Surface Modification of 3D Printed Microfluidic Devices for Controlled Wetting in Two-Phase Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Custom 3D Printer
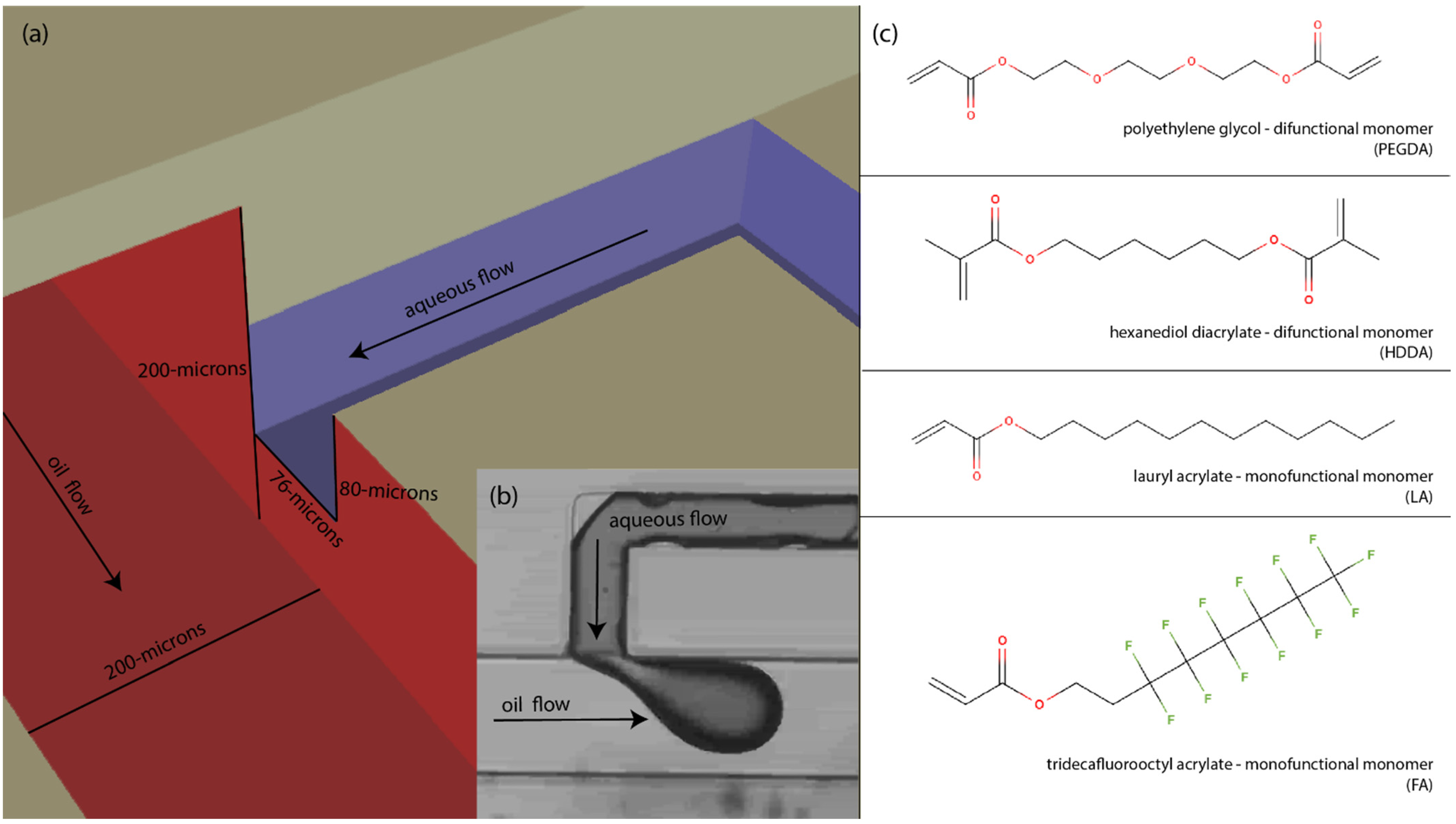
2.2. Materials
2.3. Optical System
2.4. 3D Printed T-Junction
2.5. Acrylate Monomer Treatment and Optical Exposure
3. Results and Discussion
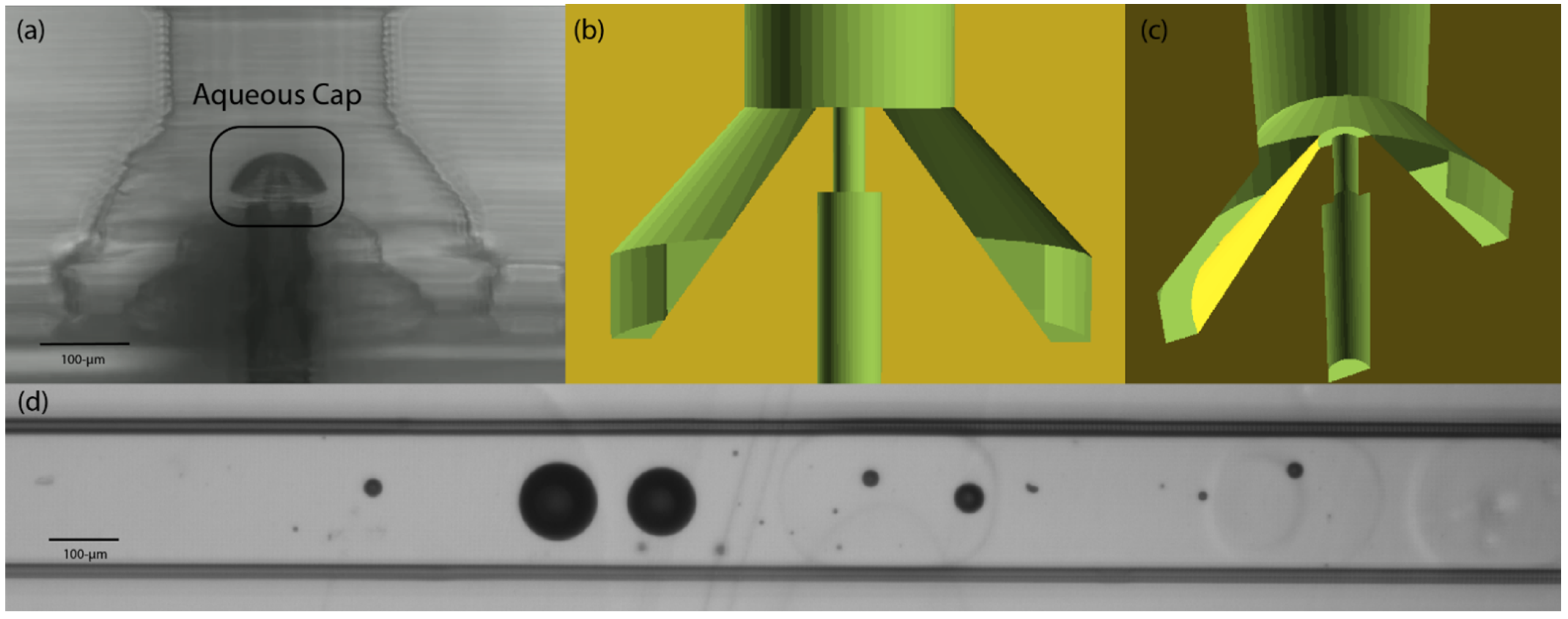
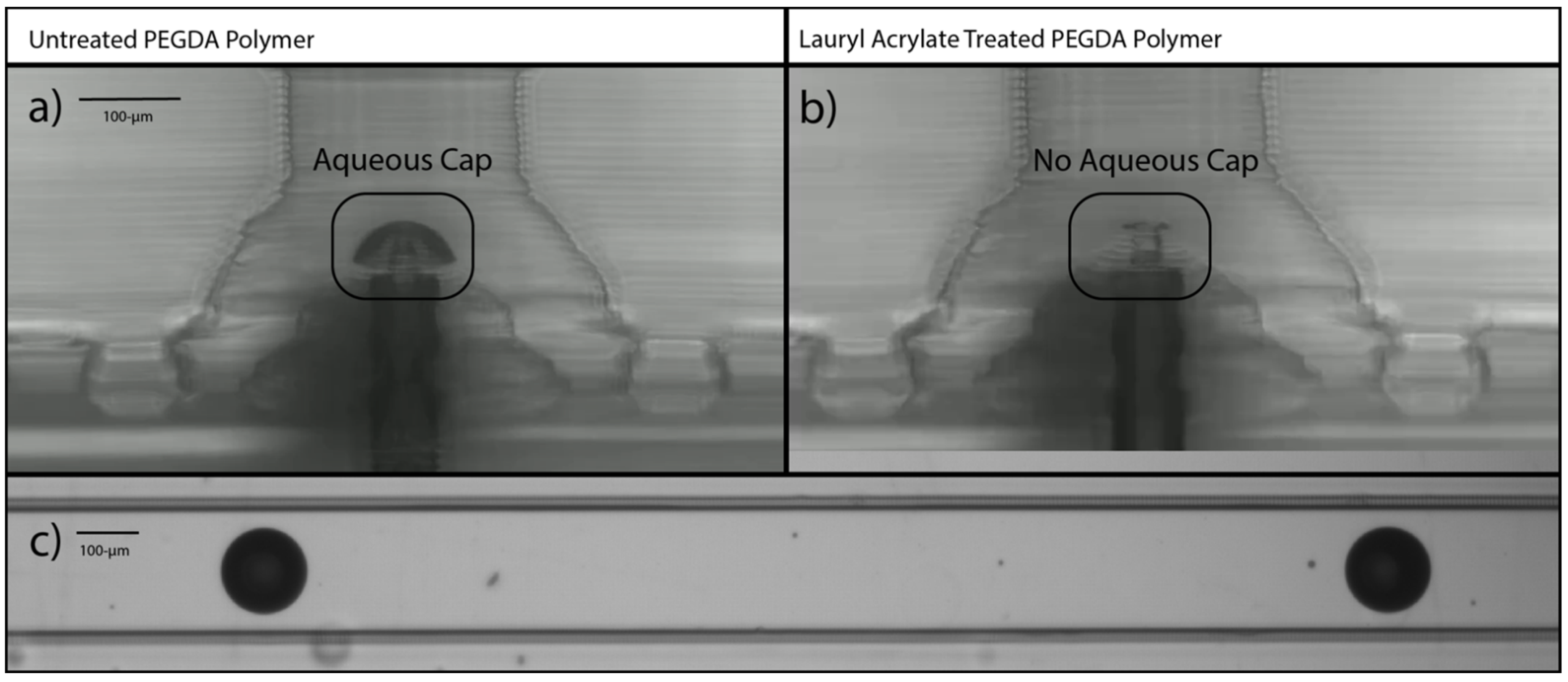
3.1. Treatment Conditions Using Acrylate Monomers with Residual Initiator
3.2. Application in Annular Channel-in-Channel Droplet Generator
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgment
Conflicts of Interest
References
- Eduati, F.; Utharala, R.; Madhavan, D.; Neumann, U.P.; Longerich, T.; Cramer, T.; Saez-Rodriguez, J.; Merten, C.A. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 2018, 9, 2434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cherukury, H.; Labanieh, L.; Zhao, W.; Kang, D.K. Rapid Detection of beta-Lactamase-Producing Bacteria Using the Integrated Comprehensive Droplet Digital Detection (IC 3D) System. Sensors 2020, 20, 4667. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 mum × 20 mum microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Noriega, J.L.; Chartrand, N.A.; Valdoz, J.C.; Cribbs, C.G.; Jacobs, D.A.; Poulson, D.; Viglione, M.S.; Woolley, A.T.; Van Ry, P.M.; Christensen, K.A.; et al. Spatially and optically tailored 3D printing for highly miniaturized and integrated microfluidics. Nat. Commun. 2021, 12, 5509. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chidambara, V.A.; Andreasen, S.Z.; Golabi, M.; Huynh, V.N.; Linh, Q.T.; Bang, D.D.; Wolff, A. Point-of-care devices for pathogen detections: The three most important factors to realise towards commercialization. TrAC-Trends Anal. Chem. 2020, 131, 116004. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. 3D printed high density, reversible, chip-to-chip microfluidic interconnects. Lab Chip 2018, 18, 639–647. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H.; Zhang, Y.V. Microfluidic systems in clinical diagnosis. Electrophoresis 2022, 1–29. [Google Scholar] [CrossRef]
- Warr, C.A.; Hinnen, H.S.; Avery, S.; Cate, R.J.; Nordin, G.P.; Pitt, W.G. 3D-Printed Microfluidic Droplet Generator with Hydrophilic and Hydrophobic Polymers. Micromachines 2021, 12, 91. [Google Scholar] [CrossRef]
- Agnihotri, S.N.; Ugolini, G.S.; Sullivan, M.R.; Yang, Y.; De Ganzo, A.; Lim, J.W.; Konry, T. Droplet microfluidics for functional temporal analysis and cell recovery on demand using microvalves: Application in immunotherapies for cancer. Lab Chip 2022, 22, 3258–3267. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Liu, F.; Chen, Y.; Yao, J.; Li, Z.; Huang, Y.; Wang, J. Comparative Analysis of Droplet-Based Ultra-High-Throughput Single-Cell RNA-Seq Systems. Mol. Cell 2019, 73, 130–142.e135. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Mach, K.E.; Zhang, P.; Liao, J.C.; Wang, T.H. Combating Antimicrobial Resistance via Single-Cell Diagnostic Technologies Powered by Droplet Microfluidics. Acc. Chem. Res. 2022, 55, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Postek, W.; Garstecki, P. Droplet Microfluidics for High-Throughput Analysis of Antibiotic Susceptibility in Bacterial Cells and Populations. Acc. Chem. Res. 2022, 55, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Ben Arye, T.; Avesar, J.; Kang, J.H.; Fine, A.; Super, M.; Meller, A.; Ingber, D.E.; Levenberg, S. Stationary nanoliter droplet array with a substrate of choice for single adherent/nonadherent cell incubation and analysis. Proc. Natl. Acad. Sci. USA 2014, 111, 11293–11298. [Google Scholar] [CrossRef]
- Gach, P.C.; Shih, S.C.; Sustarich, J.; Keasling, J.D.; Hillson, N.J.; Adams, P.D.; Singh, A.K. A Droplet Microfluidic Platform for Automating Genetic Engineering. ACS Synth. Biol. 2016, 5, 426–433. [Google Scholar] [CrossRef]
- Subramanian, B.; Kim, N.; Lee, W.; Spivak, D.A.; Nikitopoulos, D.E.; McCarley, R.L.; Soper, S.A. Surface modification of droplet polymeric microfluidic devices for the stable and continuous generation of aqueous droplets. Langmuir 2011, 27, 7949–7957. [Google Scholar] [CrossRef]
- Taylor, D.; Verdon, N.; Lomax, P.; Allen, R.J.; Titmuss, S. Tracking the stochastic growth of bacterial populations in microfluidic droplets. Phys. Biol. 2022, 19, 026003. [Google Scholar] [CrossRef]
- Li, H.; Torab, P.; Mach, K.E.; Surrette, C.; England, M.R.; Craft, D.W.; Thomas, N.J.; Liao, J.C.; Puleo, C.; Wong, P.K. Adaptable microfluidic system for single-cell pathogen classification and antimicrobial susceptibility testing. Proc. Natl. Acad. Sci. USA 2019, 116, 10270–10279. [Google Scholar] [CrossRef]
- Zhang, P.; Kaushik, A.M.; Hsieh, K.; Li, S.; Lewis, S.; Mach, K.E.; Liao, J.C.; Carroll, K.C.; Wang, T.H. A Cascaded Droplet Microfluidic Platform Enables High-Throughput Single Cell Antibiotic Susceptibility Testing at Scale. Small Methods 2022, 6, e2101254. [Google Scholar] [CrossRef]
- Zhang, P.; Kaushik, A.; Hsieh, K.; Wang, T.H. Customizing droplet contents and dynamic ranges via integrated programmable picodroplet assembler. Microsyst. Nanoeng. 2019, 5, 22. [Google Scholar] [CrossRef]
- Pitt, W.G. The Free Energy of Wetting. Chem. Eng. Educ. 1993, 27, 184–193. [Google Scholar]
- Ruiz-Cabello, F.J.M.; Rodríguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A. Contact Angle Hysteresis on Polymer Surfaces: An Experimental Study. J. Adhes. Sci. Technol. 2011, 1, 2039–2049. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Contact angle hysteresis explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Tretinnikov, O.N.; Ikada, Y. Dynamic Wetting and Contact Angle Hysteresis of Polymer Surfaces Studied with the Modified Wilhelmy Balance Method. Langmuir 1994, 10, 1606–1614. [Google Scholar] [CrossRef]
- Beauchamp, M.J.; Gong, H.; Woolley, A.T.; Nordin, G.P. 3D Printed Microfluidic Features Using Dose Control in X, Y, and Z Dimensions. Micromachines 2018, 9, 326. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. 3D printed selectable dilution mixer pumps. Biomicrofluidics 2019, 13, 014106. [Google Scholar] [CrossRef]
- Mannel, M.J.; Weigel, N.; Hauck, N.; Heida, T.; Thiele, J. Combining Hydrophilic and Hydrophobic Materials in 3D Printing for Fabricating Microfluidic Devices with Spatial Wettability. Adv. Mater. Technol.-Us 2021, 6, 2100094. [Google Scholar] [CrossRef]
- Ji, Q.L.; Zhang, J.M.; Liu, Y.; Li, X.Y.; Lv, P.Y.; Jin, D.P.; Duan, H.L. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef]
- Gong, H.; Beauchamp, M.; Perry, S.; Woolley, A.T.; Nordin, G.P. Optical Approach to Resin Formulation for 3D Printed Microfluidics. RSC Adv. 2015, 5, 106621–106632. [Google Scholar] [CrossRef]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; Van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA Resin for 3D Printing. ACS Appl. Bio. Mater. 2020, 3, 2239–2244. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warr, C.A.; Crawford, N.G.; Nordin, G.P.; Pitt, W.G. Surface Modification of 3D Printed Microfluidic Devices for Controlled Wetting in Two-Phase Flow. Micromachines 2023, 14, 6. https://doi.org/10.3390/mi14010006
Warr CA, Crawford NG, Nordin GP, Pitt WG. Surface Modification of 3D Printed Microfluidic Devices for Controlled Wetting in Two-Phase Flow. Micromachines. 2023; 14(1):6. https://doi.org/10.3390/mi14010006
Chicago/Turabian StyleWarr, Chandler A., Nicole G. Crawford, Gregory P. Nordin, and William G. Pitt. 2023. "Surface Modification of 3D Printed Microfluidic Devices for Controlled Wetting in Two-Phase Flow" Micromachines 14, no. 1: 6. https://doi.org/10.3390/mi14010006
APA StyleWarr, C. A., Crawford, N. G., Nordin, G. P., & Pitt, W. G. (2023). Surface Modification of 3D Printed Microfluidic Devices for Controlled Wetting in Two-Phase Flow. Micromachines, 14(1), 6. https://doi.org/10.3390/mi14010006






