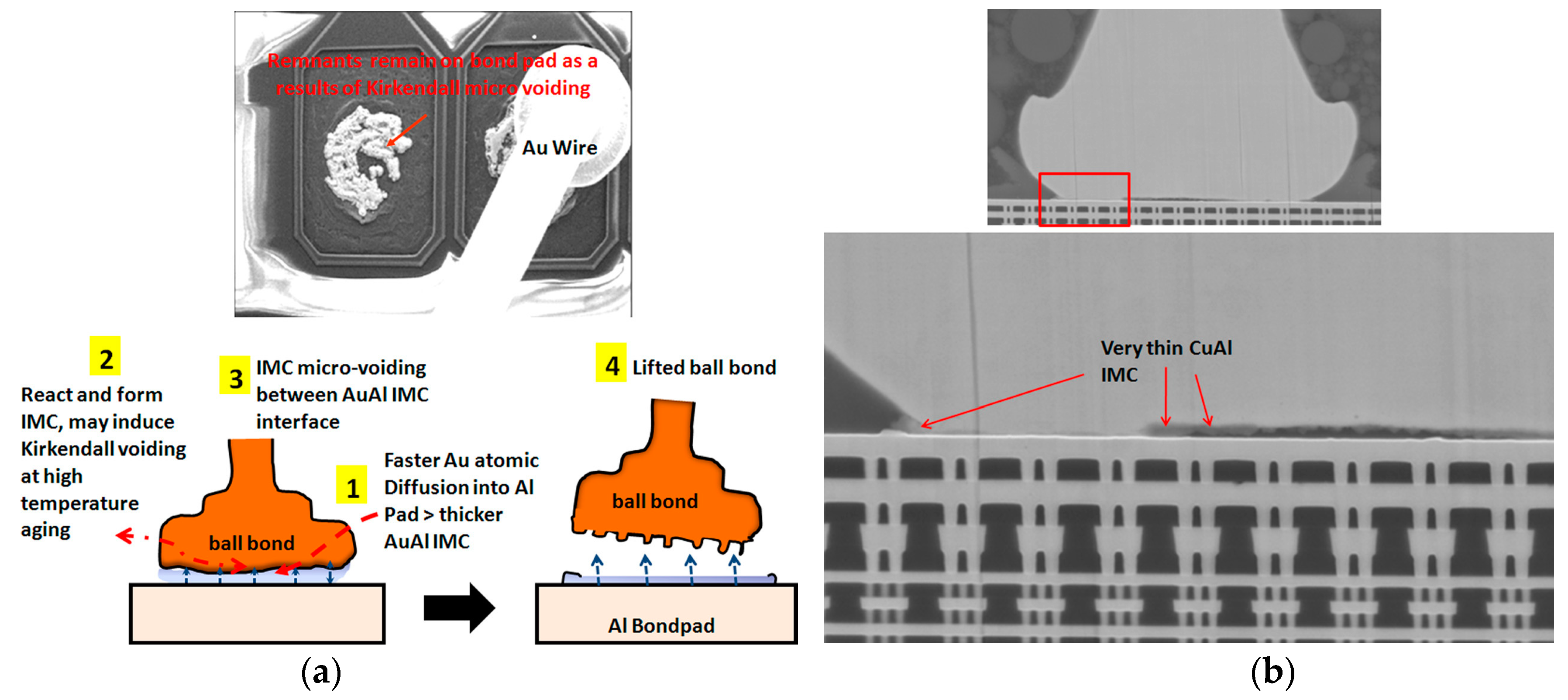
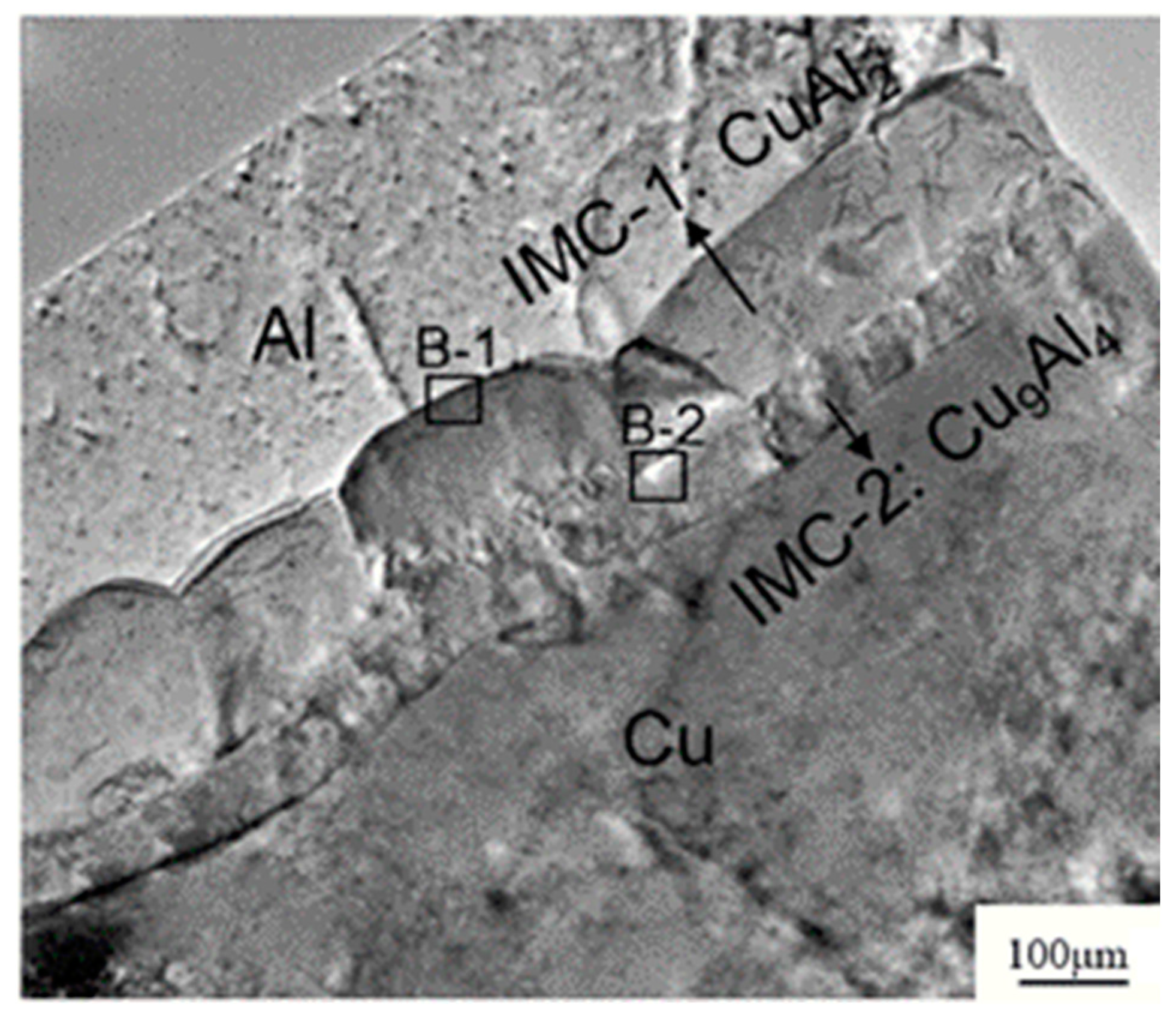



| Alloy Wire Type | Advantages |
|---|---|
| Au-Pd system | Higher strength, better free air ball (FAB) morphology, lower IMCs growth |
| Au-Cu system | Higher strength, lower environmental requirements, lower IMCs growth |
| Au-In system | Better wire loop, lower break rate, higher bonding strength |
| Au-Cu-Ca system | Higher strength, better wire thinning effect, lower IMCs growth |
| Test | Duration | Failures by Wire Type | ||
|---|---|---|---|---|
| Pd-Coated Cu wire | Au Wire | Cu Wire | ||
| Temperature Cycle (−65 to −150 °C) | 1000 cycles | 0% | 0% | 91% |
| Temperature humidity bias (85 °C, 85%RH, 10V) | 1000 h | 0% | 0% | 3% |
| Pressure cooker test (PCT) (121 °C, 100%RH, 2 atom) | 125 h | 0% | 0% | 95% |
| Solder reflow (260 °C) | 3 times | 0% | 0% | 0% |
| Properties | Au | Cu | Ag |
|---|---|---|---|
| Electrical Conductivity (% IACS) | 73.4 | 103.1 | 108.4 |
| Resistivity (×10−9 Ω·m) | 23.5 | 16.7 | 14.7 |
| Thermal Conductivity (W/m·K) | 317.9 | 398.0 | 428.0 |
| Thermal Expansion Coefficient (μm/m·K) | 14.2 | 16.7 | 19.0 |
| Tensile Strength (MPa) | 103.0 | 209.0 | 125.0 |
| Yield Strength (MPa) | 30.0–40.0 | 33.3 | 35.0 |
| Elastic Modulus (GPa) | 78.0 | 128.0 | 71.0 |
| Brinell Hardness (HB) | 18.0 | 37.0 | 25.0 |
| Metal Activity | Cu > Ag > Au | ||
| Type | Technical Challenges | Solution |
|---|---|---|
| Au | Poor loop formability and easy to collapse during wire bonding. | Add alloying elements such as Cu and Pd; use Cu bonding wire to replace it. |
| Easy to generate Kirkendall voids under long-term high temperature storage life (HTSL) tests. | Add trace alloying elements to slow down the diffusion rate of Au atoms. | |
| Cu | Easy to be oxidized and corroded. | Chose alloying and plating treatment; use inert shielding gas. |
| High hardness, causing defects such as pad peeling and cratering. | Use softer copper wire; increase pad thickness; set dummy microvias beneath pad metallization to stabilize and strengthen pad structure. | |
| Low hardness and strength of the HAZ, resulting in wire breakage near the FAB. | Adjust the EFO parameters. | |
| Short circuit and tail defects. | Use Pd-coated Cu wire instead of bare Cu wire. | |
| Cu-Al IMC corrosion, causing microcracks. | Use low halogen of molding compound and solder resist in the pad. | |
| Al(OH)3 forms during bonding process, galvanic and pitting corrosion occur with the presence of chloride halides in sodium chloride solutions. | Control humidity and the temperature of production workshop [176]. | |
| Ag | Easy to be oxidized and vulcanized. | Coat Au, Pd, etc. on bare Ag wire; add alloying elements to make Ag alloy wire. |
| Ag+ migration. | Add Pd to inhibit Ag+ migration. | |
| Low PCT reliability of bare Ag wire. | Increase Pd content reasonably. | |
| Complex bonding process with narrow process window. | Optimize the bonding process; add alloying elements such as Au and Pd. | |
| Short tailing during wire bonding. | Reduce the second bonding force by 10%. | |
| Poor FAB size repeatability and concentricity of Ag alloy wire. | Adopt lower FAB flow rates and EFO currents [72]. | |
| FAB surface deformation and defects. | Increase Pd content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, Y.; Cao, J.; Su, C.; Li, C.; Chang, A.; An, B. Research Progress on Bonding Wire for Microelectronic Packaging. Micromachines 2023, 14, 432. https://doi.org/10.3390/mi14020432
Zhou H, Zhang Y, Cao J, Su C, Li C, Chang A, An B. Research Progress on Bonding Wire for Microelectronic Packaging. Micromachines. 2023; 14(2):432. https://doi.org/10.3390/mi14020432
Chicago/Turabian StyleZhou, Hongliang, Yingchong Zhang, Jun Cao, Chenghao Su, Chong Li, Andong Chang, and Bin An. 2023. "Research Progress on Bonding Wire for Microelectronic Packaging" Micromachines 14, no. 2: 432. https://doi.org/10.3390/mi14020432
APA StyleZhou, H., Zhang, Y., Cao, J., Su, C., Li, C., Chang, A., & An, B. (2023). Research Progress on Bonding Wire for Microelectronic Packaging. Micromachines, 14(2), 432. https://doi.org/10.3390/mi14020432






