Magnetically Actuated Microscaffold with Controllable Magnetization and Morphology for Regeneration of Osteochondral Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Magnetic Microscaffolds
2.3. Characterization of Magnetic Microscaffolds
2.4. Cell Culture
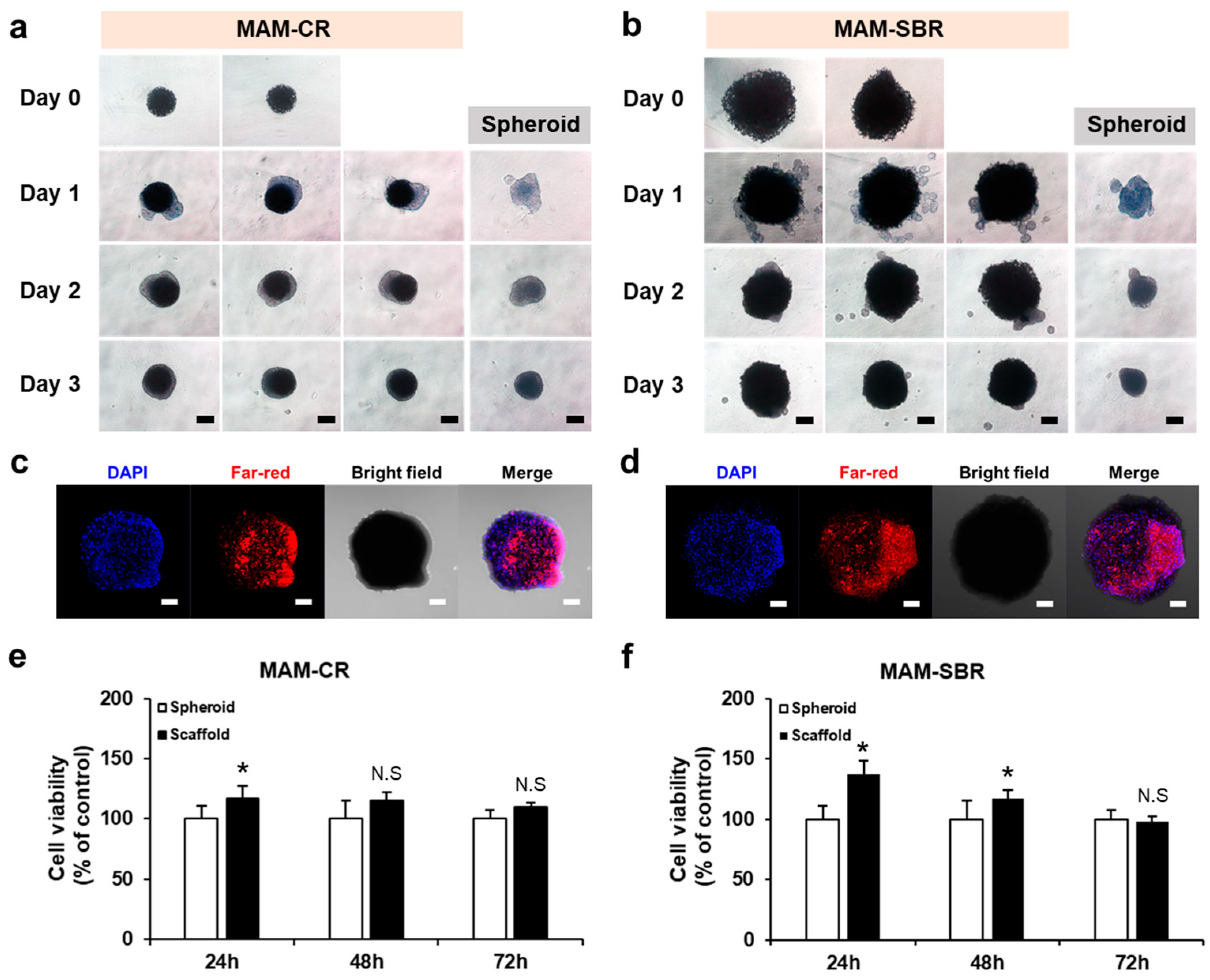
2.5. Cell Viability
2.6. Magnetic Actuation of Microscaffold
2.7. Statistics and Data Analysis
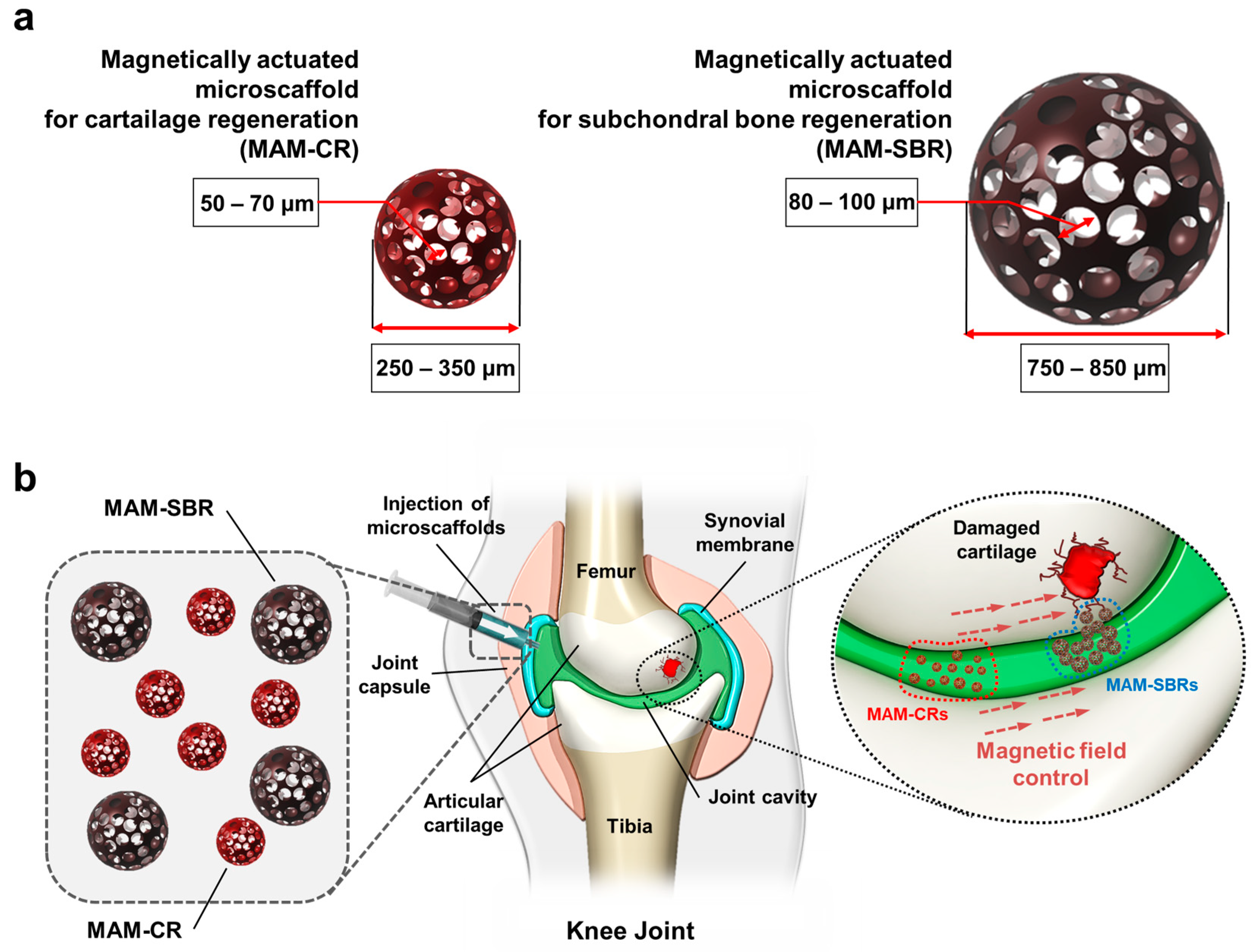
3. Results and Discussion
3.1. Characterization of Magnetic Microscaffold
3.2. Cell Loading and Viability Tests of the Magnetic Microscaffolds
3.3. Mobility Test of Magnetic Microscaffold
3.4. Targeting Validation of Magnetic Microscaffold
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Nobili, A.; Garattini, S.; Mannucci, P.M. Multiple diseases and polypharmacy in the elderly: Challenges for the internist of the third millennium. J. Comorb. 2011, 1, 28–44. [Google Scholar] [CrossRef]
- Smith, B.D.; Grande, D.A. The current state of scaffolds for musculoskeletal regenerative applications. Nat. Rev. Rheumatol. 2015, 11, 213–222. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharm. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef]
- Koh, Y.G.; Jo, S.B.; Kwon, O.R.; Suh, D.S.; Lee, S.W.; Park, S.H.; Choi, Y.J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef]
- Sato, M.; Uchida, K.; Nakajima, H.; Miyazaki, T.; Guerrero, A.R.; Watanabe, S.; Roberts, S.; Baba, H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res. 2012, 14, R31. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Go, G.; Jeong, S.G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.; Nguyen, K.T.; Jin, Z.; Kim, C.S.; Seo, Y.R.; et al. Human adipose-derived mesenchymal stem cell-based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020, 5, eaay6626. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.H.; Sun, D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef]
- Yasa, I.C.; Tabak, A.F.; Yasa, O.; Ceylan, H.; Sitti, M. 3D-Printed Microrobotic Transporters with Recapitulated Stem Cell Niche for Programmable and Active Cell Delivery. Adv. Funct. Mater. 2019, 29, 1808992. [Google Scholar] [CrossRef]
- Wang, B.; Chan, K.F.; Yuan, K.; Wang, Q.; Xia, X.; Yang, L.; Ko, H.; Wang, Y.-X.J.; Sung, J.J.Y.; Chiu, P.W.Y.; et al. Endoscopy-assisted magnetic navigation of biohybrid soft microrobots with rapid endoluminal delivery and imaging. Sci. Robot. 2021, 6, eabd2813. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeon, S.; Yang, Y.-S.; Jin, C.; Kim, J.-Y.; Oh, Y.-S.; Rah, J.-C.; Choi, H. A Neurospheroid-Based Microrobot for Targeted Neural Connections in a Hippocampal Slice. Adv. Mater. 2023, 2208747. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Huh, S.J.; Kang, B.-J.; Shin, H. Directed Regeneration of Osteochondral Tissue by Hierarchical Assembly of Spatially Organized Composite Spheroids. Adv. Sci. 2022, 9, 2103525. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J.; Ding, J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. A 2014, 102, 180–192. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Weeber, R.; Hermes, M.; Schmidt, A.M.; Holm, C. Polymer architecture of magnetic gels: A review. J. Phys. Condens. Matter 2018, 30, 063002. [Google Scholar] [CrossRef] [PubMed]
- Sokolsky, S.A.; Solovyova, A.Y.; Zverev, V.S.; Hess, M.; Schmidt, A.; Elfimova, E.A. Analysis of the ferrofluid microstructure based on the static magnetic measurements. J. Magn. Magn. Mater. 2021, 537, 168169. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Kim, S.M.; Shin, H. Size-controlled human adipose-derived stem cell spheroids hybridized with single-segmented nanofibers and their effect on viability and stem cell differentiation. Biomater. Res. 2021, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Descamps, L.; Audry, M.-C.; Howard, J.; Mekkaoui, S.; Albin, C.; Barthelemy, D.; Payen, L.; Garcia, J.; Laurenceau, E.; Le Roy, D.; et al. Self-Assembled Permanent Micro-Magnets in a Polymer-Based Microfluidic Device for Magnetic Cell Sorting. Cells 2021, 10, 1734. [Google Scholar] [CrossRef]
- Yoo, A.; Go, G.; Nguyen, K.T.; Lee, K.; Min, H.-K.; Kang, B.; Kim, C.-S.; Han, J.; Park, J.-O.; Choi, E. Magnetoresponsive stem cell spheroid-based cartilage recovery platform utilizing electromagnetic fields. Sens. Actuators B Chem. 2020, 307, 127569. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Cells in the Non-Uniform Magnetic World: How Cells Respond to High-Gradient Magnetic Fields. BioEssays 2018, 40, 1800017. [Google Scholar] [CrossRef] [PubMed]
- Rubio Ayala, M.; Syrovets, T.; Hafner, S.; Zablotskii, V.; Dejneka, A.; Simmet, T. Spatiotemporal magnetic fields enhance cytosolic Ca2+ levels and induce actin polymerization via activation of voltage-gated sodium channels in skeletal muscle cells. Biomaterials 2018, 163, 174–184. [Google Scholar] [CrossRef]
- Go, G.; Yoo, A.; Nguyen, K.T.; Nan, M.; Darmawan, B.A.; Zheng, S.; Kang, B.; Kim, C.S.; Bang, D.; Lee, S.; et al. Multifunctional microrobot with real-time visualization and magnetic resonance imaging for chemoembolization therapy of liver cancer. Sci. Adv. 2022, 8, eabq8545. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Song, H.-W.; Nguyen, K.T.; Kim, S.; Nan, M.; Park, J.-O.; Go, G.; Choi, E. Magnetically Actuated Microscaffold with Controllable Magnetization and Morphology for Regeneration of Osteochondral Tissue. Micromachines 2023, 14, 434. https://doi.org/10.3390/mi14020434
Lee J, Song H-W, Nguyen KT, Kim S, Nan M, Park J-O, Go G, Choi E. Magnetically Actuated Microscaffold with Controllable Magnetization and Morphology for Regeneration of Osteochondral Tissue. Micromachines. 2023; 14(2):434. https://doi.org/10.3390/mi14020434
Chicago/Turabian StyleLee, Junhyeok, Hyeong-Woo Song, Kim Tien Nguyen, Seokjae Kim, Minghui Nan, Jong-Oh Park, Gwangjun Go, and Eunpyo Choi. 2023. "Magnetically Actuated Microscaffold with Controllable Magnetization and Morphology for Regeneration of Osteochondral Tissue" Micromachines 14, no. 2: 434. https://doi.org/10.3390/mi14020434
APA StyleLee, J., Song, H.-W., Nguyen, K. T., Kim, S., Nan, M., Park, J.-O., Go, G., & Choi, E. (2023). Magnetically Actuated Microscaffold with Controllable Magnetization and Morphology for Regeneration of Osteochondral Tissue. Micromachines, 14(2), 434. https://doi.org/10.3390/mi14020434






