Perovskite Light-Emitting Devices Based on Solid-State Diffusion In Situ Dynamic Thermal Crystallization
Abstract
:1. Introduction
2. Experimental Section
Device Fabrication
- When the temperature has risen to the preset temperature, the substrate was placed on a suction cup, and the calibrated thermocouple was used to directly contact the substrate to measure its temperature.
- We added the precursor solution of PbBr2 to the surface of ITO/PEDOT:PSS quickly. The spin-coating speed of the PbBr2 precursor solution was 5000 rpm, and spin-coating acceleration was 2500 rpm/s. The spin-coating time was 30 s.
- The PbBr2 film was annealed at 90 °C for 30 min.
- Then, we transferred the film to the vapor thermal deposition system to deposit the CsBr.
- After preparation, the film was annealed at 170 °C for 10 min.
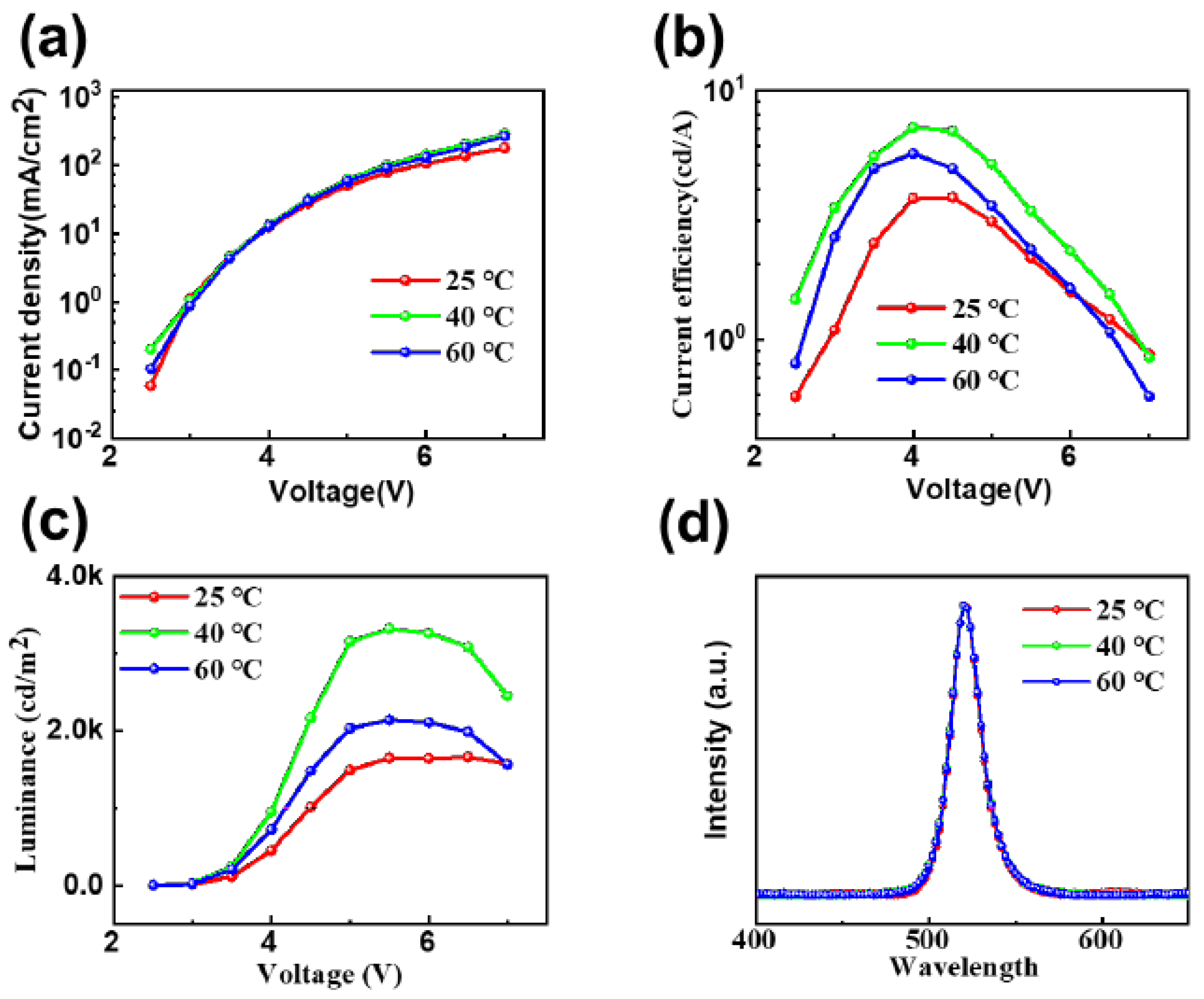
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Kerner, R.A.; Zhao, L.F.; Tran, N.L.; Lee, K.M.; Koh, T.W.; Scholes, G.D.; Rand, B.P. Efficient perovskite light-emitting diodes featuring nanometre-sized crystallites. Nat. Photonics 2017, 11, 108–115. [Google Scholar] [CrossRef]
- Wang, N.N.; Cheng, L.; Ge, R.; Zhang, S.T.; Miao, Y.F.; Zou, W.; Yi, C.; Sun, Y.; Cao, Y.; Yang, R.; et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. [Google Scholar] [CrossRef]
- Tong, G.; Li, H.; Li, D.; Zhu, Z.; Xu, E.; Li, G.; Yu, L.; Xu, J.; Jiang, Y. Dual-Phase CsPbBr3–CsPb2Br5 Perovskite Thin Films via Vapor Deposition for High-Performance Rigid and Flexible Photodetectors. Small 2018, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chang, S.P.; Ansay, A.G.; Wang, Z.H.; Yang, C.C. Ambient-Processed, Additive-Assisted CsPbBr3 Perovskite Light-Emitting Diodes with Colloidal NiOx Nanoparticles for Efficient Hole Transporting. Coatings 2020, 10, 8. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.Y.; Wang, G.R.; Wang, J.T.; Zhang, J.; Chen, P.; Li, C.N.; Duan, Y. Multifunctional tyrosine modified SnO2 to improve the performance of perovskite solar cells. Appl. Phys. Lett. 2022, 121, 073501. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.Y.; Chen, C.; Wang, J.T.; Zhang, J.; Chen, P.; Li, C.N.; Duan, Y. Stable and highly efficient perovskite solar cells: Doping hydrophobic fluoride into hole transport material PTAA. Nano Res. 2022, 15, 4431–4438. [Google Scholar] [CrossRef]
- Chen, C.; Han, T.H.; Tan, S.; Xue, J.; Zhao, Y.; Liu, Y.; Wang, H.; Hu, W.; Bao, C.; Mazzeo, M.; et al. Efficient Flexible Inorganic Perovskite Light-Emitting Diodes Fabricated with CsPbBr3 Emitters Prepared via Low-Temperature in Situ Dynamic Thermal Crystallization. Nano Lett. 2020, 20, 4673–4680. [Google Scholar] [CrossRef]
- Chen, C.; Wu, D.; Yuan, M.; Yu, C.; Zhang, J.; Li, C.N.; Duan, Y. Spectroscopic ellipsometry study of CsPbBr3 perovskite thin films prepared by vacuum evaporation. J. Phys. D Appl. Phys. 2021, 54, 224002. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y.; et al. Trifluoroacetate induced small-grained CsPbBr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Han, X.; Li, J.; Xu, Y.; Kang, L.; Wang, Y.; Yang, Y.; Yu, T. Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, L.X.; Hu, Y.S.; Guo, X.Y.; Liu, X.Y.; Geng, C.; Xu, S.; Luan, N.N.; Bi, W.G.; Wang, L.S. Synergistic morphology control and non-radiative defect passivation using a crown ether for efficient perovskite light-emitting devices. J. Mater. Chem. C 2020, 8, 9986–9992. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, H.R.; Cao, F.; Wang, S.; Wu, J.L.; Dou, Y.J.; Zhang, J.H.; Chen, J.; Zhao, D.W.; Yang, X.Y. Efficient All-Solution-Processed Perovskite Light-Emitting Diodes Enabled by Small-Molecule Doped Electron Injection Layers. Adv. Opt. Mater. 2020, 8, 1900567. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72% Efficiency. Angew. Chem. Int. Ed. Engl. 2018, 57, 3787–3791. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, J.M.; Yang, P.X.; Liu, L.L.; Yang, S.E.; Xia, T.Y.; Guo, H.Z.; Chen, Y.S. All-inorganic CsPbBr3 perovskite: A promising choice for photovoltaics. Mater. Adv. 2021, 2, 646–683. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wolf, C.; Kim, Y.T.; Cho, H.; Kwon, W.; Do, S.; Sadhanala, A.; Park, C.G.; Rhee, S.W.; Im, S.H.; et al. Highly Efficient Light-Emitting Diodes of Colloidal Metal-Halide Perovskite Nanocrystals beyond Quantum Size. ACS Nano 2017, 11, 6586–6593. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat Commun 2014, 5, 5404. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Bi, C.; Shao, Y.C.; Dong, Q.F.; Wang, Q.; Yuan, Y.B.; Wang, C.G.; Gao, Y.L.; Huang, J.S. Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution-processed precursor stacking layers. Energy Environ. Sci. 2014, 7, 2619–2623. [Google Scholar] [CrossRef]
- Ko, H.S.; Lee, J.W.; Park, N.G. 15.76% efficiency perovskite solar cells prepared under high relative humidity: Importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 2015, 3, 8808–8815. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, A.; Jacimovic, J.; Barisic, O.S.; Spina, M.; Gaal, R.; Forro, L.; Horvath, E. Ultra-Low Thermal Conductivity in Organic-Inorganic Hybrid Perovskite CH3NH3PbI3. J. Phys. Chem. Lett. 2014, 5, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.P.; Pan, X.; Zhang, X.H.; Ye, J.J.; Zhu, L.Z.; Li, Y.; Ma, Y.M.; Huang, Y.; Zhu, J.; Hu, L.H.; et al. Influence of Structure and Morphology of Perovskite Films on the Performance of Perovskite Solar Cells. Acta Chim. Sin. 2015, 73, 267–271. [Google Scholar] [CrossRef]
- Pei, Y.X.; Zou, X.P.; Qi, X.L.; Teng, G.Q.; Li, Q.; Guo, D.D.; Zeng, S.X. Effect of Perovskite Film Preparation on Performance of Solar Cells. J. Chem. 2016, 2016, 1975763. [Google Scholar] [CrossRef]

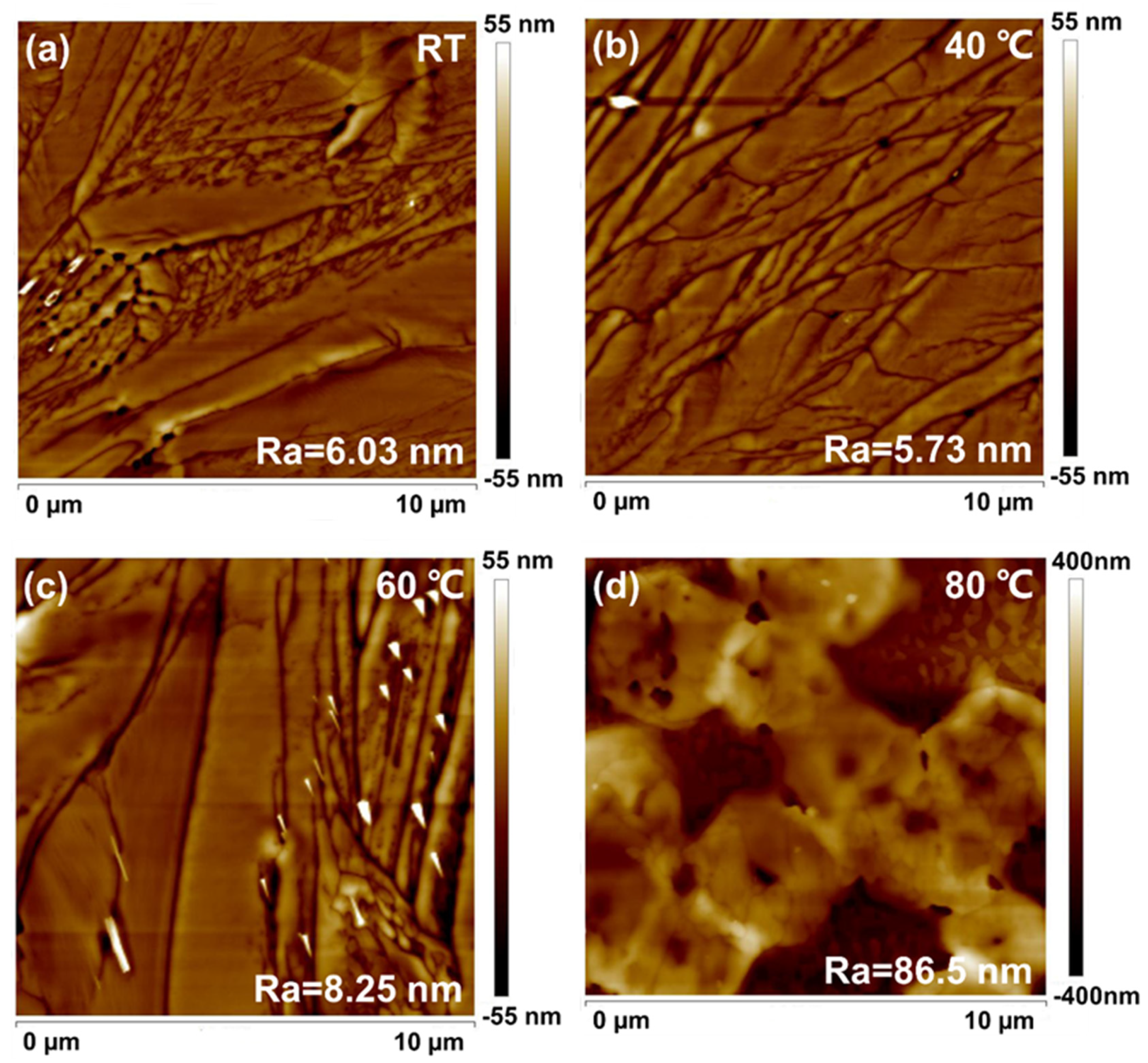
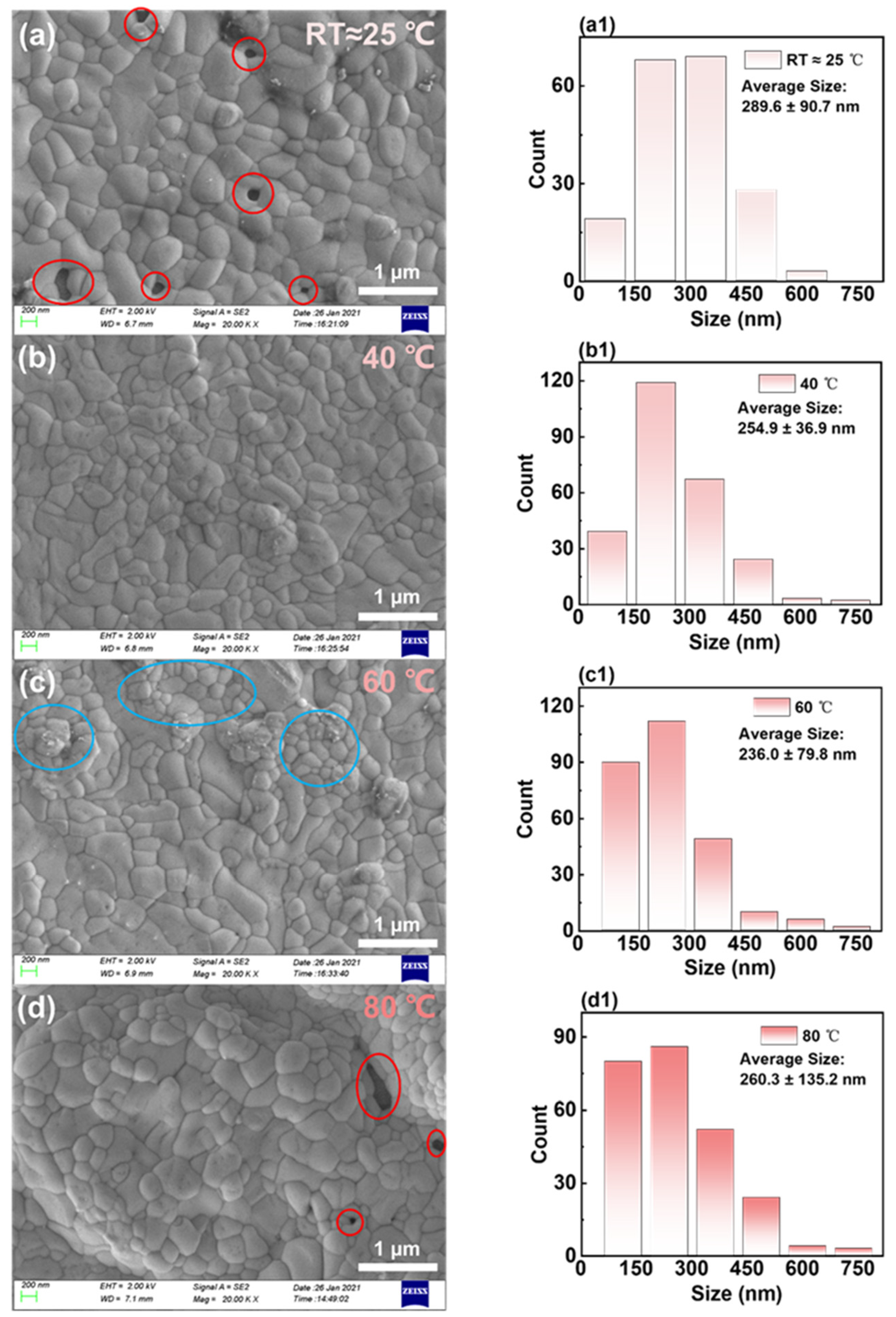
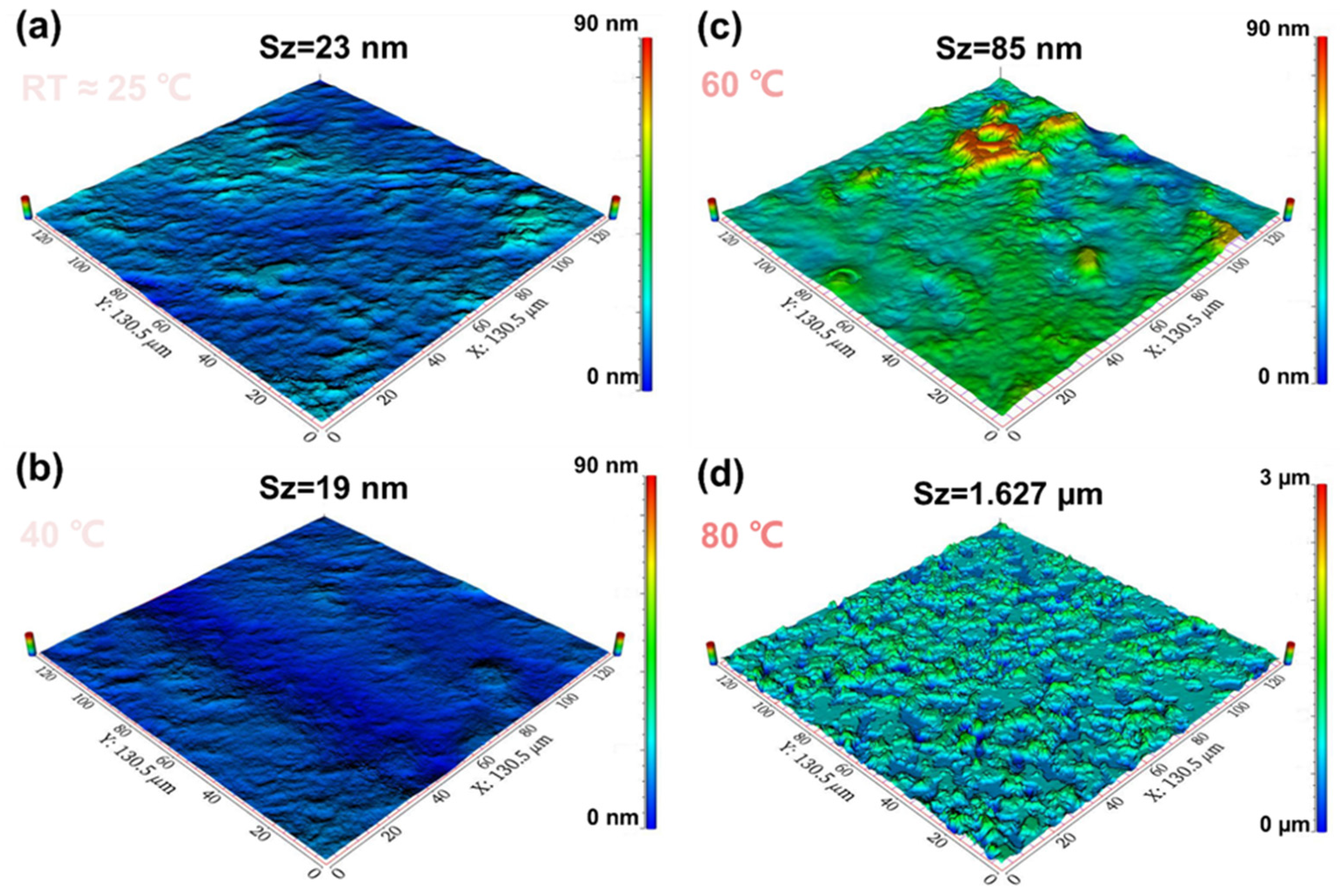

| RT | 40 °C | 60 °C | 80 °C | |
|---|---|---|---|---|
| Sa (nm) | 3 | 2 | 13 | 148 |
| Sq (nm) | 4 | 2 | 10 | 208 |
| Size(nm) | 289.6 ± 90.7 | 254.9 ± 36.9 | 236.0 ± 79.8 | 260.3 ± 135.2 |
| RT | 40 °C | 60 °C | 80 °C | |
|---|---|---|---|---|
| (020) FWHM | 0.3° | 0.18° | 0.16° | 0.12° |
| (040) FWHM | 0.3° | 0.26° | 0.22° | 0.22° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhu, Y.; Dou, K.; Liu, C.; Yu, C.; Ji, S.; Wang, J. Perovskite Light-Emitting Devices Based on Solid-State Diffusion In Situ Dynamic Thermal Crystallization. Micromachines 2023, 14, 2084. https://doi.org/10.3390/mi14112084
Chen C, Zhu Y, Dou K, Liu C, Yu C, Ji S, Wang J. Perovskite Light-Emitting Devices Based on Solid-State Diffusion In Situ Dynamic Thermal Crystallization. Micromachines. 2023; 14(11):2084. https://doi.org/10.3390/mi14112084
Chicago/Turabian StyleChen, Chen, Yanni Zhu, Kainan Dou, Chuang Liu, Chao Yu, Sihang Ji, and Jin Wang. 2023. "Perovskite Light-Emitting Devices Based on Solid-State Diffusion In Situ Dynamic Thermal Crystallization" Micromachines 14, no. 11: 2084. https://doi.org/10.3390/mi14112084
APA StyleChen, C., Zhu, Y., Dou, K., Liu, C., Yu, C., Ji, S., & Wang, J. (2023). Perovskite Light-Emitting Devices Based on Solid-State Diffusion In Situ Dynamic Thermal Crystallization. Micromachines, 14(11), 2084. https://doi.org/10.3390/mi14112084






