Study on SAW Methane Sensor Based on Cryptophane-A Composite Film
Abstract
1. Introduction
2. Sensor Development
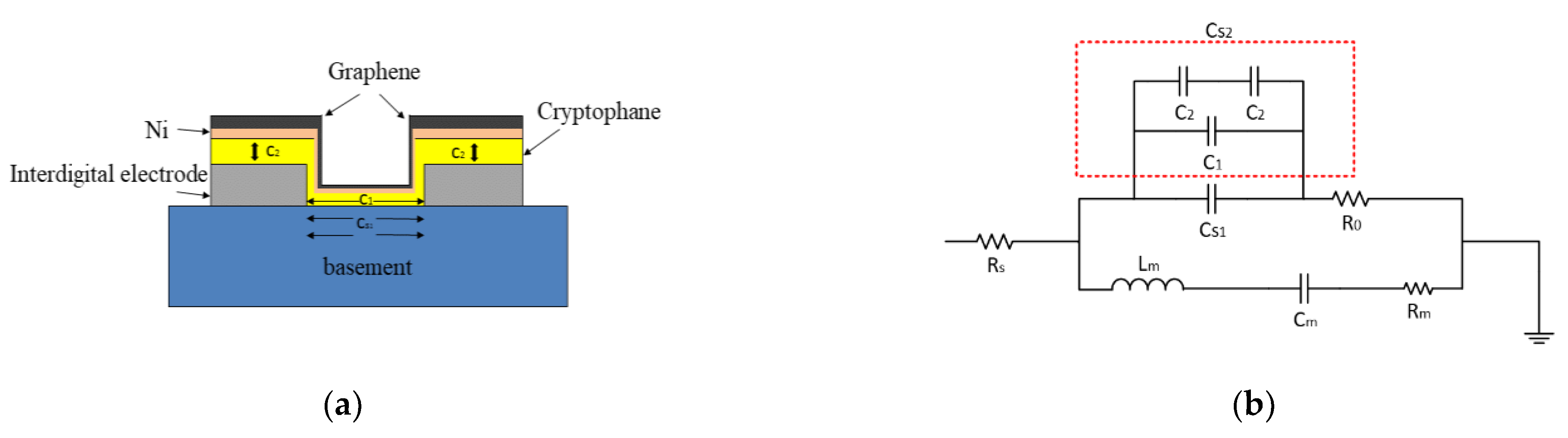
2.1. The Sensing Mechanism
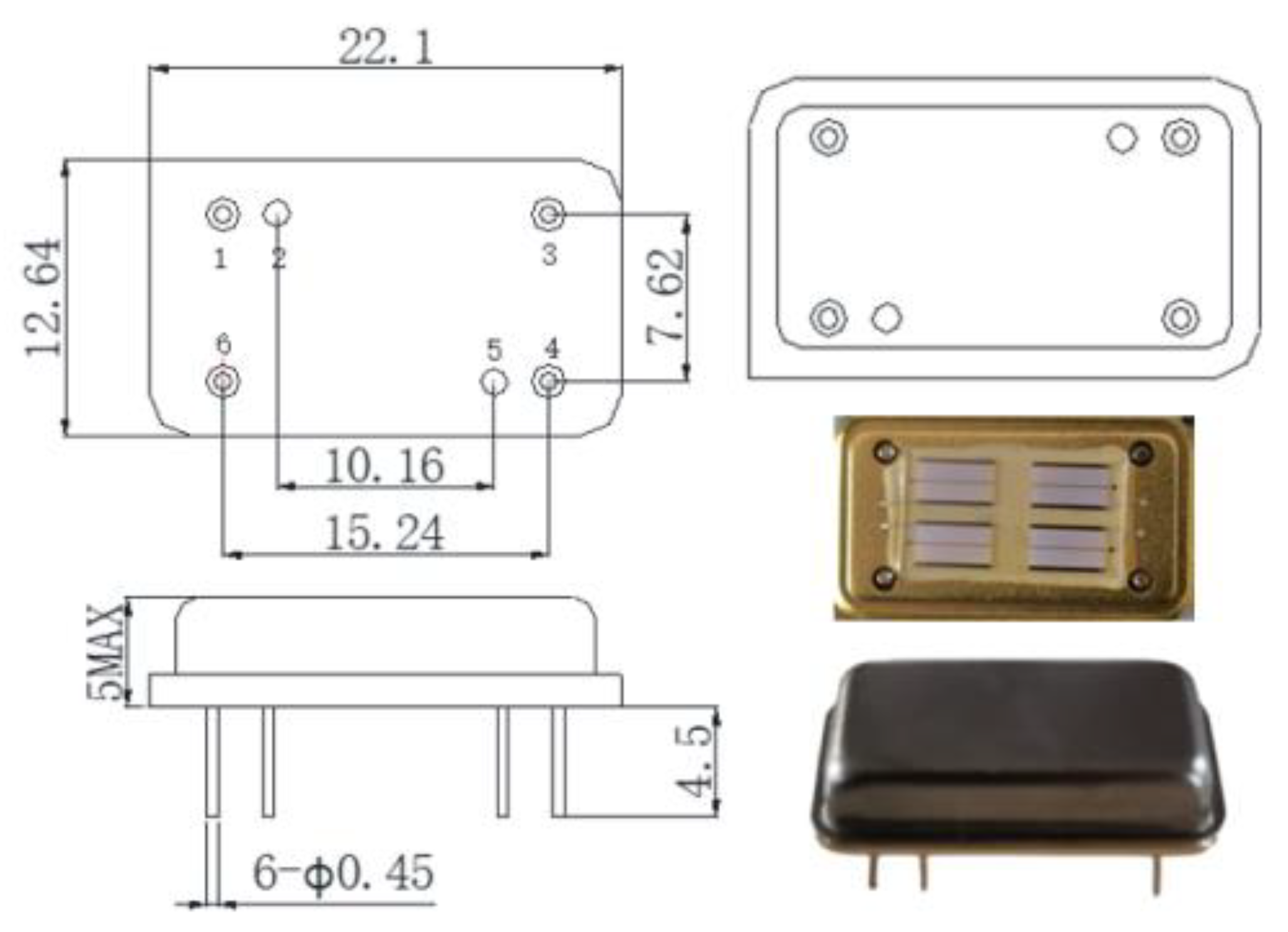
2.2. Design of Sensor Parts
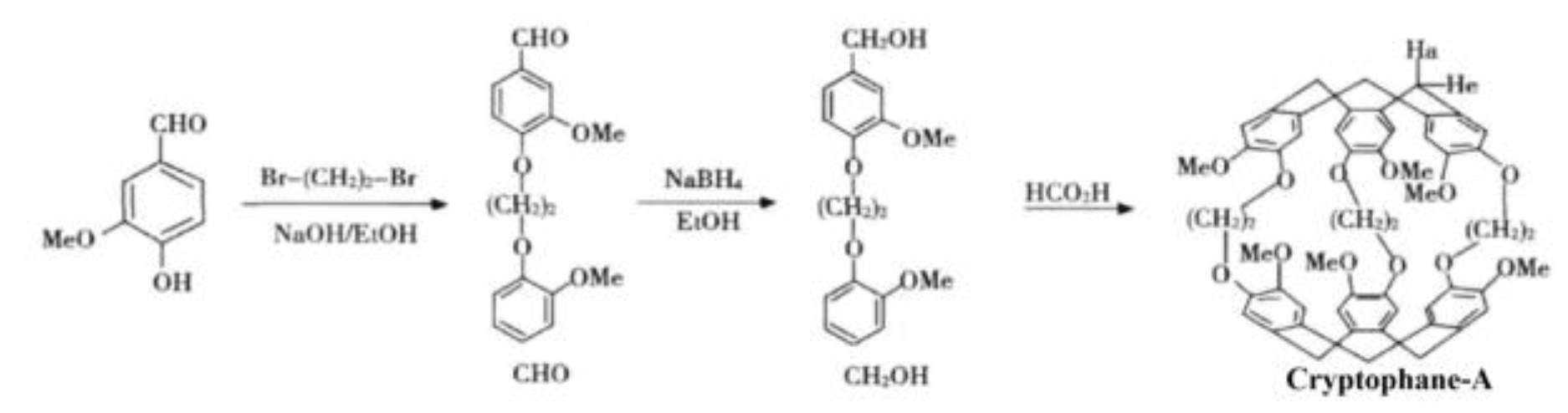
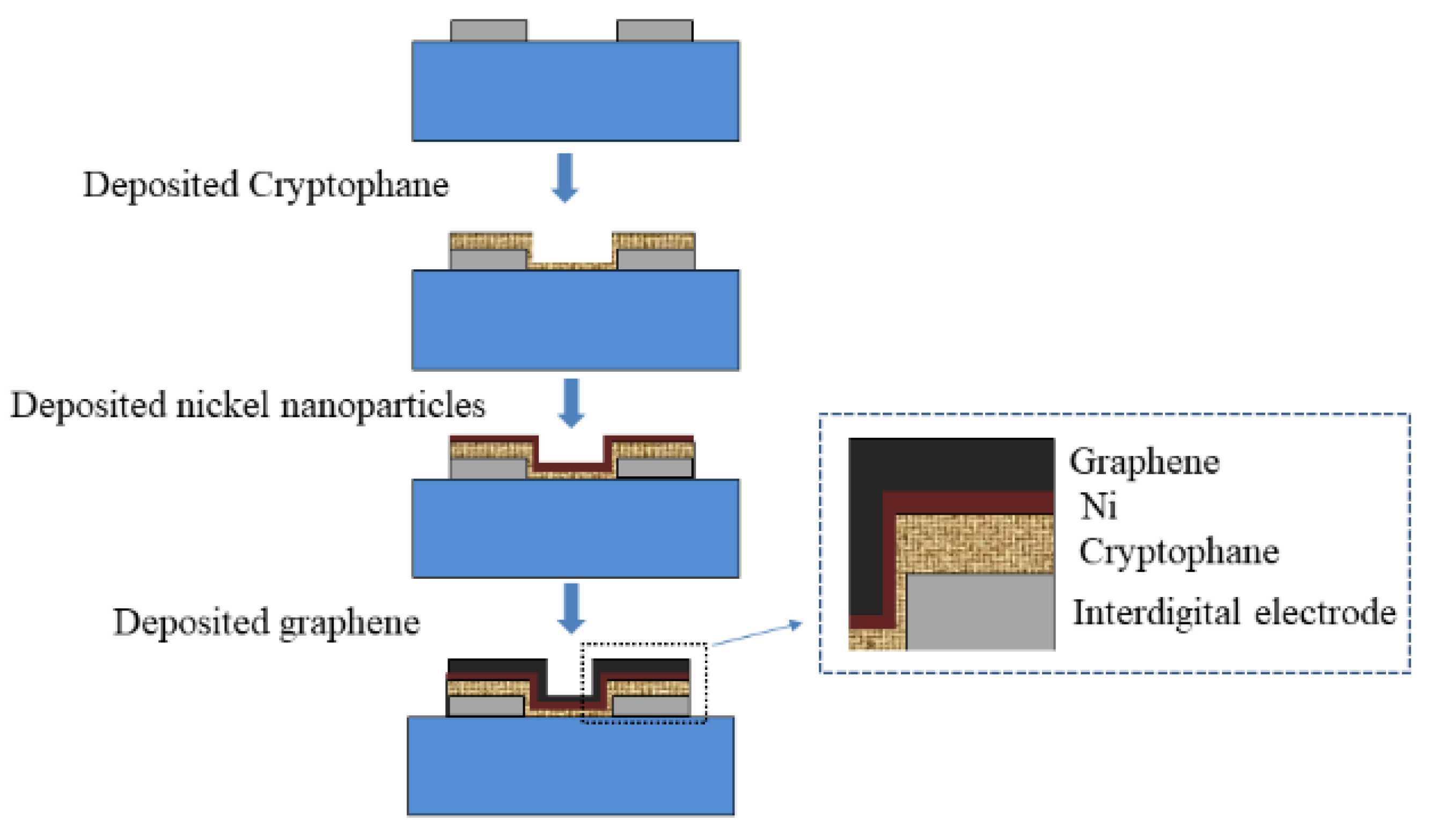
2.3. Preparation of Sensitive Film
3. Results and Discussion
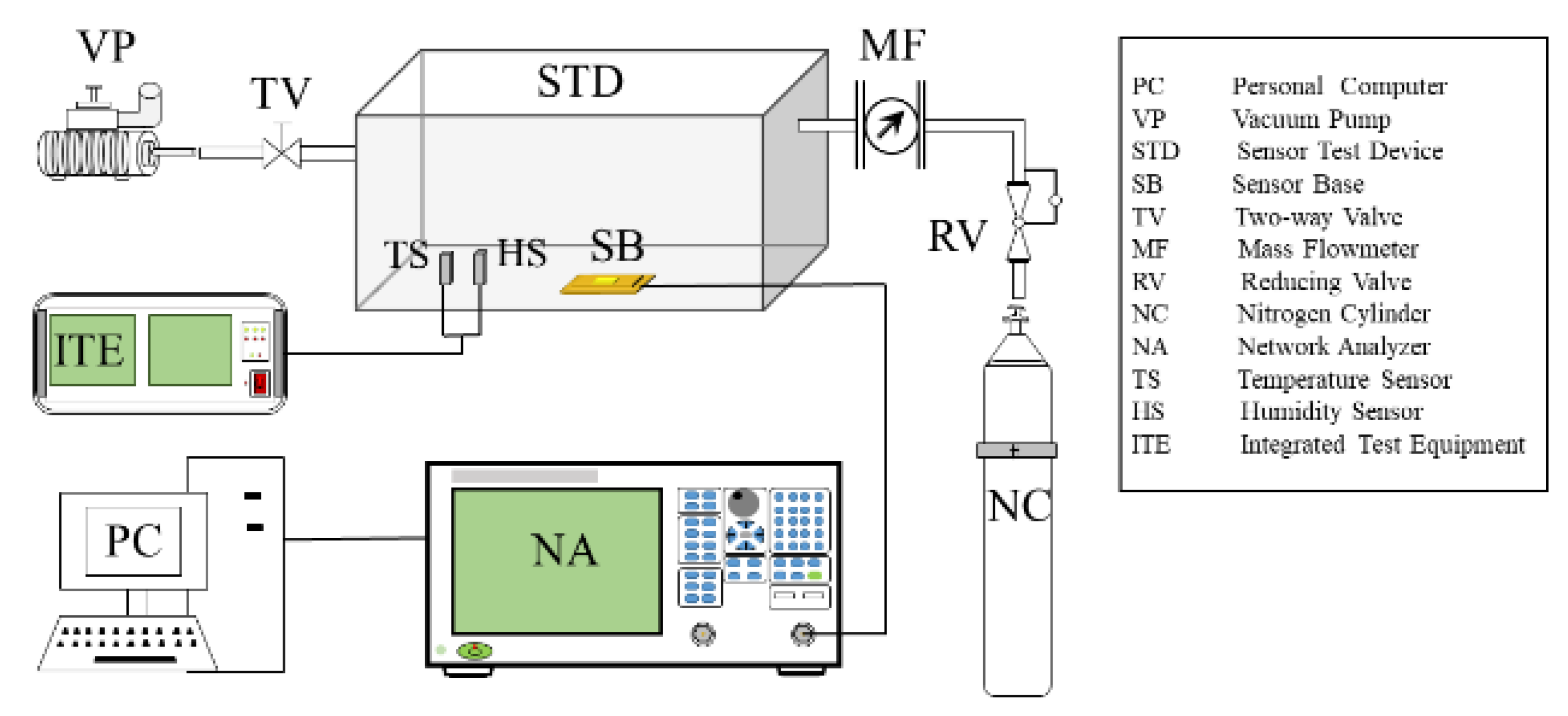
3.1. Performance Testing System of SAW Device
3.2. Consistency Characteristics of Sensor Response
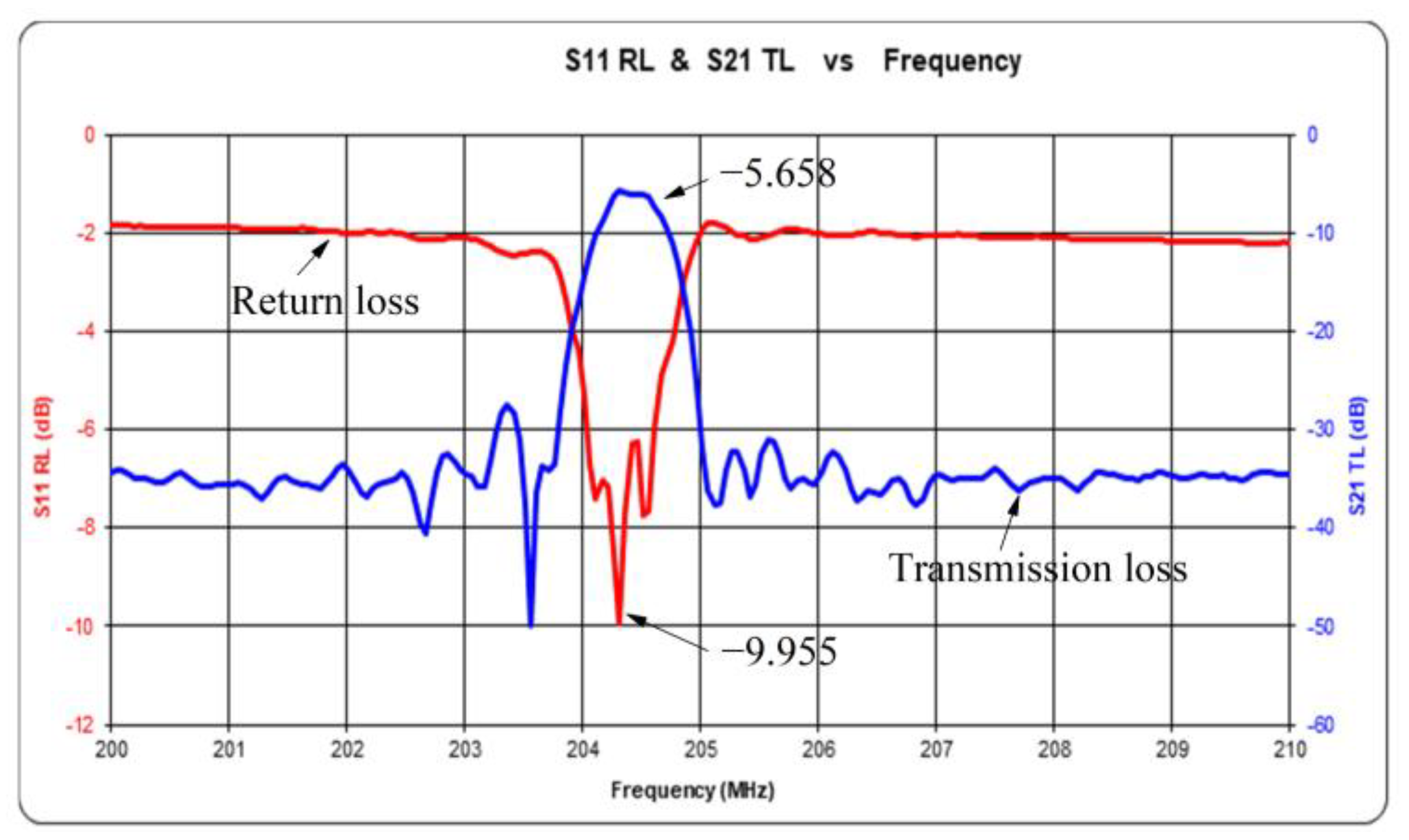
3.3. Sensor Insertion Loss Test
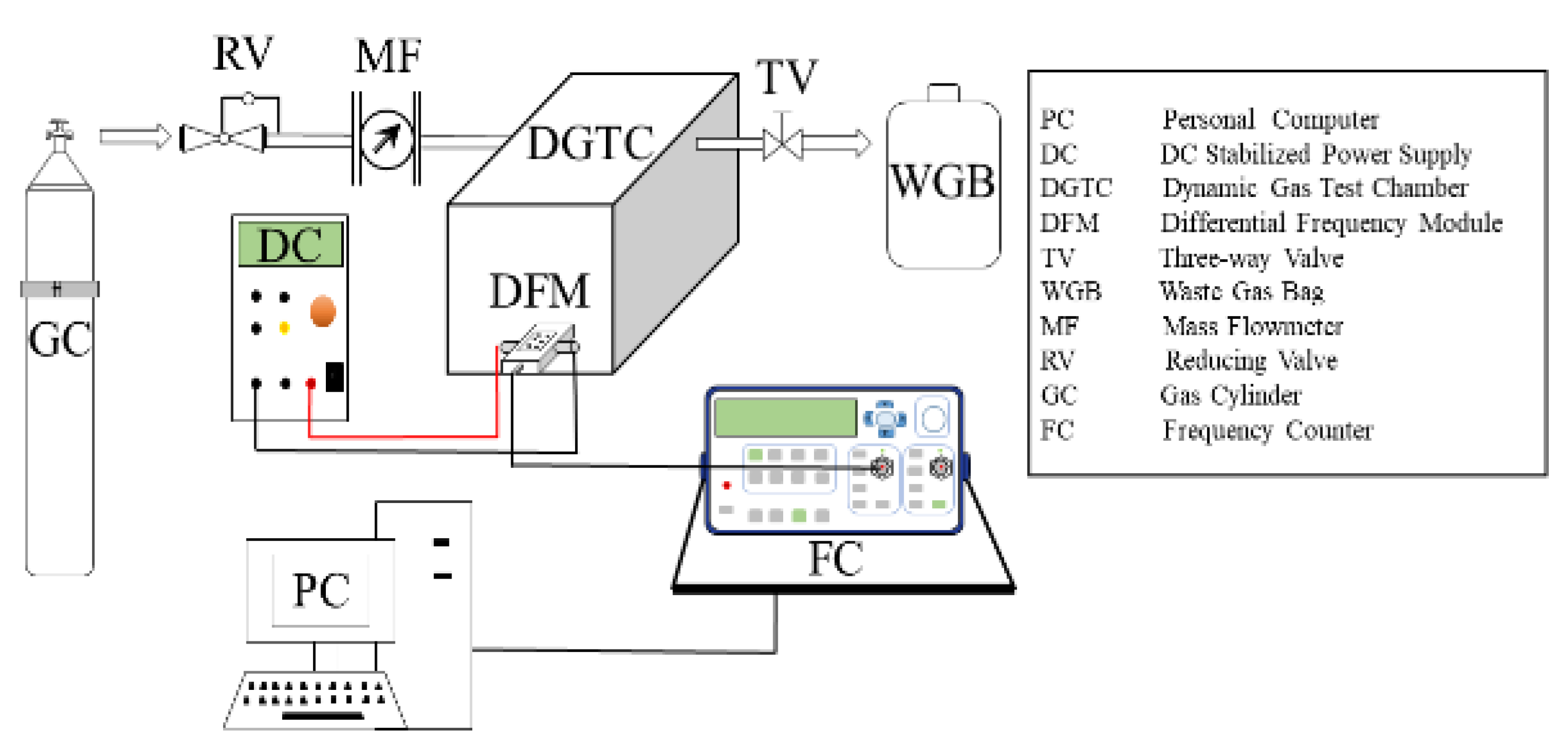
3.4. Gas Sensing Test System
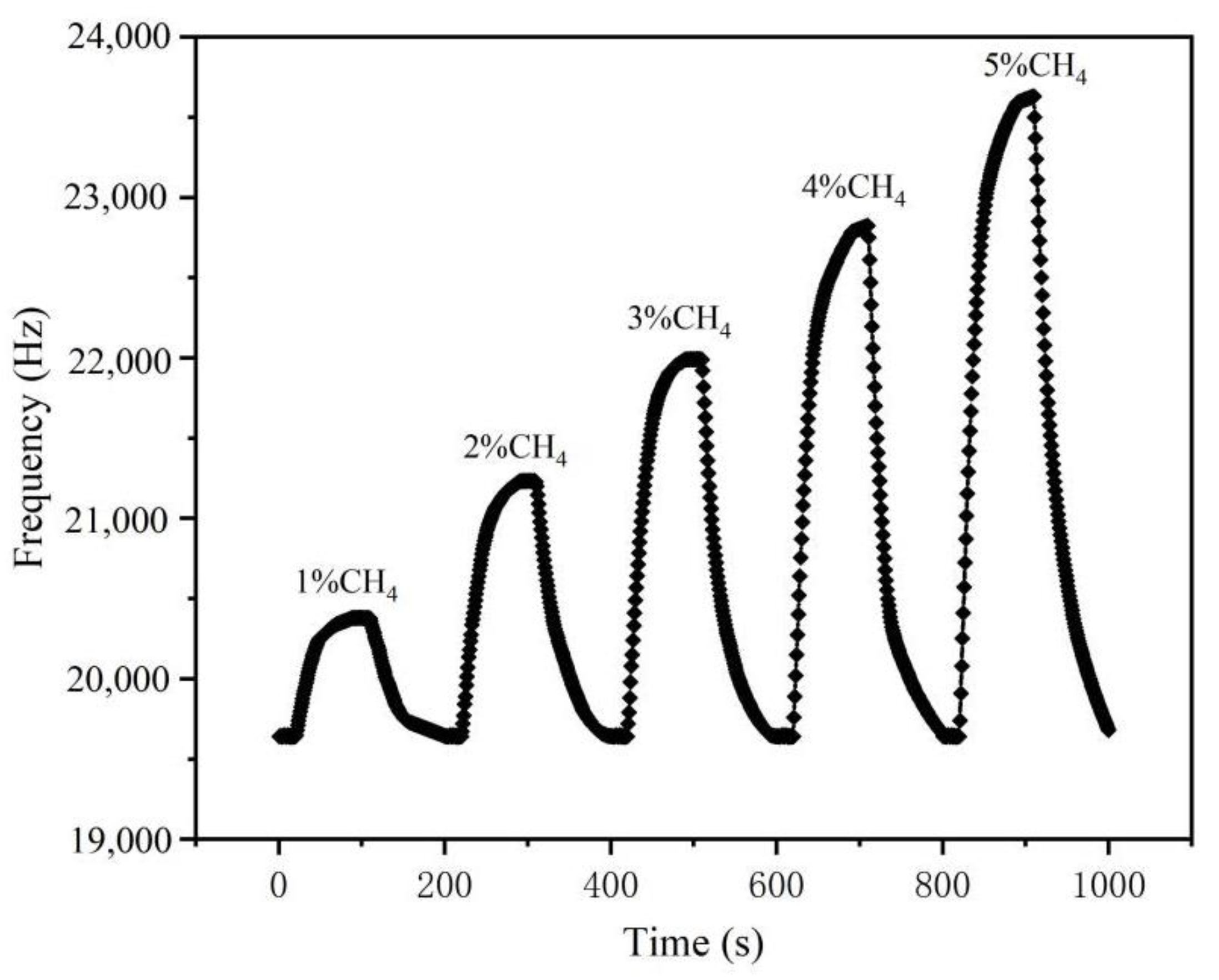
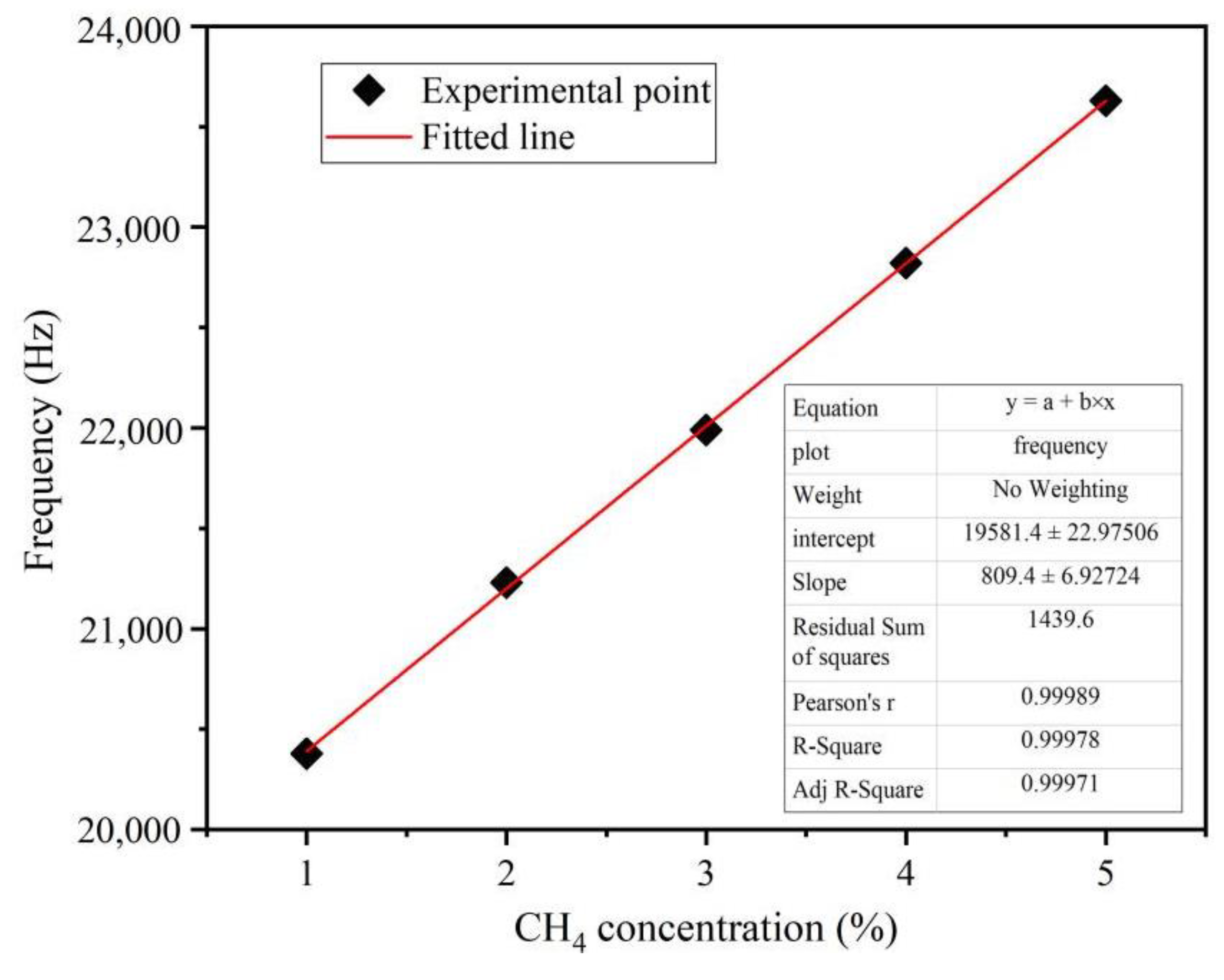
3.5. Methane Response Characteristics of the Sensor
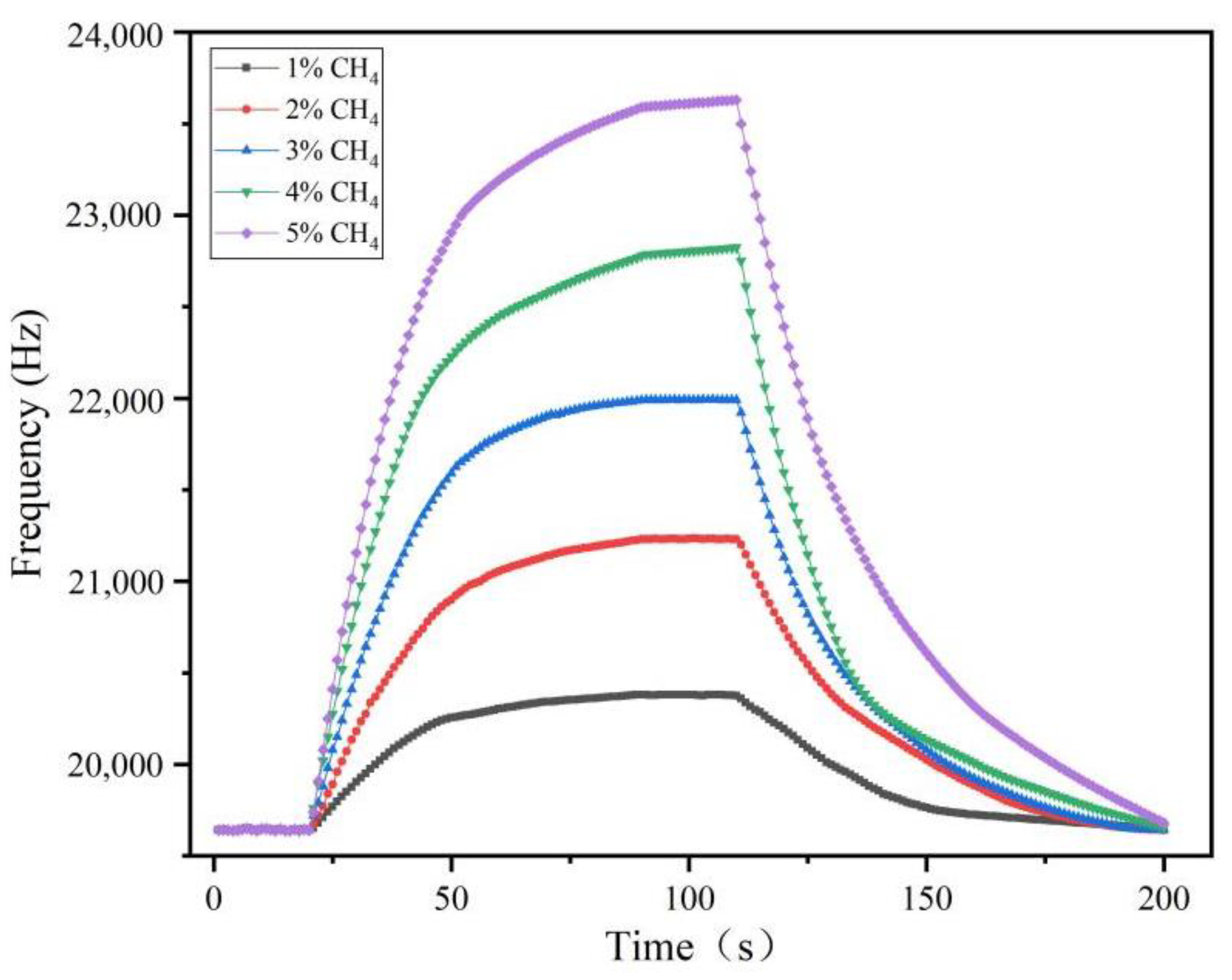
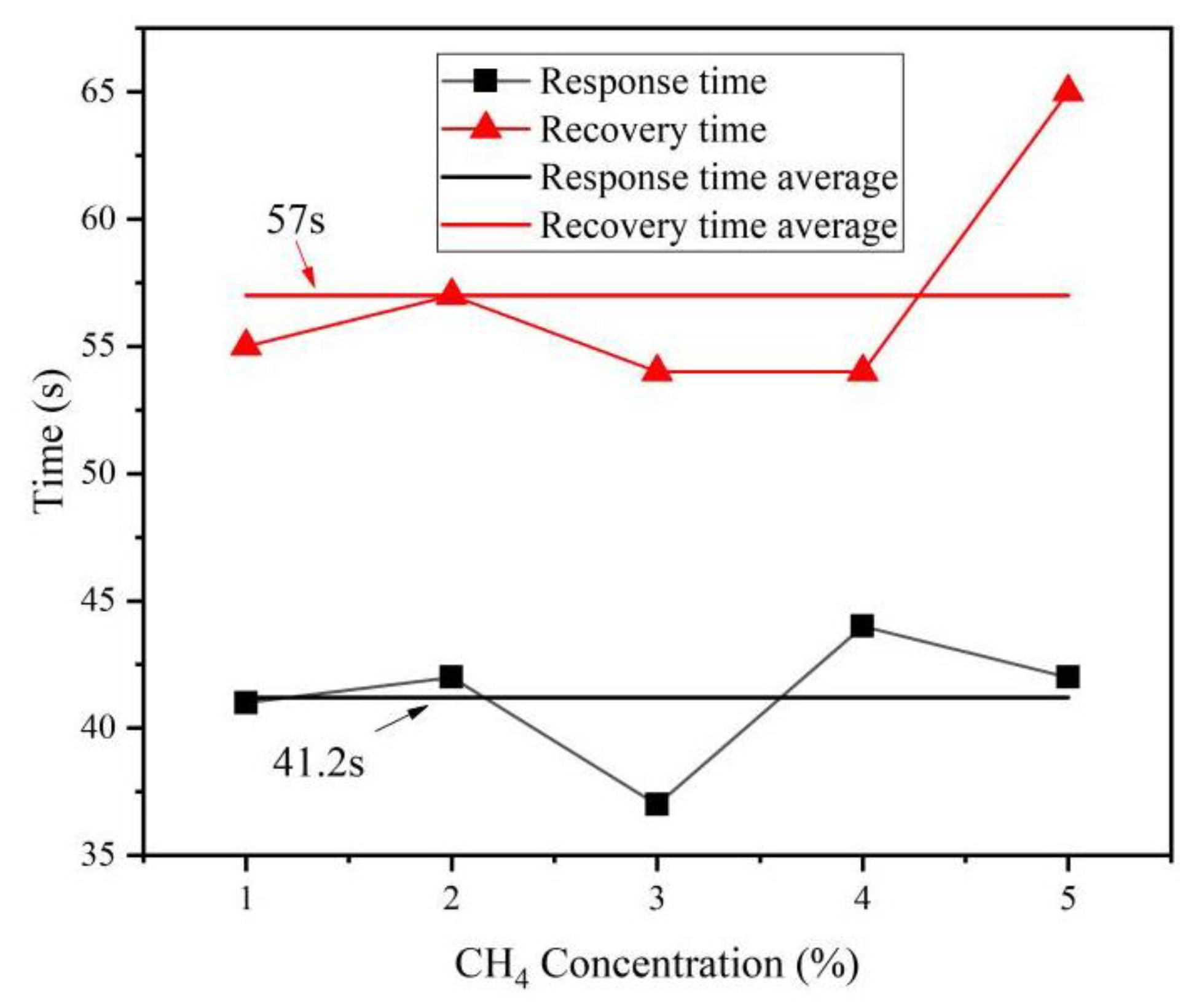
3.6. Characteristics of the Sensor Response Time
3.7. Characteristics of the Sensor Selection
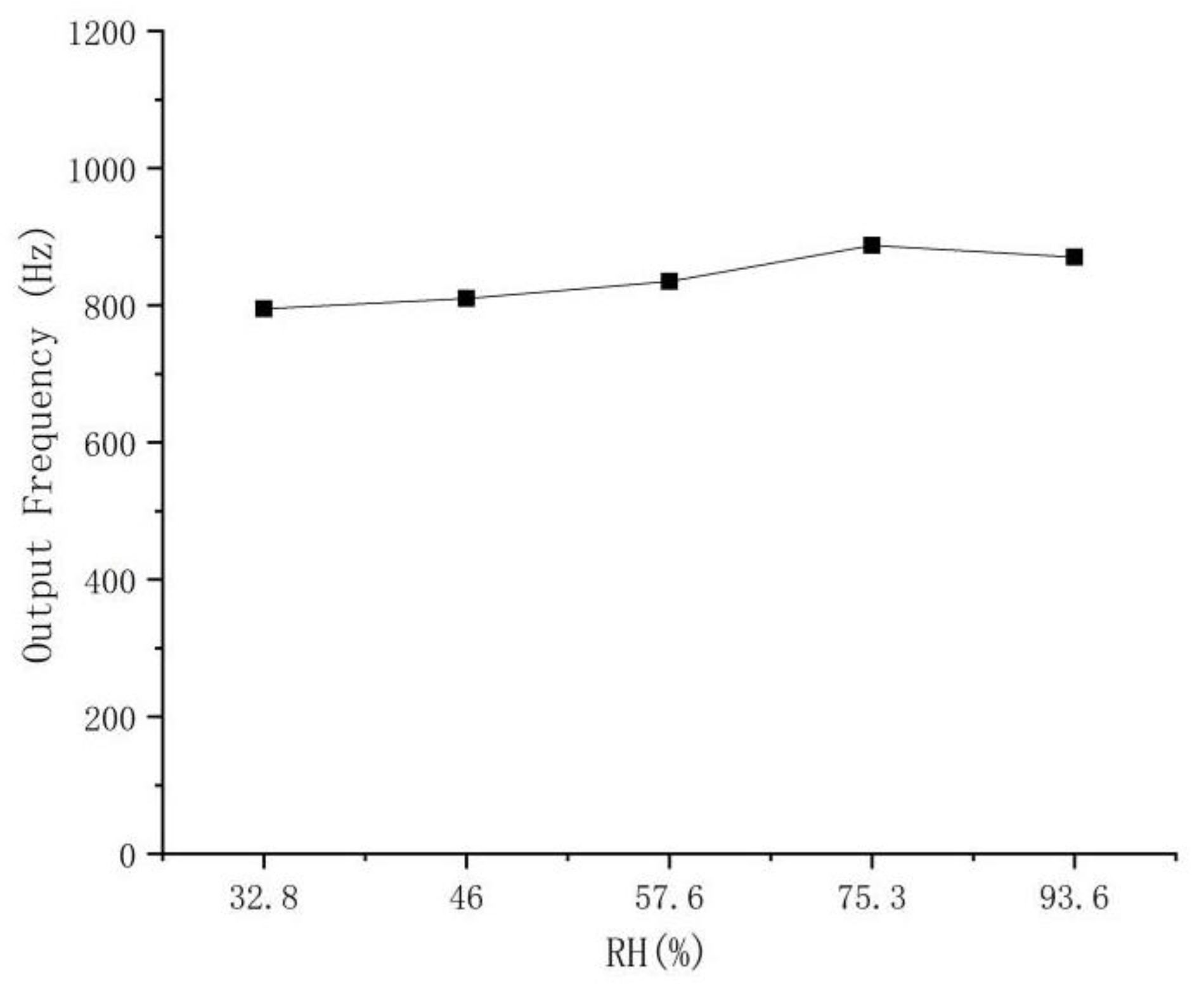
3.8. Humidity Influence on the Response of the Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Gao, W.; Song, Z.; An, Y.; Li, L.; Song, Z.; Yu, W.W.; Wang, Y. Design of a novel gas sensor structure based on mid–infrared absorption spectrum. Sens. Actuators B Chem. 2010, 147, 5–9. [Google Scholar] [CrossRef]
- Jörgen, S.; Huib, B.; Timo, R.; Arjen, B. Capacitive and Infrared Gas Sensors for the Assessment of the Methane Number of LNG Fuels. Sensors 2020, 20, 3345. [Google Scholar] [CrossRef]
- Zhao, Q. Development of an Infrared Methane Sensor Based on Double Narrow–band LED Light Source. Acta Photonica Sin. 2020, 49, 154–162. [Google Scholar] [CrossRef]
- Jang, W.; Park, J.-S.; Lee, K.-W.; Roh, Y. Methane and hydrogen sensing properties of catalytic combustion type single–chip micro gas sensors with two different Pt film thicknesses for heaters. Micro Nano Syst. Lett. 2018, 6, 7. [Google Scholar] [CrossRef]
- Su, J.; Cao, L.; Li, L.; Wei, J.; Li, G.; Yuan, Y. Highly sensitive methane catalytic combustion micro–sensor based on mesoporous structure and nano–catalyst. Nanoscale 2013, 5, 9720–9725. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; RoyChaudhuri, C.; Bhattacharya, R.; Saha, H.; Mukherjee, N. Palladium–silver–activated ZnO surface: Highly selective methane sensor at reasonably low operating temperature. Acs. Appl. Mater. Interfaces 2014, 6, 3879–3887. [Google Scholar] [CrossRef]
- Ludmila, O.; George, F.; Nelly, M. Effect of heterogeneous catalytic methane oxidation on kinetics of conductivity response of adsorption semiconductor sensors based on Pd/SnO2 nanomaterial. Res. Chem. Intermed. 2019, 45, 4101–4111. [Google Scholar] [CrossRef]
- Teng, L.; En, L.; Lei, Y.; Fan, J.; Li, Z. María del Carmen Horrillo. Analysis and Design of an Effective Light Interference Methane Sensor Based on Three–Dimensional Optical Path Model. J. Sens. 2018, 2018, 1342593. [Google Scholar] [CrossRef]
- Lin, H.; Liang, Z.; Li, E.; Yang, M.; Zhai, B. Analysis and design of an improved light interference methane sensor. In Proceedings of the 11th IEEE International Conference on Control & Automation (ICCA), Taichung, Taiwan, 18–20 June 2014. [Google Scholar] [CrossRef]
- Wang, W.; Hu, H.; He, S.; Pan, Y.; Zhang, C.; Dong, C. A room–temperature SAW methane sensor with Cryptophane–A film. In Proceedings of the 2015 IEEE International Ultrasonics Symposium (IUS), Taipei, Taiwan, 21–24 October 2015. [Google Scholar] [CrossRef]
- Hu, H.; Wang, W.; He, S.; Pan, Y.; Zhang, C.; Dong, C. A Novel Surface Acoustic Wave Methane Sensor Coated with Cryptophane–A. Chin. J. Sens. Actuators 2016, 29, 5. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three–dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sens. Actuators B. Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Shen, B.; Yang, H.; Jiang, L.; Liu, X. Simulation Study of FEUDT Structure Optimization and Sensitive Film Loading of SAW Devices. Micromachines 2022, 13, 1643. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic waves in strain–engineered K0.7Na0.3NbO3 thin films. Appl. Phys. Lett. 2018, 113, 052901. [Google Scholar] [CrossRef]
- Long, G.; Guo, Y.; Li, W.; Tang, Q.; Zu, X.; Ma, J.; Du, B.; Fu, Y. Surface acoustic wave ammonia sensor based on ZnS mucosal–like nanostructures. Microelectron. Eng. 2020, 222, 111201. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Tang, Y.; Zu, X.; Ma, J.; Wang, L.; Fu, Y. Room–Temperature Ammonia Sensor Based on ZnO Nanorods Deposited on ST–Cut Quartz Surface Acoustic Wave Devices. Sensors 2017, 17, 1142. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; Fu, Q.; Zhou, D.; Hu, Y.; Gong, S.; Zheng, Z. Nanocrystalline SnO2 film prepared by the aqueous sol–gel method and its application as sensing films of the resistance and SAW H2S sensor. Sens. Actuators B Chem. 2015, 217, 119–128. [Google Scholar] [CrossRef]
- Ippolito, S.; Ponzoni, A.; Kalantar-Zadeh, K.; Wlodarski, W.; Comini, E.; Faglia, G.; Sberveglieri, G. Layered WO3/ZnO/36° LiTaO3 SAW gas sensor sensitive towards ethanol vapour and humidity. Sens. Actuators B Chem. 2006, 117, 442–450. [Google Scholar] [CrossRef]
- Raj, V.B.; Singh, H.; Nimal, A.; Sharma, M.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E–nose for the detection of chemical warfare agents. Sens. Actuators B. Chem. 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, H.; Fu, C.; Ke, Y.; He, S. Corrigendum to “Development of a SnO2/CuO–coated surface acoustic wave–based H2S sensor with switch–like response and recovery” [Sens. Actuators: B. Chem. 169C (2012) 10–16]. Sens. Actuators B Chem. 2013, 176, 1204. [Google Scholar] [CrossRef]
- Si, P.; John, M.; Alexei, K.; Jens, D.; Preben, J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Sergi, A.; Sterzo, C.; Russo, M. SAW chemical sensing using poly–ynes and organometallic polymer films. Sens. Actuators B. Chem. 2001, 8, 88–98. [Google Scholar] [CrossRef]
- Chiang, C.; Tsai, K.; Lee, Y.; Lin, H.; Yi, L.; Shih, C.; Lin, C.; Jeng, H.; Weng, Y.; Cheng, Y.; et al. In situ fabrication of conducting polymer composite film as a chemical resistive CO2 gas sensor. Microelectron. Eng. 2013, 111, 409–415. [Google Scholar] [CrossRef]
- Özmen, A.; Tekce, F.; Ebeoğlu, M.; Taşaltın, C.; Öztürk, Z. Finding the composition of gas mixtures by a phthalocyanine–coated QCM sensor array and an artificial neural network. Sens. Actuators B. Chem. 2005, 115, 450–454. [Google Scholar] [CrossRef]
- Koshets, I.; Kazantseva, Z.; Shirshov, Y.; Cherenok, S.; Kalchenko, V. Calixarene films as sensitive coatings for QCM–based gas sensors. Sens. Actuators B. Chem. 2004, 106, 177–181. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two–dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Poole, T.; Nash, G. Acoustoelectric Current in Graphene Nanoribbons. Sci. Rep. 2017, 7, 1767. [Google Scholar] [CrossRef]
- Bandhu, L.; Lawton, L.; Nash, G. Macroscopic acoustoelectric charge transport in graphene. Appl. Phys. Lett. 2013, 103, 133101. [Google Scholar] [CrossRef]
- Poole, T.; Nash, G. Acoustoelectric photoresponse of graphene nanoribbons. J. Phys. D Appl. Phys. 2018, 51, 154001. [Google Scholar] [CrossRef]
- Chivukula, V.; Kritzinger, C.; Yavari, F.; Ciplys, D.; Koratkar, N.; Shur, M.S. Detection of CO2 absorption in graphene using surface acoustic waves. In Proceedings of the EEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010. [Google Scholar] [CrossRef]
- Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P.; Gilje, S.; Kaner, R.; Kalantar, K.; Wlodarski, W. Graphene–like nano–sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2008, 467, 344–347. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Zhao, C.; Hu, P.; Zu, X.; Fu, Y. Graphene/LiNbO3 surface acoustic wave device based relative humidity sensor. Optik 2014, 125, 5800–5802. [Google Scholar] [CrossRef]
- Lokeshwar, B.; Nash, G.R. Controlling the properties of surface acoustic waves using graphene. Nano Res. 2016, 9, 685–691. [Google Scholar] [CrossRef]
- Whitehead, E.F.; Chick, E.M.; Bandhu, L.; Lawton, L.M.; Nash, G.R. Gas loading of graphene–quartz surface acoustic wave devices. Appl. Phys. Lett. 2013, 103, 063110. [Google Scholar] [CrossRef]
- Preciado, E.; Schülein, F.J.; Nguyen, A.E.; Barroso, D.; Isarraraz, M.; von Son, G.; Lu, I.-H.; Michailow, W.; Möller, B.; Klee, V.; et al. Scalable fabrication of a hybrid field–effect and acousto–electric device by direct growth of monolayer MoS2/LiNbO3. Nat. Commun. 2015, 6, 8593. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J.; Chen, J.; Gong, J.; Nie, Q. MOF–derived in situ growth of carbon nanotubes entangled Ni/NiO porous polyhedrons for high performance glucose sensor. Mater. Lett. 2018, 221, 267–270. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Gong, W.; Jiao, P.; Chen, C.; Yang, Y.; Wang, M.; Wang, G. Effect of modified graphene sheets on the structure and pyrolysis properties of phenolic resin. Carbon Tech. 2014. [Google Scholar] [CrossRef]
- Xu, P.; Shen, X.; Xin, G. Electro–brush plating Ni–Graphene oxide composite coating enhanced corrosion resistance of fluoride ion. Mater. Res. Express 2021, 8, 026406. [Google Scholar] [CrossRef]
- Nie, H.; Yao, Z.; Zhou, X.; Yang, Z.; Huang, S. Nonenzymatic electrochemical detection of glucose using well–distributed nickel nanoparticles on straight multi–walled carbon nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Garel, L.; Dutasta, J. Complexation of Methane and Chlorofluorocarbons by Cryptophane–A in Organic Solution (p1169–1171). Angew. Chem. Int. Ed. 2010, 32, 1169–1171. [Google Scholar] [CrossRef]
- Benounis, M.; Jaffrezic, N.; Dutasta, J.; Cherif, K.; Abdelghani, A. Study of a new evanescent wave optical fibre sensor for methane detection based on cryptophane molecules. Sens. Actuators B Chem. 2004, 107, 32–39. [Google Scholar] [CrossRef]
- Boulart, C.; Mowlem, M.; Connelly, D.; Dutasta, J.; German, C. A novel, low–cost, high performance dissolved methane sensor for aqueous environments. Opt. Express 2008, 16, 12607–12617. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Yang, J.; Shi, Y. Optical fiber sensing element based on luminescence quenching of silica nanowires modified with cryptophane–A for the detection of methane. Sens. Actuators B Chem. 2011, 156, 553–558. [Google Scholar] [CrossRef]
- El, G.; Holman, K. Cryptophanes–ScienceDirect. Compr. Supramol. Chem. II 2017, 199–249. [Google Scholar] [CrossRef]
- Xu, S.; Li, C.; Li, H.; Li, M.; Qu, C.; Yang, B. Carbon dioxide sensors based on a surface acoustic wave device with a graphene–nickel–L–alanine multilayer film. J. Mater. Chem. C 2015, 3, 3882–3890. [Google Scholar] [CrossRef]
- Shen, B.; Yang, P.; Liu, X.; Zhang, H.; Cao, S. Fabrication and characterizations of SAW methane sensor based on cryptophane–E membrane. J. Ambient. Intell. Humaniz. Comput. 2018, prepublish. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Lu, X. Simulation and design of SAW temperature sensor. Piezoelectrics Acoustooptics 2014, 36, 8–11. [Google Scholar] [CrossRef]
- Bostan, C.; Serban, B.; Avramescu, V.; Georgescu, I. Simulation of SAW gas sensors with polymer layers using the finite element method. In Proceedings of the Semiconductor Conference, Sinaia, Romania, 11–13 October 2010; pp. 503–506. [Google Scholar] [CrossRef]

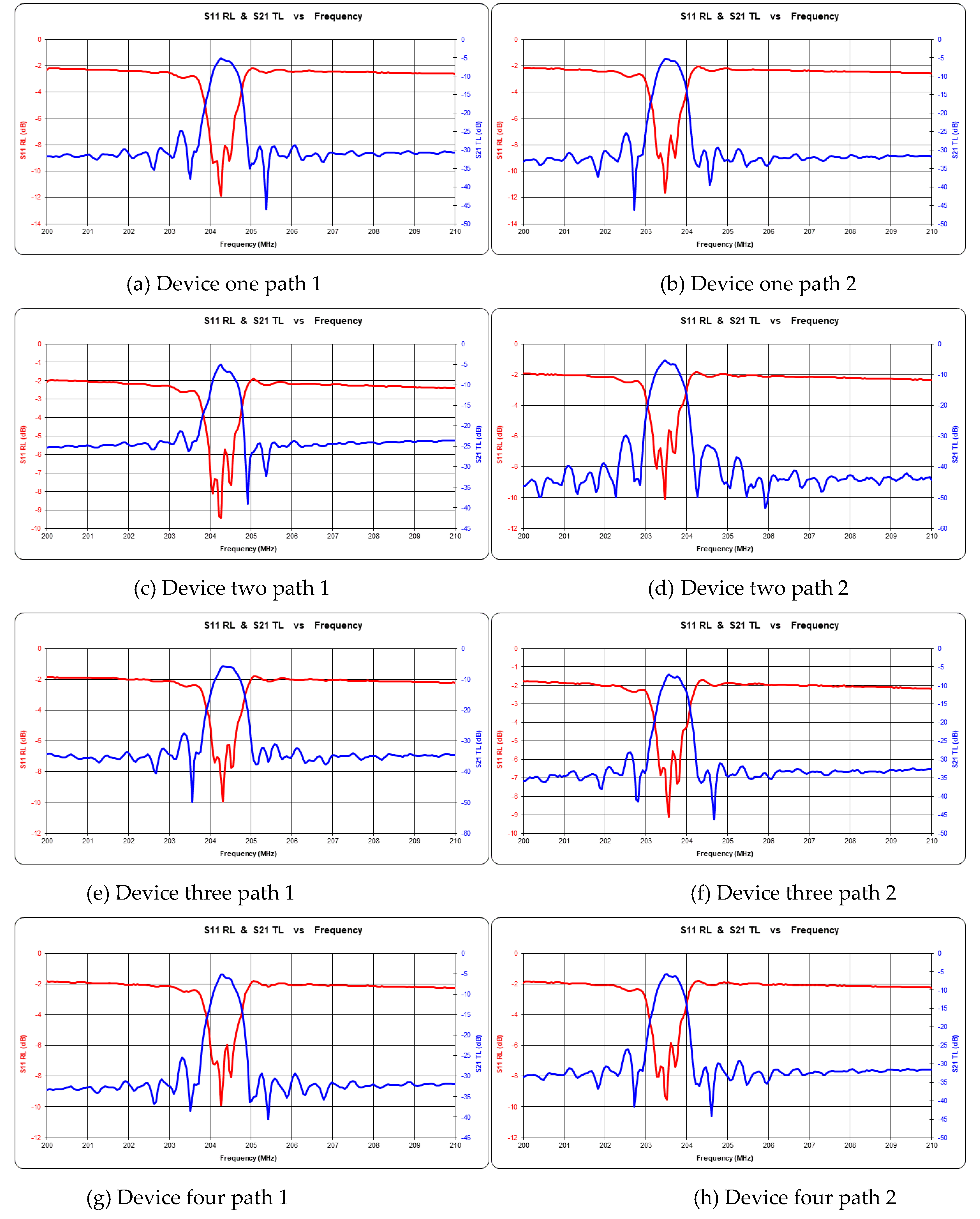

| Device | Path 1 (MHz) | Path 2 (MHz) |
|---|---|---|
| 1 | 204.26 | 203.46 |
| 2 | 204.26 | 203.46 |
| 3 | 204.31 | 203.56 |
| 4 | 204.26 | 203.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shen, B.; Jiang, L.; Yang, H.; Jin, C.; Zhou, T. Study on SAW Methane Sensor Based on Cryptophane-A Composite Film. Micromachines 2023, 14, 266. https://doi.org/10.3390/mi14020266
Liu X, Shen B, Jiang L, Yang H, Jin C, Zhou T. Study on SAW Methane Sensor Based on Cryptophane-A Composite Film. Micromachines. 2023; 14(2):266. https://doi.org/10.3390/mi14020266
Chicago/Turabian StyleLiu, Xinlei, Bin Shen, Leiming Jiang, Haiyang Yang, Chunbo Jin, and Tianshun Zhou. 2023. "Study on SAW Methane Sensor Based on Cryptophane-A Composite Film" Micromachines 14, no. 2: 266. https://doi.org/10.3390/mi14020266
APA StyleLiu, X., Shen, B., Jiang, L., Yang, H., Jin, C., & Zhou, T. (2023). Study on SAW Methane Sensor Based on Cryptophane-A Composite Film. Micromachines, 14(2), 266. https://doi.org/10.3390/mi14020266







