Material Removal Mechanism in Photocatalytic−Assisted Jet Electrochemical Machining of SiCp/Al
Abstract
:1. Introduction
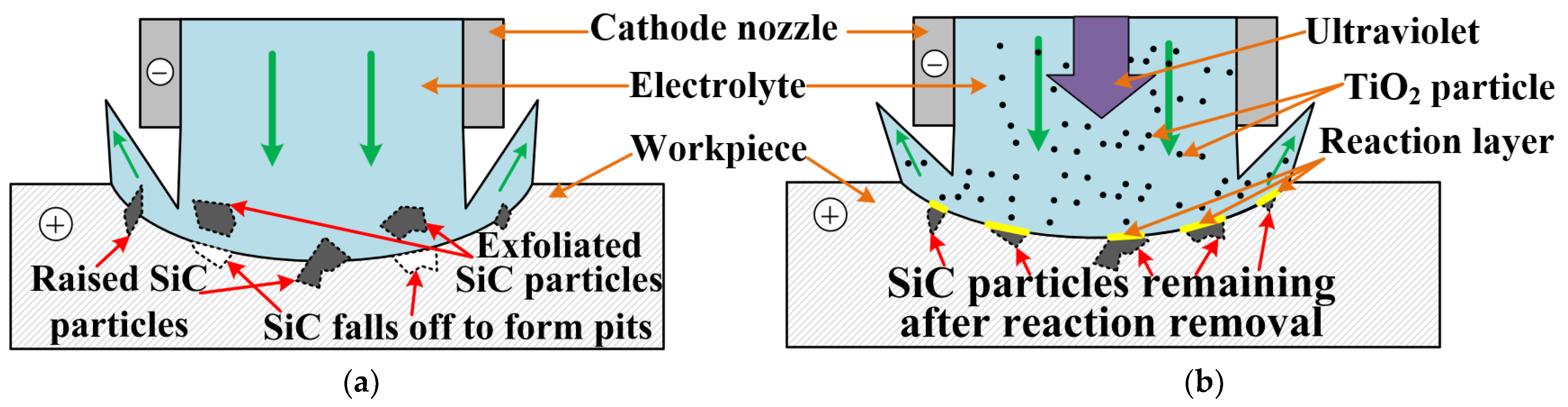
2. Principle of PAJECM Process
3. Experimental Setup
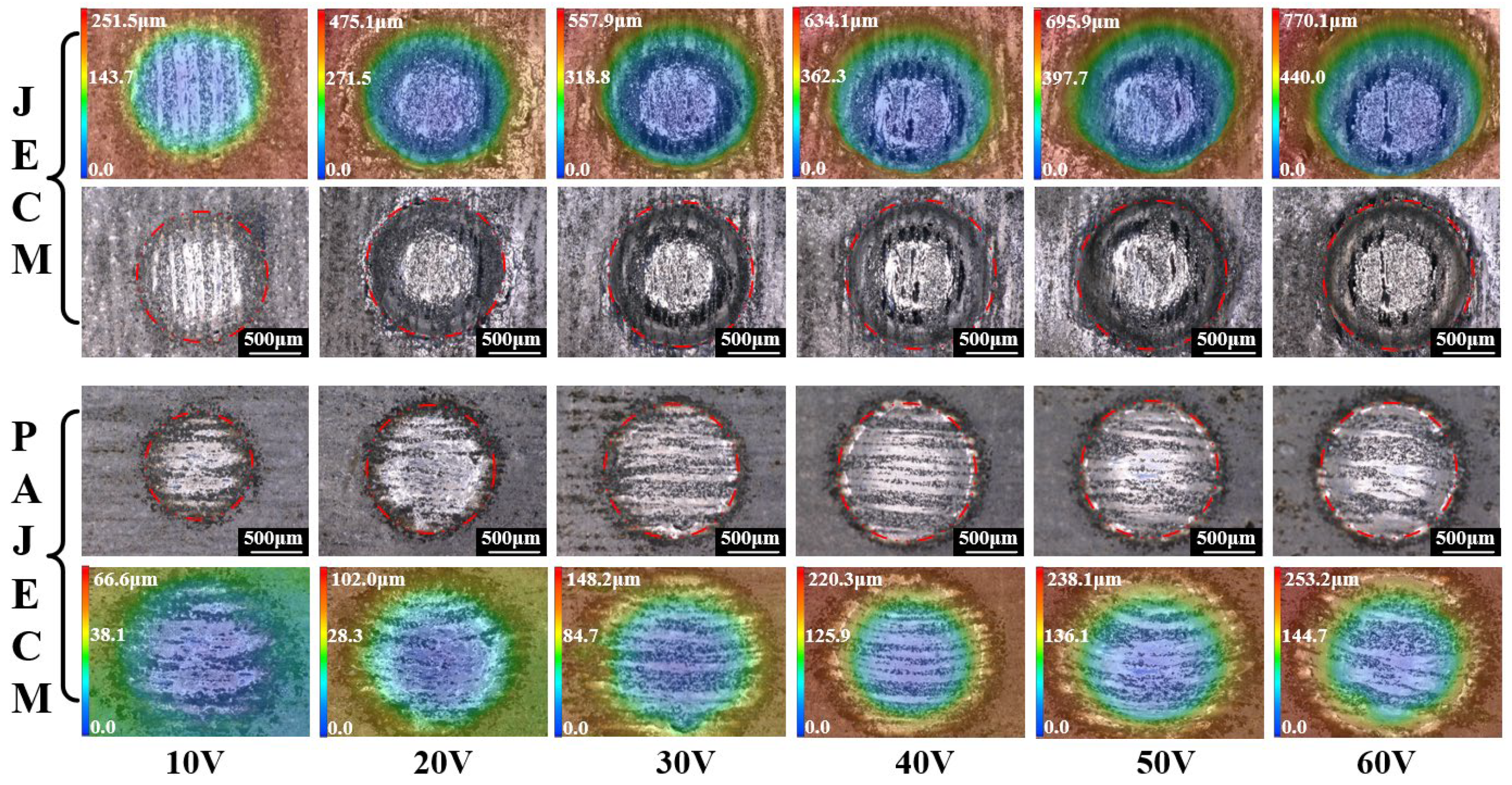
4. Experimental Results
5. Analysis and Discussion
5.1. Electrochemical Characterization Analysis
5.2. Elemental Energy Spectrum Analysis
5.3. Discussion
6. Conclusions
- (1)
- The material removal morphology of SiCp/Al in PAJECM significantly changed due to the addition of photocatalysis. The observation of the machined micro surface confirmed that the SiC particles with poor conductivity are effectively removed. Compared with JECM, the surface roughness value of PAJECM decreases from Ra 2.5 to Ra 1.5 μm when the processing voltage is 10 V, and the surface roughness value decreased from Ra 5.7 to Ra 2.7 μm when the processing voltage is 60 V. PAJECM significantly improves the surface quality of SiCp/Al machining.
- (2)
- By observing the material removal phenomenon, we found that the SiCp/Al material removal mechanism of PAJECM is significantly different from that of JECM. In PAJECM, the aluminum matrix and SiC particles are simultaneously removed. The aluminum matrix is removed by anodic dissolution. The SiC particles are removed by two steps: the hard SiC particles form a SiO2 layer under the photocatalytic reaction; the comparatively soft SiO2 layer is removed by the abrasive particle impact. In JECM, the aluminum matrix is also removed by anodic dissolution, while the SiC particles either fall off to form a pit or remain on the machined surface to form a protrusion.
- (3)
- The material removal mechanism of PAJECM was verified by its electrochemical polarization curves and the EDS results. After adding photocatalysis, the self−corrosion potential and decomposition potential of SiCp/Al significantly increase, which proves that the intense oxidizing substance •OH is generated during processing. After processing, the oxygen content on the surface is obviously increased, and the distribution of oxygen and silicon is consistent, which proves the formation of Si−containing oxide SiO2.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Gu, L.; He, G. A Review on Conventional and Nonconventional Machining of SiC Particle−Reinforced Aluminium Matrix Composites. Adv. Manuf. 2020, 8, 279–315. [Google Scholar] [CrossRef]
- Suryanarayanan, K.; Praveen, R.; Raghuraman, S. Silicon Carbide Reinforced Aluminum Metal Matrix Composites for Aerospace Applications: A Literature Review. International Journal of Innovative Research in Science. Eng. Technol. 2013, 2, 6336–6344. [Google Scholar]
- Zhang, Z.; Xiang, D.; Wu, B.; Feng, H.; Shi, Z.; Hu, Y.; Zhao, B. Finite Element Simulation Study of Ultrasonic Vibration−Assisted Tensile High−Volume Fraction SiCp/Al Composite. Materials 2019, 12, 3841. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Monaghan, J. Non−Conventional Machining of Particle Reinforced Metal Matrix Composite. Int. J. Mach. Tools Manuf. 2000, 40, 1351–1366. [Google Scholar] [CrossRef]
- Ge, Y.F.; Xu, J.H.; Yang, H.; Luo, S.B.; Fu, Y.C. Workpiece Surface Quality When Ultra−Precision Turning of SiCp/Al Composites. J. Mater. Process. Tech. 2008, 203, 166–175. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, J.; Lu, M.; Jing, X.; Jin, Y.; Song, D. Analyzing the Effect of Particle Shape on Deformation Mechanism During Cutting Simulation of SiC P/Al Composites. Micromachines 2021, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Ablyaz, T.R.; Bains, P.S.; Sidhu, S.S.; Muratov, K.R.; Shlykov, E.S. Impact of Magnetic Field Environment on the EDM Performance of Al−SiC Metal Matrix Composite. Micromachines 2021, 12, 469. [Google Scholar] [CrossRef]
- Dandekar, C.R.; Shin, Y.C. Experimental Evaluation of Laser−Assisted Machining of Silicon Carbide Particle−Reinforced Aluminum Matrix Composites. Int. J. Adv. Manuf. Technol. 2013, 66, 1603–1610. [Google Scholar] [CrossRef]
- Frank, M.; John, M. Non−Conventional Machining of Particle Reinforced Metal Matrix Composites. J. Mater. Process. Technol. 2001, 118, 278–285. [Google Scholar]
- Saxena, K.K.; Qian, J.; Reynaerts, D. A Tool−Based Hybrid Laser−Electrochemical Micromachining Process: Experimental Investigations and Synergistic Effects. Int. J. Mach. Tools Manuf. 2020, 155, 103569. [Google Scholar] [CrossRef]
- Kawanaka, T.; Kunieda, M. Mirror−Like Finishing by Electrolyte Jet Machining. CIRP Ann. 2015, 64, 237–240. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Ming, P.; Li, X.; Zeng, Y.; Cai, J. The Effect of Electrolytic Jet Orientation on Machining Characteristics in Jet Electrochemical Machining. Micromachines 2019, 10, 404. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Chen, X.; Saxena, K.K.; Liu, J.; Guo, Z. Jet Electrochemical Micromachining of Micro−Grooves with Conductive−Masked Porous Cathode. Micromachines 2020, 11, 557. [Google Scholar] [CrossRef]
- Hackert−Oschätzchen, M.; Lehnert, N.; Martin, A.; Schubert, A. Jet Electrochemical Machining of Particle Reinforced Aluminum Matrix Composites with Different Neutral Electrolytes. IOP Conf. Ser. Mater. Sci. Eng. 2016, 118, 12036. [Google Scholar] [CrossRef]
- Arumugam, R.; Rajalingam, M. A Review on the Effect of Electro Chemical Machining on Metal Matrix Composite Materials. Mater. Today Proc. 2020, 33, 3854–3857. [Google Scholar] [CrossRef]
- Schubert, A.; Götze, U.; Hackert−Oschätzchen, M.; Lehnert, N.; Herold, F.; Meichsner, G.; Schmidt, A. Evaluation of the Technical−Economic Potential of Particle−Reinforced Aluminum Matrix Composites and Electrochemical Machining. IOP Conf. Ser. Mater. Sci. Eng. 2016, 118, 12035. [Google Scholar] [CrossRef]
- Hackert−Oschätzchen, M.; Lehnert, N.; Martin, A.; Meichsner, G.; Schubert, A. Surface Characterization of Particle Reinforced Aluminum−Matrix Composites Finished by Pulsed Electrochemical Machining. Procedia CIRP 2016, 45, 351–354. [Google Scholar] [CrossRef]
- Lehnert, N.; Hackert−Oschätzchen, M.; Martin, A.; Schubert, A. Derivation of Guidelines for Reliable Finishing of Aluminium Matrix Composites by Jet Electrochemical Machining. Procedia CIRP 2018, 68, 471–476. [Google Scholar] [CrossRef]
- Ao, S.; Qin, X.; Li, K.; Luo, Z. Effects of Process Parameters on Jet Electrochemical Machining of SiC Particle−Reinforced Aluminum Matrix Composites. Int. J. Adv. Manuf. Technol. 2021, 112, 3351–3361. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, C.; Qiu, Y.; Gao, C. Electrochemical Jet Machining of High Volume Fraction SiCp/Al Composite using Electrolyte of Sodium Chloride. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 22006. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, C.; Guo, C.; Qiu, Y. Simulation and Experiments of Abrasive Assisted Electrochemical Jet Machining of SiC Reinforced Aluminum Matrix Composites. Procedia CIRP 2020, 95, 760–765. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, C.; Yu, X.; Guo, C. Improving Machining Localization in Abrasive−Assisted Electrochemical Jet Machining of SiCp/Al Composites using Large Standoff Distance. Arab. J. Sci. Eng. 2021, 47, 8629–8642. [Google Scholar] [CrossRef]
- Bao, X.H.; Ming, P.M.; Zhang, X.M.; Shen, J.W.; Qin, G.; Yan, L. Facile Preparation of Robust Superamphiphobic Surface by Electrochemical Etching Process Based on the SiC/Al Composites. J. Electrochem. Soc. 2018, 165, E563–E571. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, X.; Xiong, Q.; Lu, J.; Liao, B. The Influences of Technological Parameters on the Ultraviolet Photocatalytic Reaction Rate and Photocatalysis−Assisted Polishing Effect for SiC. J. Cryst. Growth 2020, 531, 125379. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Luo, Q.; Xu, X. Photocatalysis Assisting the Mechanical Polishing of a Single−Crystal SiC Wafer Utilizing an Anatase TiO2−Coated Diamond Abrasive. Precis. Eng. 2017, 49, 235–242. [Google Scholar] [CrossRef]
- Gao, B.; Zhai, W.; Zhai, Q.; Zhang, M. Novel polystyrene/CeO2−TiO2 Multicomponent Core/Shell Abrasives for High−Efficiency and High−Quality Photocatalytic−Assisted Chemical Mechanical Polishing of Reaction−Bonded Silicon Carbide. Appl. Surf. Sci. 2019, 484, 534–541. [Google Scholar] [CrossRef]
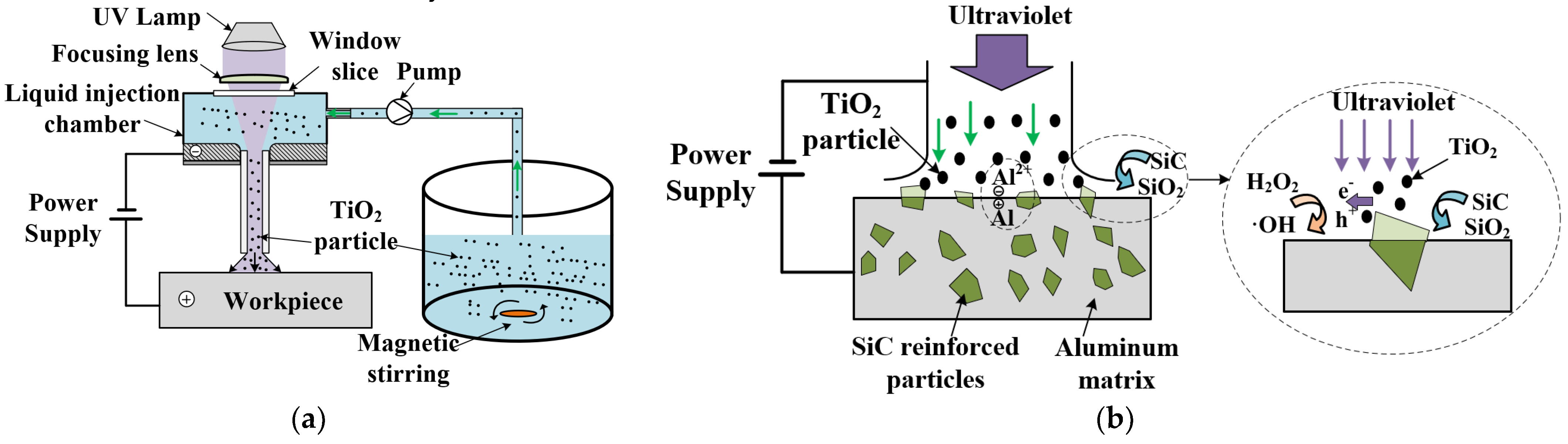

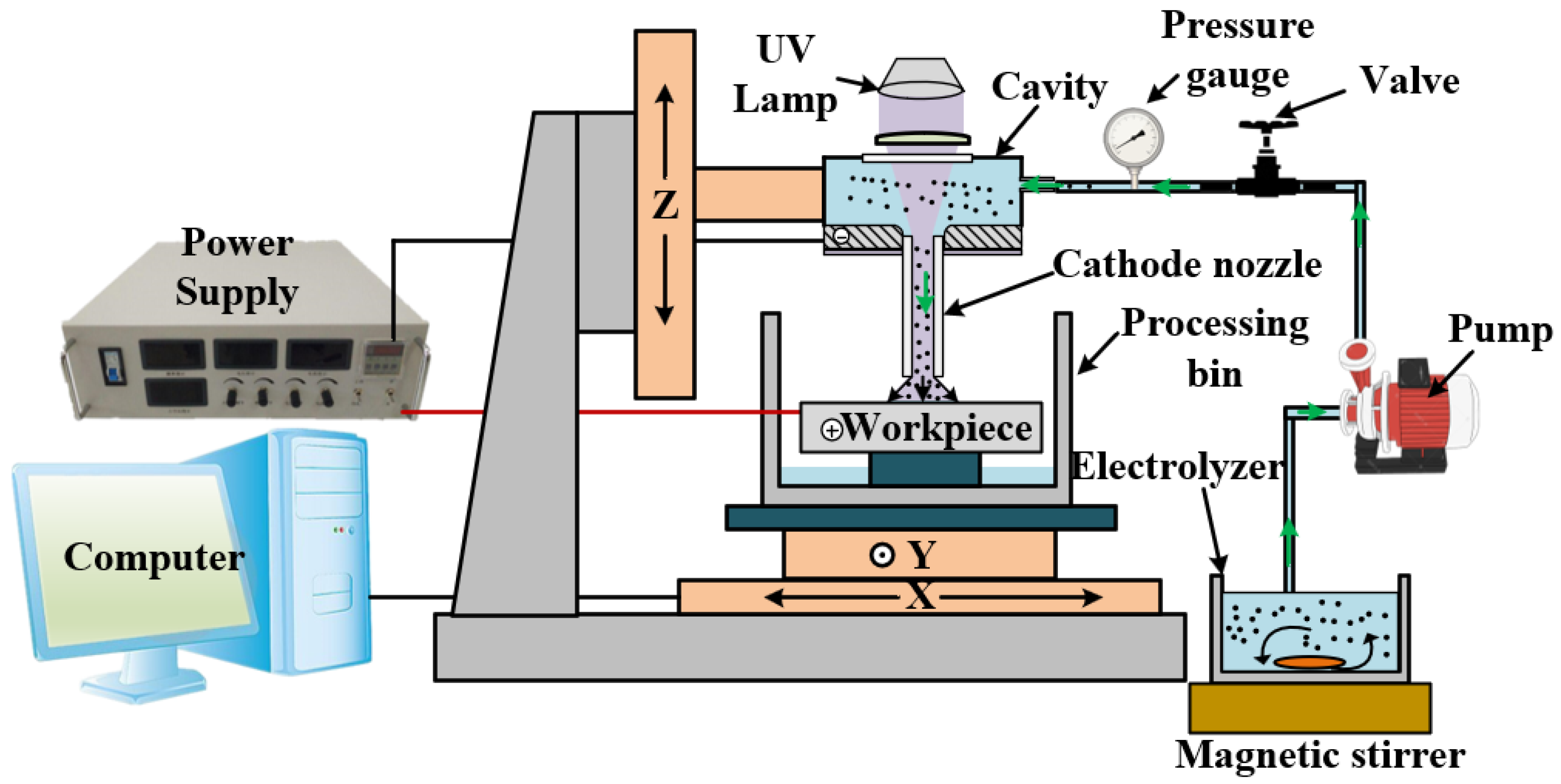
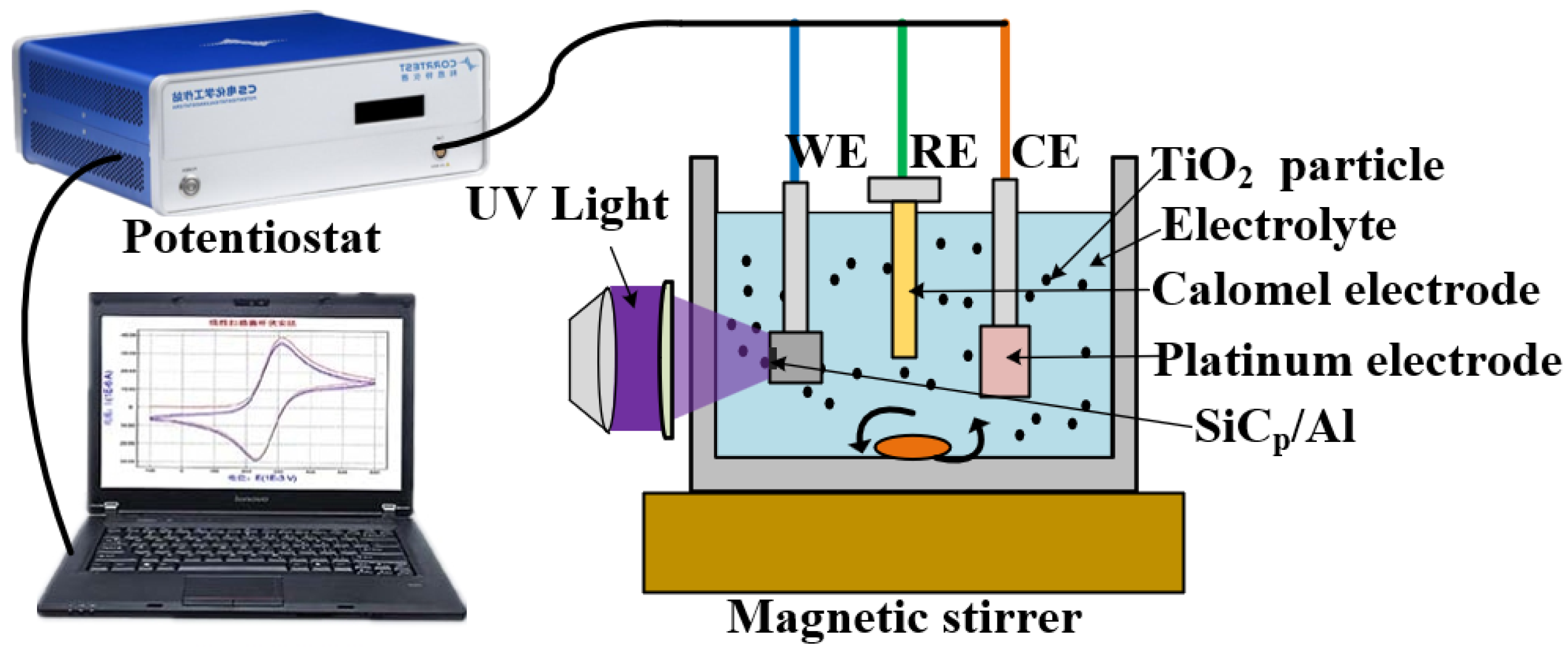

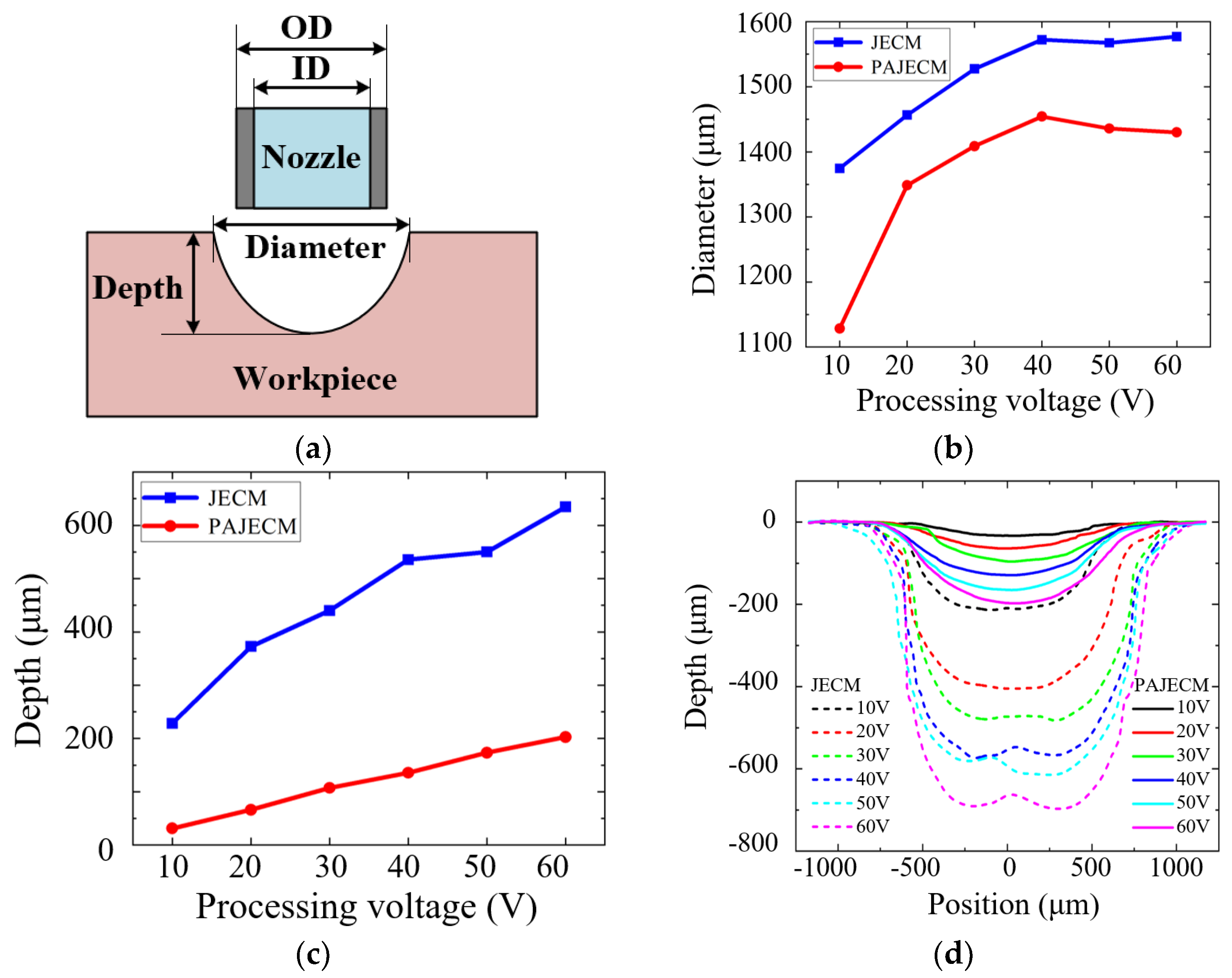
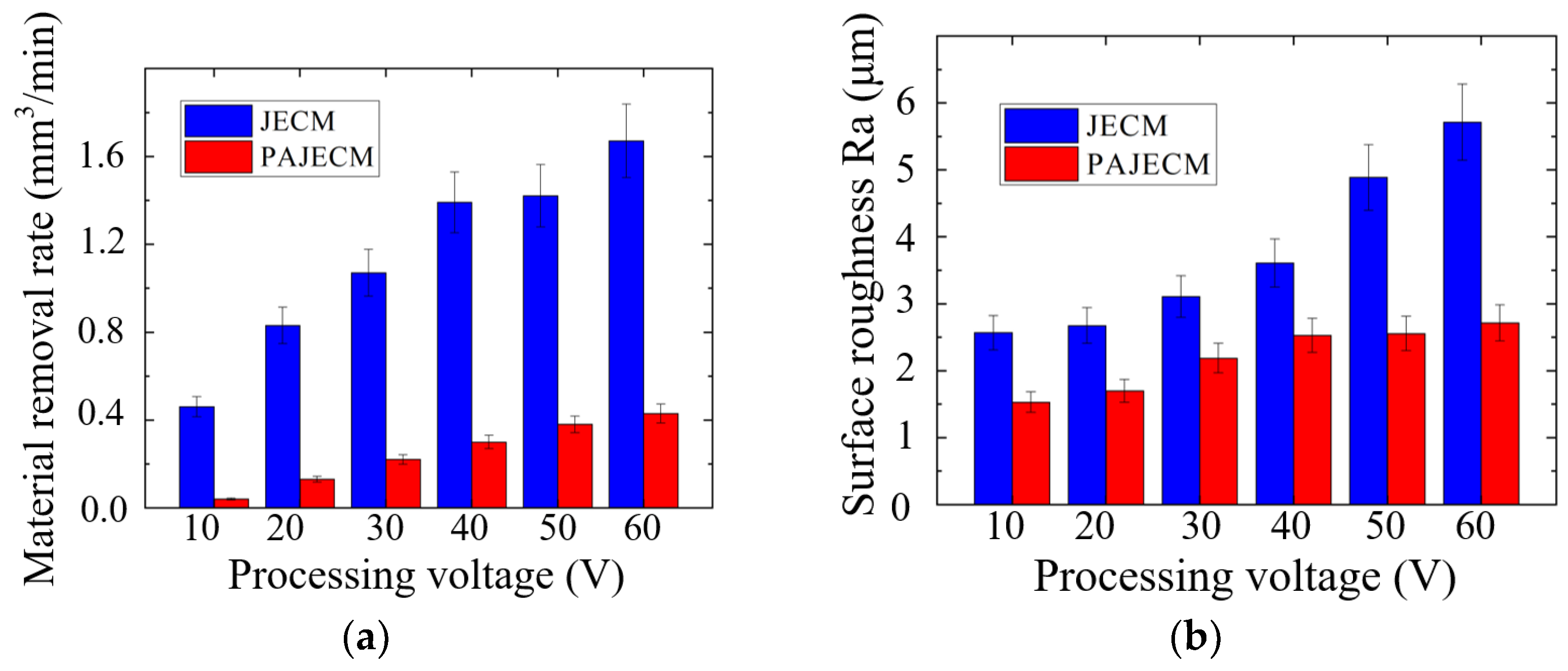

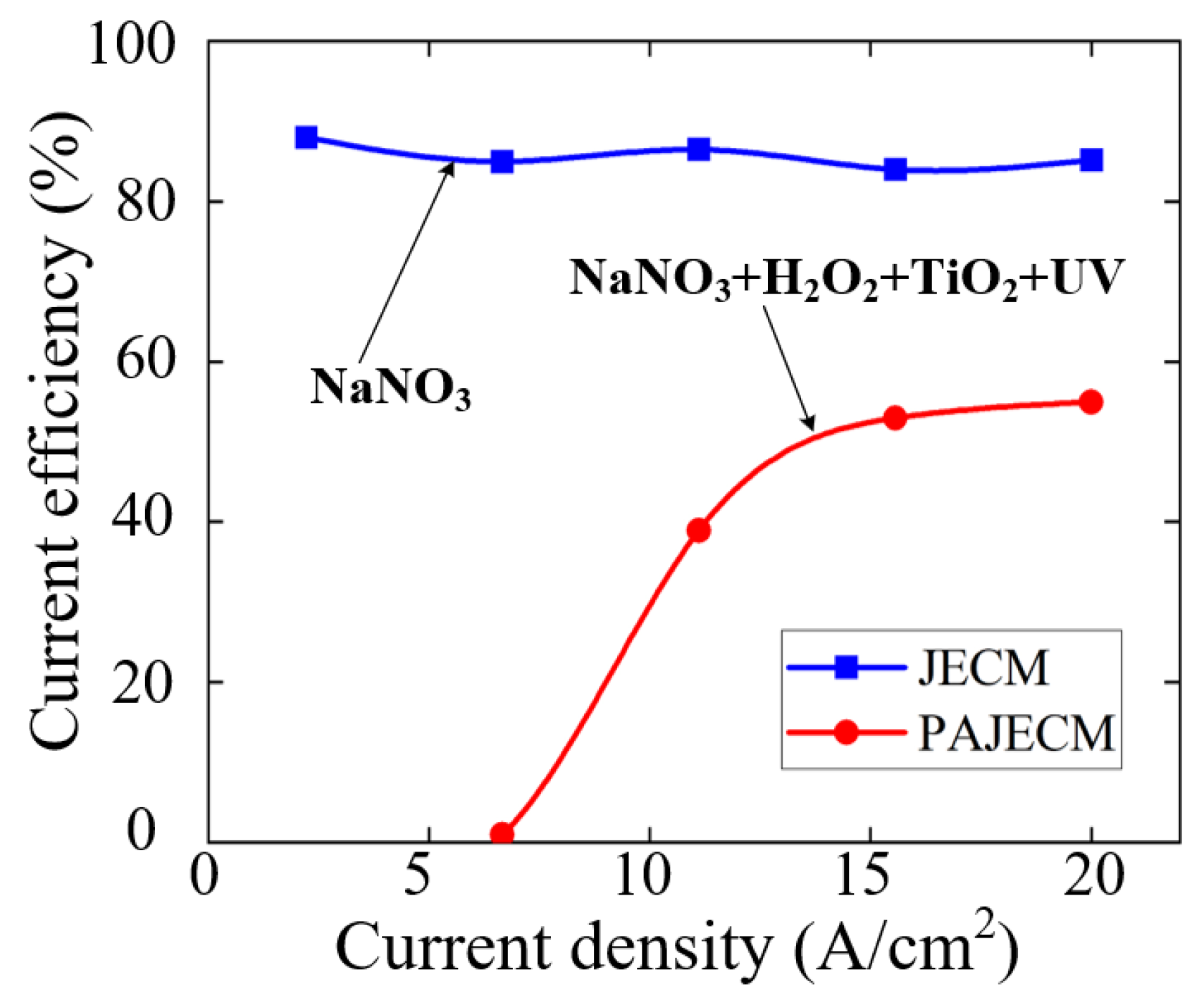
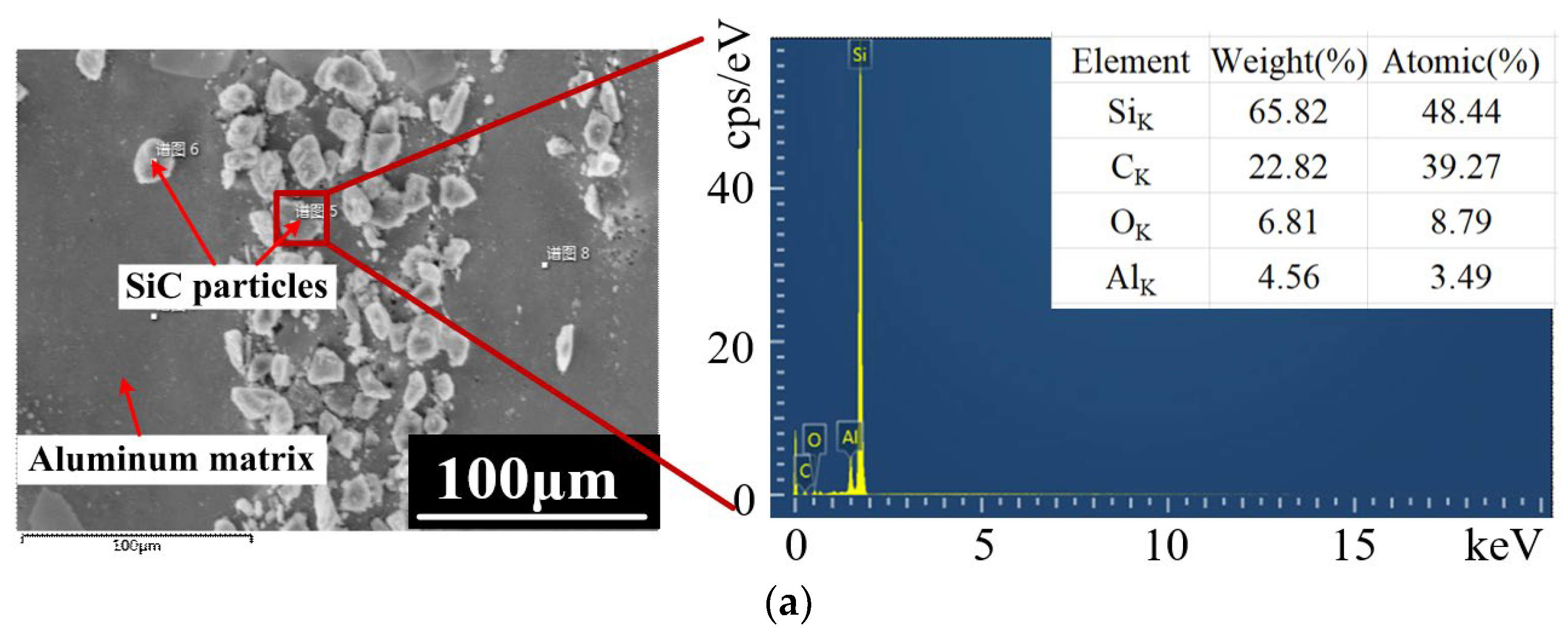
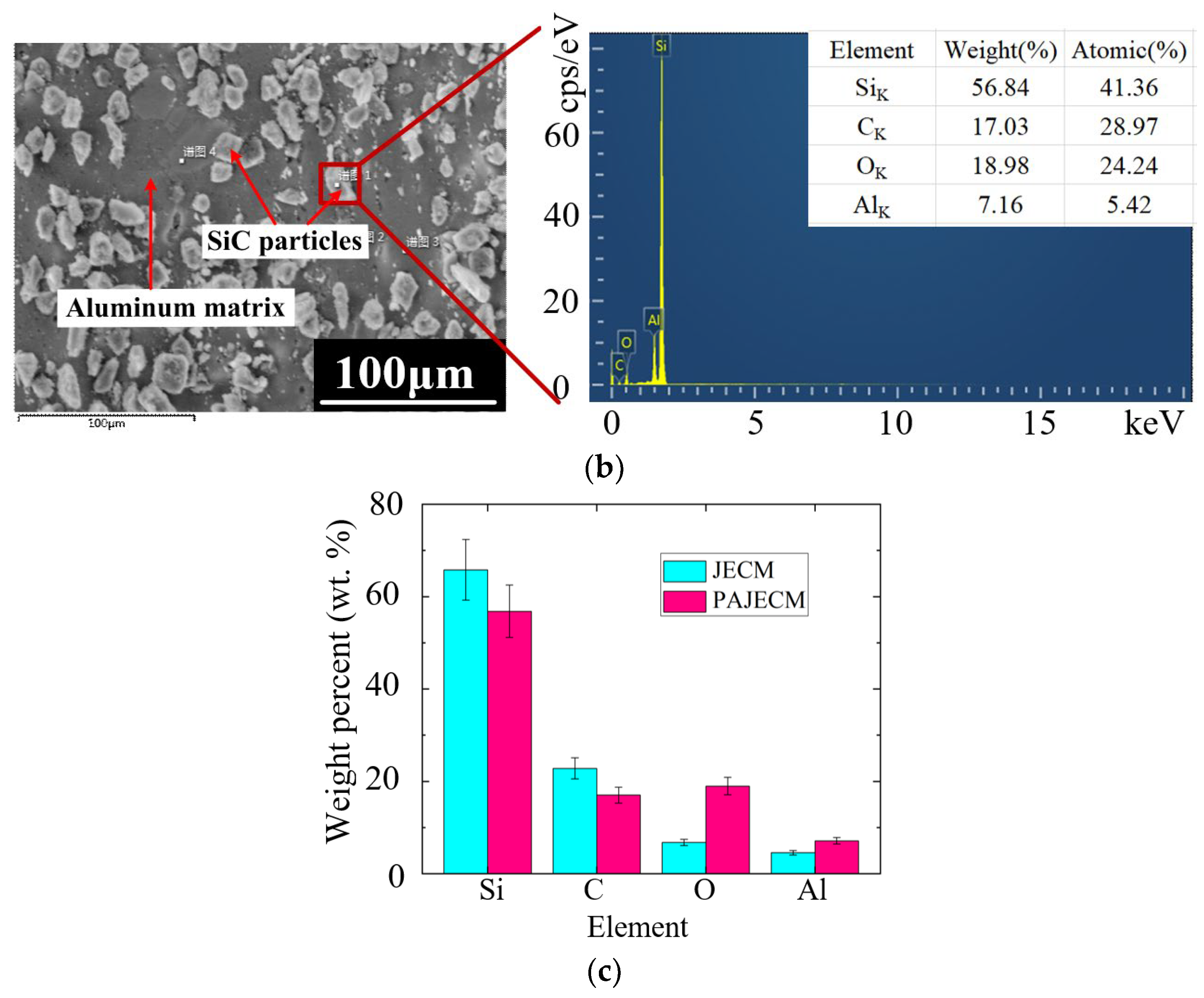

| Processing Parameters | Value |
|---|---|
| Workpiece | SiCp/Al with 20% SiC volume fraction, thickness 3 mm |
| Catalyst TiO2 concentration | 4 g/L |
| H2O2 Volume fraction | 3% |
| Electrolyte type and concentration | NaNO3, 15% |
| UV light intensity | 1500 mW/cm2, 10 W, 365 nm wavelength |
| Processing voltage | DC 10, 20, 30, 40, 50, 60 V |
| Processing time | 30 s |
| Flow rate | 200 mL/min |
| Stainless-steel capillary nozzle | Inner diameter (ID) 0.8 mm, outer diameter (OD) 1 mm |
| Inter−electrode gap | 0.3 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhou, J.; Wu, S.; Kang, X.; Gu, L.; Zhao, W. Material Removal Mechanism in Photocatalytic−Assisted Jet Electrochemical Machining of SiCp/Al. Micromachines 2022, 13, 1482. https://doi.org/10.3390/mi13091482
Wang F, Zhou J, Wu S, Kang X, Gu L, Zhao W. Material Removal Mechanism in Photocatalytic−Assisted Jet Electrochemical Machining of SiCp/Al. Micromachines. 2022; 13(9):1482. https://doi.org/10.3390/mi13091482
Chicago/Turabian StyleWang, Feng, Jing Zhou, Siyi Wu, Xiaoming Kang, Lin Gu, and Wansheng Zhao. 2022. "Material Removal Mechanism in Photocatalytic−Assisted Jet Electrochemical Machining of SiCp/Al" Micromachines 13, no. 9: 1482. https://doi.org/10.3390/mi13091482
APA StyleWang, F., Zhou, J., Wu, S., Kang, X., Gu, L., & Zhao, W. (2022). Material Removal Mechanism in Photocatalytic−Assisted Jet Electrochemical Machining of SiCp/Al. Micromachines, 13(9), 1482. https://doi.org/10.3390/mi13091482






