Synthesis, Structural, Optical, and Electrical Characterization of Biochitosan/Na0.5Bi0.5TiO3 Composite Thin-Film Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NBTO Powder
2.2. Preparation of Chitosan Polymer
2.2.1. Demineralization
2.2.2. Deproteinization
2.2.3. Decoloration
2.2.4. Deacetylation
2.3. Preparation of Films
2.4. Characterizations
3. Results and Discussion
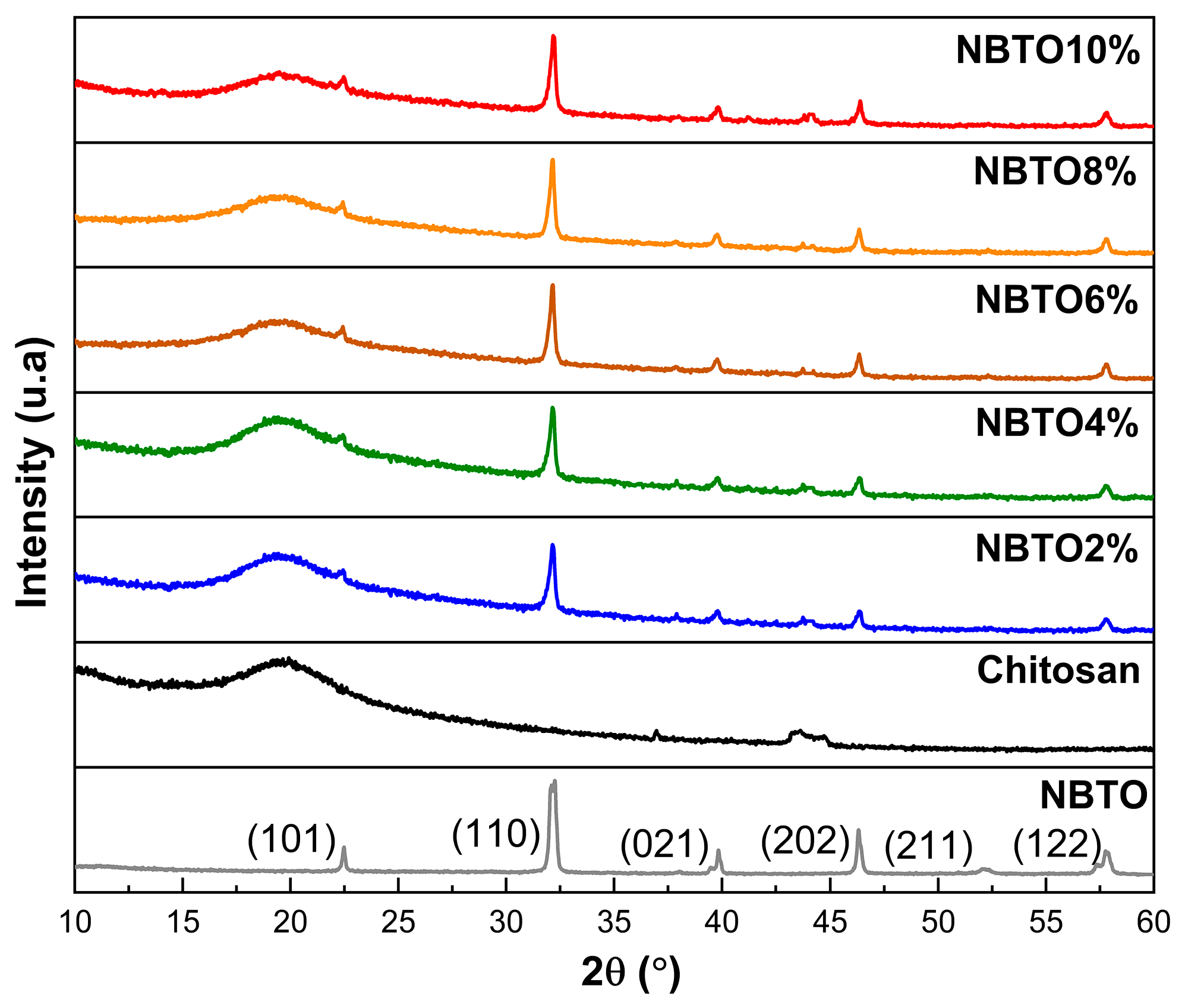
3.1. Structural Investigations
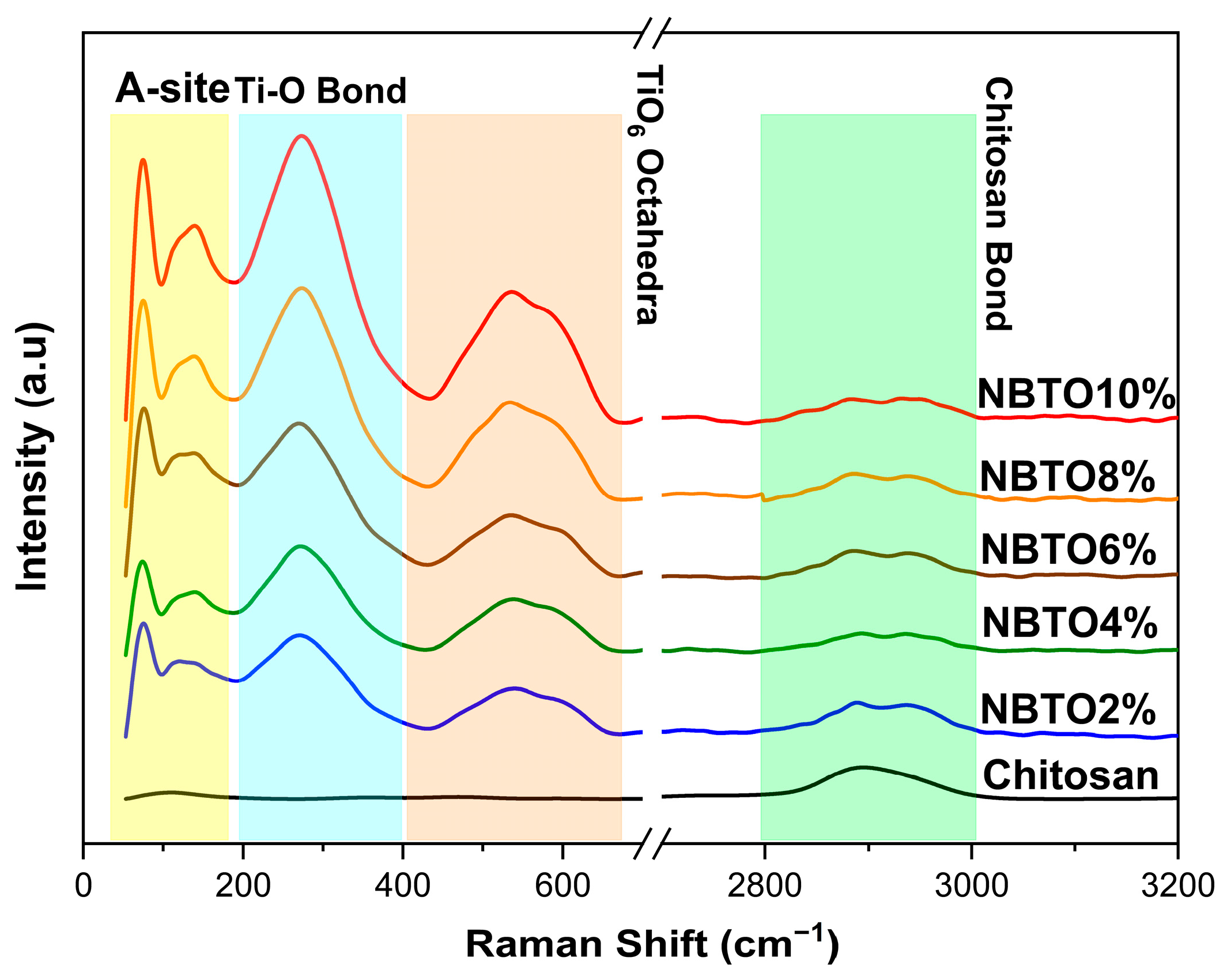
3.2. Vibrational Investigations
3.3. Microstructural Analysis
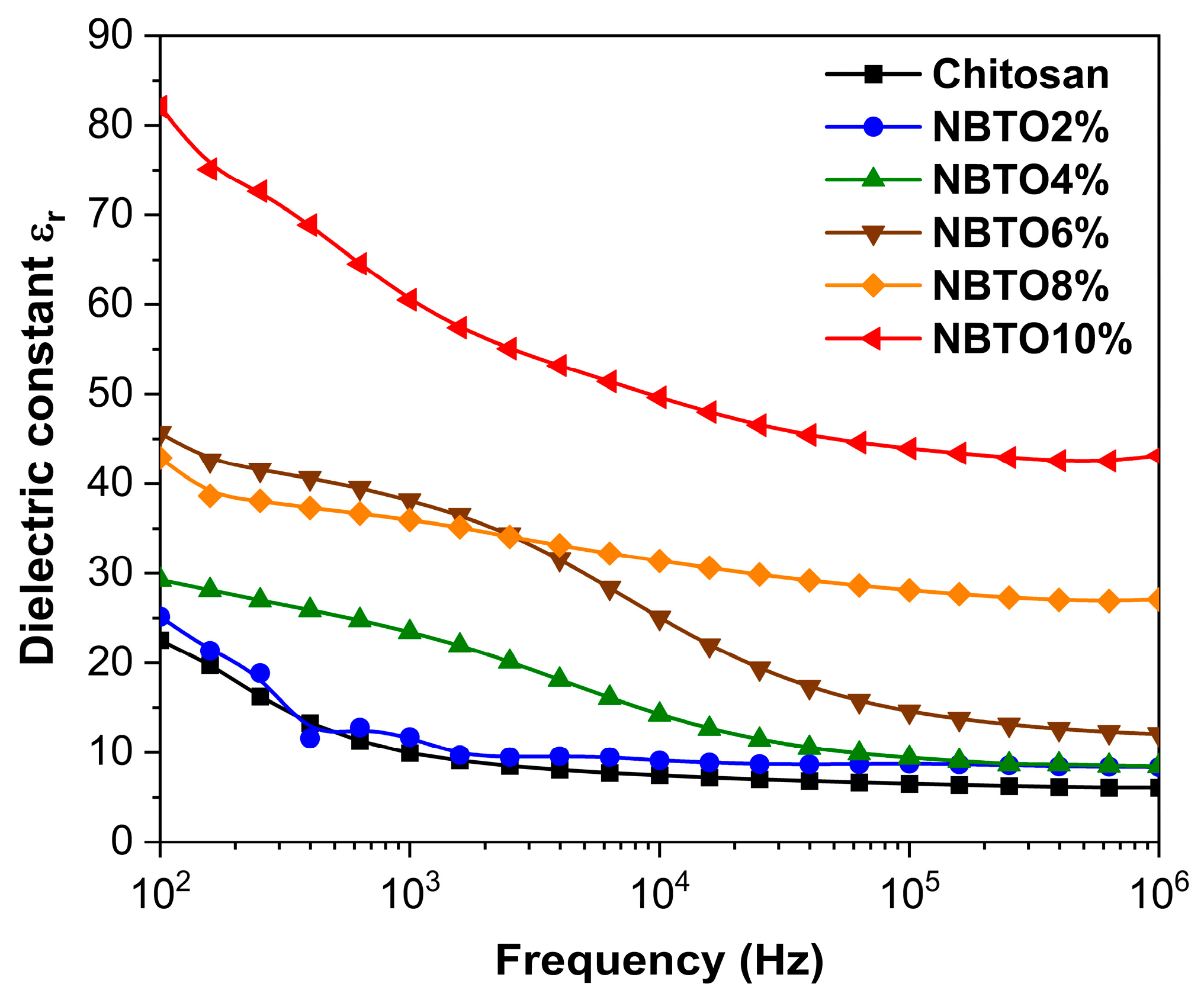
3.4. Dielectric Studies
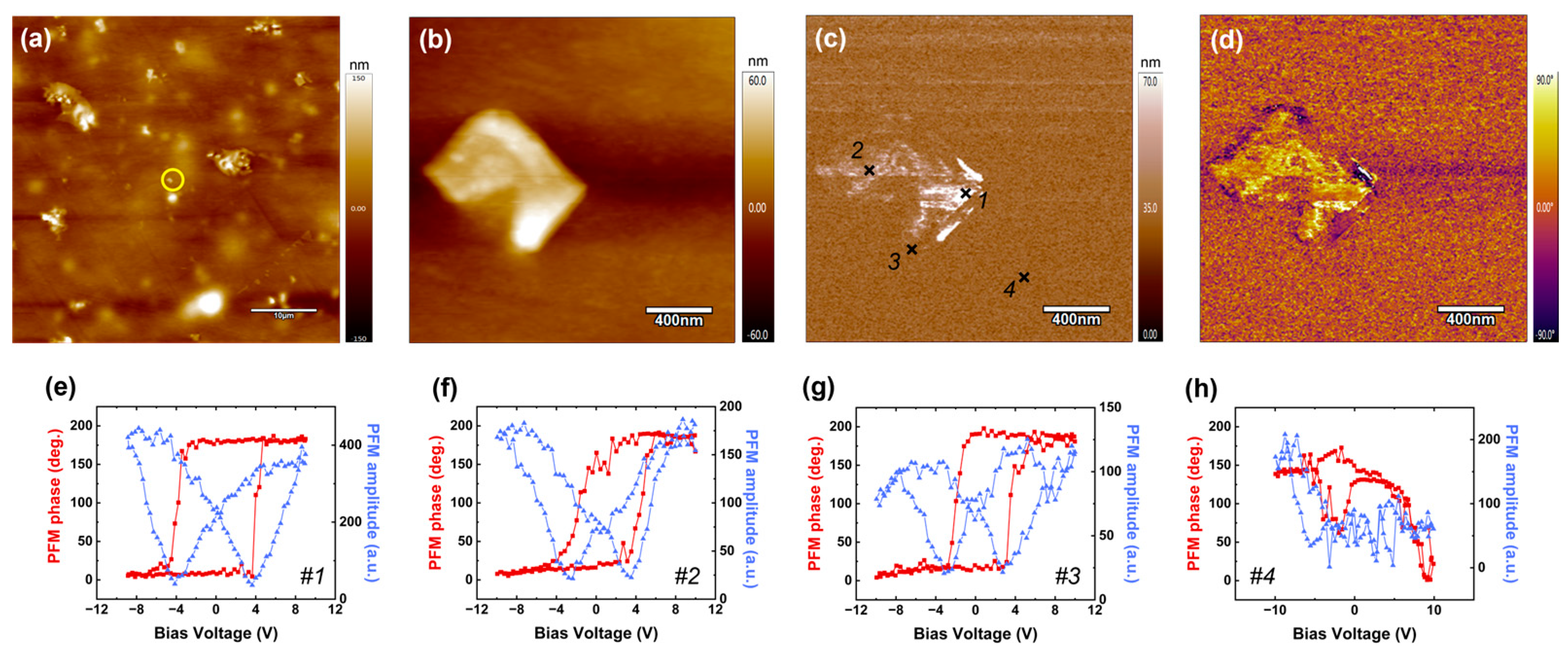
3.5. Local Piezoelectric Responses
3.6. Optical Investigations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondal, S.; Paul, T.; Maiti, S.; Das, K.K. Chattopadhyay, Human motion interactive mechanical energy harvester based on all inorganic perovskite-PVDF. Nano Energy 2020, 74, 104870. [Google Scholar] [CrossRef]
- Dudem, B.; Kim, D.H.; Bharat, L.K.; Yu, J.S. Highly-flexible piezoelectric nanogenerators with silver nanowires and barium titanate embedded composite films for mechanical energy harvesting. Appl. Energy 2018, 230, 865–874. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Hazmi, A.T.; Ahmad, F.B.; Akmal, M.H.M.; Ralib, A.A.M.; Ali, F.B. Fungal chitosan for potential application in piezoelectric energy harvesting: Review on experimental procedure of chitosan extraction. Alex. Eng. J. 2023, 67, 105–116. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Królikowski, B.; Klimiec, E.; Chylińska, M.; Bajer, D. Advances in the study of piezoelectric polymers. Russ. Chem. Rev. 2019, 88, 749–774. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Cheng, Z.; Zhang, S. The PZT/Ni unimorph magnetoelectric energy harvester for wireless sensing applications. Energy Convers. Manag. 2019, 200, 112084. [Google Scholar] [CrossRef]
- Wankhade, S.H.; Tiwari, S.; Gaur, A.; Maiti, P. PVDF–PZT nanohybrid based nanogenerator for energy harvesting applications. Energy Rep. 2020, 6, 358–364. [Google Scholar] [CrossRef]
- Hilczer, B.; Kułek, J.; Markiewicz, E.; Kosec, M.; Malič, B. Dielectric relaxation in ferroelectric PZT–PVDF nanocomposites. J. Non-Cryst. Solids 2002, 305, 167–173. [Google Scholar] [CrossRef]
- Rödel, J.; Webber, K.G.; Dittmer, R.; Jo, W.; Kimura, M.; Damjanovic, D. Transferring lead-free piezoelectric ceramics into application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. [Google Scholar] [CrossRef]
- Akmal, M.H.M.; Ahmad, F.B. Bionanomaterial Thin Film for Piezoelectric Applications. In Advances in Nanotechnology and Its Applications; Jameel, A.T., Yaser, A.Z., Eds.; Springer: Singapore, 2020; pp. 63–82. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mandal, D. Bio-assembled, piezoelectric prawn shell made self-powered wearable sensor for non-invasive physiological signal monitoring. Appl. Phys. Lett. 2017, 110, 123701. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhao, Y. Effect of Molecular Weight, Acid, and Plasticizer on the Physicochemical and Antibacterial Properties of β-Chitosan Based Films. J. Food Sci. 2012, 77, E127–E136. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ha, M.; Choi, B.; Joo, S.H.; Kang, H.S.; Park, J.H.; Gu, B.; Park, C.; Park, C.; Kim, J.; et al. Biodegradable, electro-active chitin nanofiber films for flexible piezoelectric transducers. Nano Energy 2018, 48, 275–283. [Google Scholar] [CrossRef]
- Zamli, M.I.; Ahmad, F.B.; Akmal, M.H.M. Extraction of microbial chitosan for piezoelectric application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1045, 012037. [Google Scholar] [CrossRef]
- Hänninen, A.; Rajala, S.; Salpavaara, T.; Kellomäki, M.; Tuukkanen, S. Piezoelectric Sensitivity of a Layered Film of Chitosan and Cellulose Nanocrystals. Procedia Eng. 2016, 168, 1176–1179. [Google Scholar] [CrossRef]
- Praveen, E.; Murugan, S.; Jayakumar, K. Investigations on the existence of piezoelectric property of a bio-polymer—Chitosan and its application in vibration sensors. RSC Adv. 2017, 7, 35490–35495. [Google Scholar] [CrossRef]
- Safari, A.; Janas, V.F. Processing of fine-scale piezoelectric ceramic/polymer composites for transducer applications. Ferroelectrics 1997, 196, 187–190. [Google Scholar] [CrossRef]
- Maksimovic, M. The Role of Green Internet of Things (G-IoT) and Big Data in Making Cities Smarter, Safer and More Sustainable. Int. J. Comput. Digit. Syst. 2017, 6, 175–184. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Galed, G.; Miralles, B.; Panos, I.; Santiago, A.; Heras, A. N-Deacetylation and depolymerization reactions of chitin/chitosan: Influence of the source of chitin. Carbohydr. Polym. 2005, 62, 316–320. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arab. J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Rodriguez, B.J.; Callahan, C.; Kalinin, S.V.; Proksch, R. Dual-frequency resonance-tracking atomic force microscopy. Nanotechnology 2007, 18, 475504. [Google Scholar] [CrossRef]
- Ferri, A.; Barrau, S.; Bourez, R.; Da Costa, A.; Chambrier, M.-H.; Marin, A.; Defebvin, J.; Lefebvre, J.M.; Desfeux, R. Probing the local piezoelectric behavior in stretched barium titanate/poly(vinylidene fluoride) nanocomposites. Compos. Sci. Technol. 2020, 186, 107914. [Google Scholar] [CrossRef]
- Zannen, M.; Lahmar, A.; Dietze, M.; Khemakhem, H.; Kabadou, A.; Es-Souni, M. Structural, optical, and electrical properties of Nd-doped Na0.5Bi0.5TiO3. Mater. Chem. Phys. 2012, 134, 829–833. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.A.K.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and characterization of chitosan–silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta. 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Benyoussef, M.; Zannen, M.; Belhadi, J.; Manoun, B.; Dellis, J.-L.; Lahmar, A.; El Marssi, M. Complex impedance and Raman spectroscopy of Na0.5(Bi1-xDyx)0.5TiO3 ceramics. Ceram. Int. 2020, 46, 10979–10991. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef]
- Xu, Z.; Weng, L.; Guan, L.; Zhang, X.; Wang, X.; Wu, Z. Enhanced energy storage performance of PVDF composite films with Na0.5Bi0.5TiO3 particles. J. Mater. Sci. Mater. Electron. 2021, 32, 28129–28143. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.S.S.P.; Telles, C.B.S.; Dantas-Santos, N.; Elias, S.D.O.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In Vitro Antioxidant, Anticoagulant and Antimicrobial Activity and in Inhibition of Cancer Cell Proliferation by Xylan Extracted from Corn Cobs. Int. J. Mol. Sci. 2011, 13, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs. 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Vino, A.B.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848. Asian Pac. J. Trop. Biomed. 2012, 2, S334–S341. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and chitosan–ZnO-based complex nanoparticles: Formation, characterization and antibacterial activity. J. Mater. Chem. B 2013, 1, 1968. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Behara, S.; Ghatti, L.; Kanthamani, S.; Dumpala, M.; Thomas, T. Structural, optical, and Raman studies of Gd doped sodium bismuth titanate. Ceram. Int. 2018, 44, 12118–12124. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Dhand, V.; Rhee, K.; Park, S.-J. Study on the Effect of Silanization and Improvement in the Tensile Behavior of Graphene-Chitosan-Composite. Polymers 2015, 7, 527–551. [Google Scholar] [CrossRef]
- Alvarez-Figueroa, M.J.; Narváez-Araya, D.; Armijo-Escalona, N.; Carrasco-Flores, E.A.; González-Aramundiz, J.V. Design of Chitosan Nanocapsules with Compritol 888 ATO® for Imiquimod Transdermal Administration. Evaluation of Their Skin Absorption by Raman Microscopy. Pharm. Res. 2020, 37, 195. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, G.D.; Mahale, B.; Rao, B.N.; Senyshyn, A.; Ranjan, R. Depoling phenomena in Na0.5Bi0.5TiO3−BaTiO3: A structural perspective. Phys. Rev. B 2021, 103, 184106. [Google Scholar] [CrossRef]
- Roukos, R.; Zaiter, N.; Chaumont, D. Relaxor behaviour and phase transition of perovskite ferroelectrics-type complex oxides (1–x) Na0.5Bi0.5TiO3–xCaTiO3 system. J. Adv. Ceram. 2018, 7, 124–142. [Google Scholar] [CrossRef]
- Thangavelu, K.; Asthana, S. Monoclinic Cc-phase stabilization in magnetically diluted lead free Na1/2Bi1/2TiO3—Evolution of spin glass like behavior with enhanced ferroelectric and dielectric properties. Mater. Res. Express. 2015, 2, 096301. [Google Scholar] [CrossRef]
- De La Flor, G.; Malcherek, T.; Gorfman, S.; Mihailova, B. Structural transformations in (1 − x) Na0.5Bi0.5TiO3-x BaTiO3 single crystals studied by Raman spectroscopy. Phys. Rev. B 2017, 96, 214102. [Google Scholar] [CrossRef]
- Siny, I.G.; Husson, E.; Beny, J.M.; Lushnikov, S.G.; Rogacheva, E.A.; Syrnikov, P.P. Raman scattering in the relaxor-type ferroelectric Na1/2Bi1/2TiO3. Ferroelectrics 2000, 248, 57–78. [Google Scholar] [CrossRef]
- Datta, K.; Richter, A.; Göbbels, M.; Neder, R.B.; Mihailova, B. Atomistic origin of huge response functions at the morphotropic phase boundary of (1 − x) Na0.5Bi0.5TiO3-x BaTiO3. Phys. Rev. B 2014, 90, 064112. [Google Scholar] [CrossRef]
- Maria Joseph Raj, N.P.; Ks, A.; Khandelwal, G.; Alluri, N.R.; Kim, S.-J. A lead-free ferroelectric Bi0.5Na0.5TiO3 based flexible, lightweight nanogenerator for motion monitoring applications. Sustain. Energy Fuels 2020, 4, 5636–5644. [Google Scholar] [CrossRef]
- Rout, D.; Subramanian, V.; Hariharan, K.; Murthy, V.R.K.; Sivasubramanian, V. Raman spectroscopic study of (Pb1−xBax)(Yb1∕2Ta1∕2)O3 ceramics. J. Appl. Phys. 2005, 98, 103503. [Google Scholar] [CrossRef]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron(iii) complex as promoter for alkyl oxidation reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Popielarz, R. Polymer Composites with High Dielectric Constant. Ferroelectrics 2002, 275, 1–9. [Google Scholar] [CrossRef]
- Kondawar, S.B.; Dahegaonkar, A.D.; Tabhane, V.A.; Nandanwar, D.V. Thermal And Frequency Dependance Dielectric Properties Of Conducting Polymer/Fly Ash Composites. Adv. Mater. Lett. 2014, 5, 360–365. [Google Scholar] [CrossRef]
- Devi, P.I.; Ramachandran, K. Dielectric studies on hybridised PVDF–ZnO nanocomposites. J. Exp. Nanosci. 2011, 6, 281–293. [Google Scholar] [CrossRef]
- Qureshi, A.; Mergen, A.; Eroğlu, M.S.; Singh, N.L.; Güllüoğlu, A. Dielectric Properties of Polymer Composites Filled with Different Metals. J. Macromol. Sci. Part A 2008, 45, 462–469. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Bousquet, M.; Duclère, J.-R.; Orhan, E.; Boulle, A.; Bachelet, C.; Champeaux, C. Optical properties of an epitaxial Na0.5Bi0.5TiO3 thin film grown by laser ablation: Experimental approach and density functional theory calculations. J. Appl. Phys. 2010, 107, 104107. [Google Scholar] [CrossRef]
- Evenschor, M.; Grosse, P.; Theiss, W. Optics of two-phase composites. Vib. Spectrosc. 1990, 1, 173–177. [Google Scholar] [CrossRef]
- Theiß, W. The Use of Effective Medium Theories in Optical Spectroscopy. In Advances in Solid State Physics 33; Helbig, R., Ed.; Springer: Berlin, Heidelberg, 1993; pp. 149–176. [Google Scholar] [CrossRef]
- WTheiss Hardware and Software. n.d. Available online: https://www.wtheiss.com/ (accessed on 6 September 2023).
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Badawi, A.; Alharthi, S.S.; Althobaiti, M.G.; Alharbi, A.N. The effect of iron oxide content on the structural and optical parameters of polyvinyl alcohol/graphene nanocomposite films. J. Vinyl. Addit. Technol. 2022, 28, 235–246. [Google Scholar] [CrossRef]
- Badawi, A. Enhancement of the optical properties of PVP using Zn1−xSnxS for UV-region optical applications. Appl. Phys. A 2021, 127, 51. [Google Scholar] [CrossRef]
- Alharthi, S.S.; Badawi, A. Modification of the Structure and Linear/Nonlinear Optical Characteristics of PVA/Chitosan Blend through CuO Doping for Eco-Friendly Applications. Polymers 2023, 15, 2391. [Google Scholar] [CrossRef] [PubMed]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M. Effect of nano CdS/Mg on linear and nonlinear optical characteristic of PVA/PVP/PEG film. J. Mater. Sci. Mater. Electron. 2022, 33, 17235–17248. [Google Scholar] [CrossRef]

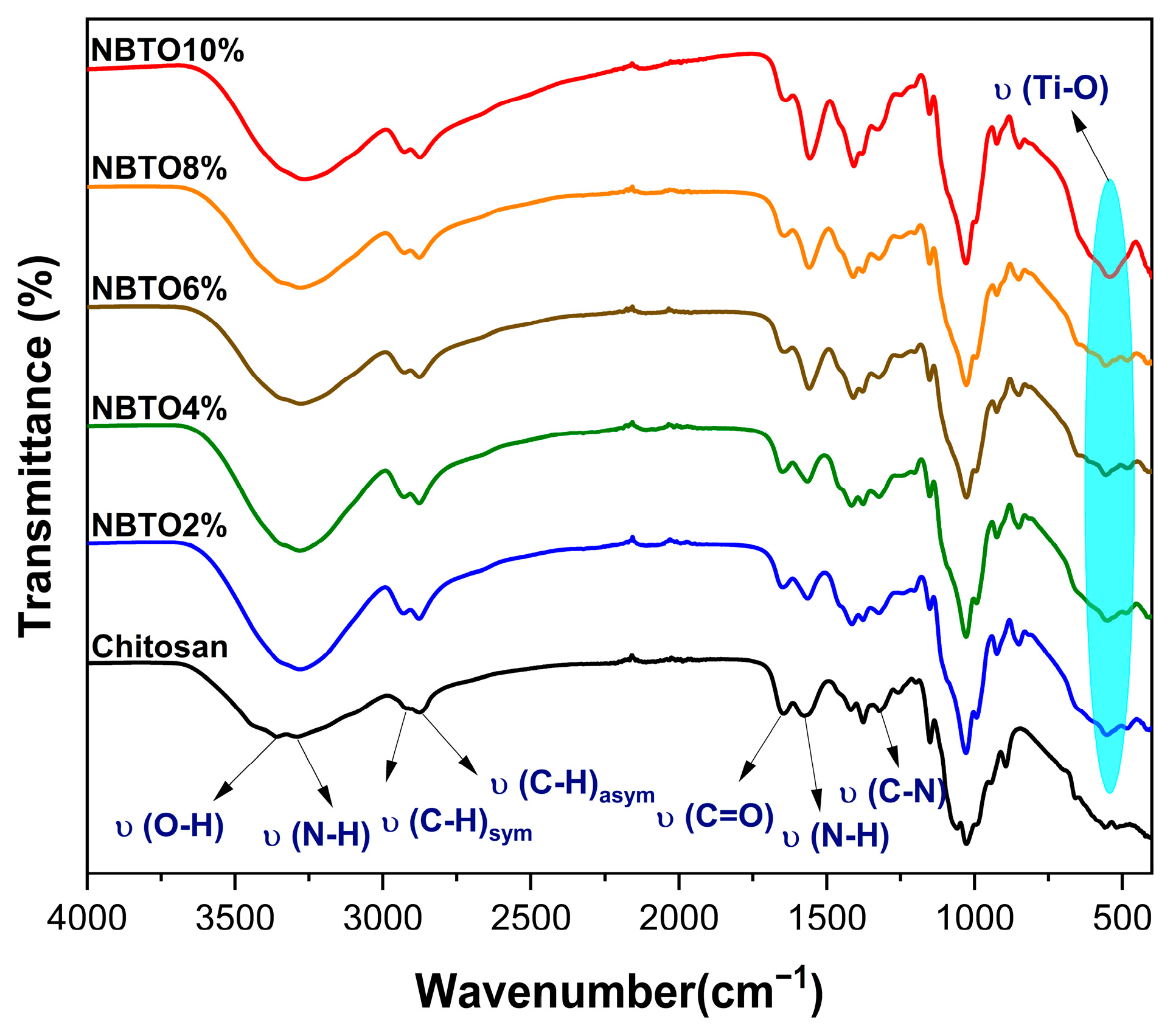




| Wave Number (cm−1) | Band Vibration |
|---|---|
| 3361 | O–H stretching |
| 3286 | N–H stretching |
| 2916 | C–H symmetric |
| 2864 | C–H asymmetric stretching |
| 1650 | C=O stretching |
| 1562 | N–H bending |
| 1317 | C–N stretching |
| 541 | Ti–O stretching |
| Nanocomposites | Egdir. (eV) | Egindir. (eV) |
|---|---|---|
| Chitosan (CS) | 5.81 | 4.94 |
| CS/NBTO2% | 5.78 | 4.90 |
| CS/NBTO4% | 5.75 | 4.82 |
| CS/NBTO6% | 5.68 | 4.78 |
| CS/NBTO8% | 5.65 | 4.74 |
| CS/NBTO10% | 5.57 | 4.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidani, J.; Hassine, K.; Zannen, M.; Zeinert, A.; Da Costa, A.; Ferri, A.; Belhadi, J.; Majdoub, M.; El Marssi, M.; Lahmar, A. Synthesis, Structural, Optical, and Electrical Characterization of Biochitosan/Na0.5Bi0.5TiO3 Composite Thin-Film Materials. Micromachines 2023, 14, 1841. https://doi.org/10.3390/mi14101841
Zidani J, Hassine K, Zannen M, Zeinert A, Da Costa A, Ferri A, Belhadi J, Majdoub M, El Marssi M, Lahmar A. Synthesis, Structural, Optical, and Electrical Characterization of Biochitosan/Na0.5Bi0.5TiO3 Composite Thin-Film Materials. Micromachines. 2023; 14(10):1841. https://doi.org/10.3390/mi14101841
Chicago/Turabian StyleZidani, Jacem, Khaoula Hassine, Moneim Zannen, Andreas Zeinert, Antonio Da Costa, Anthony Ferri, Jamal Belhadi, Mustapha Majdoub, Mimoun El Marssi, and Abdelilah Lahmar. 2023. "Synthesis, Structural, Optical, and Electrical Characterization of Biochitosan/Na0.5Bi0.5TiO3 Composite Thin-Film Materials" Micromachines 14, no. 10: 1841. https://doi.org/10.3390/mi14101841
APA StyleZidani, J., Hassine, K., Zannen, M., Zeinert, A., Da Costa, A., Ferri, A., Belhadi, J., Majdoub, M., El Marssi, M., & Lahmar, A. (2023). Synthesis, Structural, Optical, and Electrical Characterization of Biochitosan/Na0.5Bi0.5TiO3 Composite Thin-Film Materials. Micromachines, 14(10), 1841. https://doi.org/10.3390/mi14101841








