Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material
Abstract
:1. Introduction
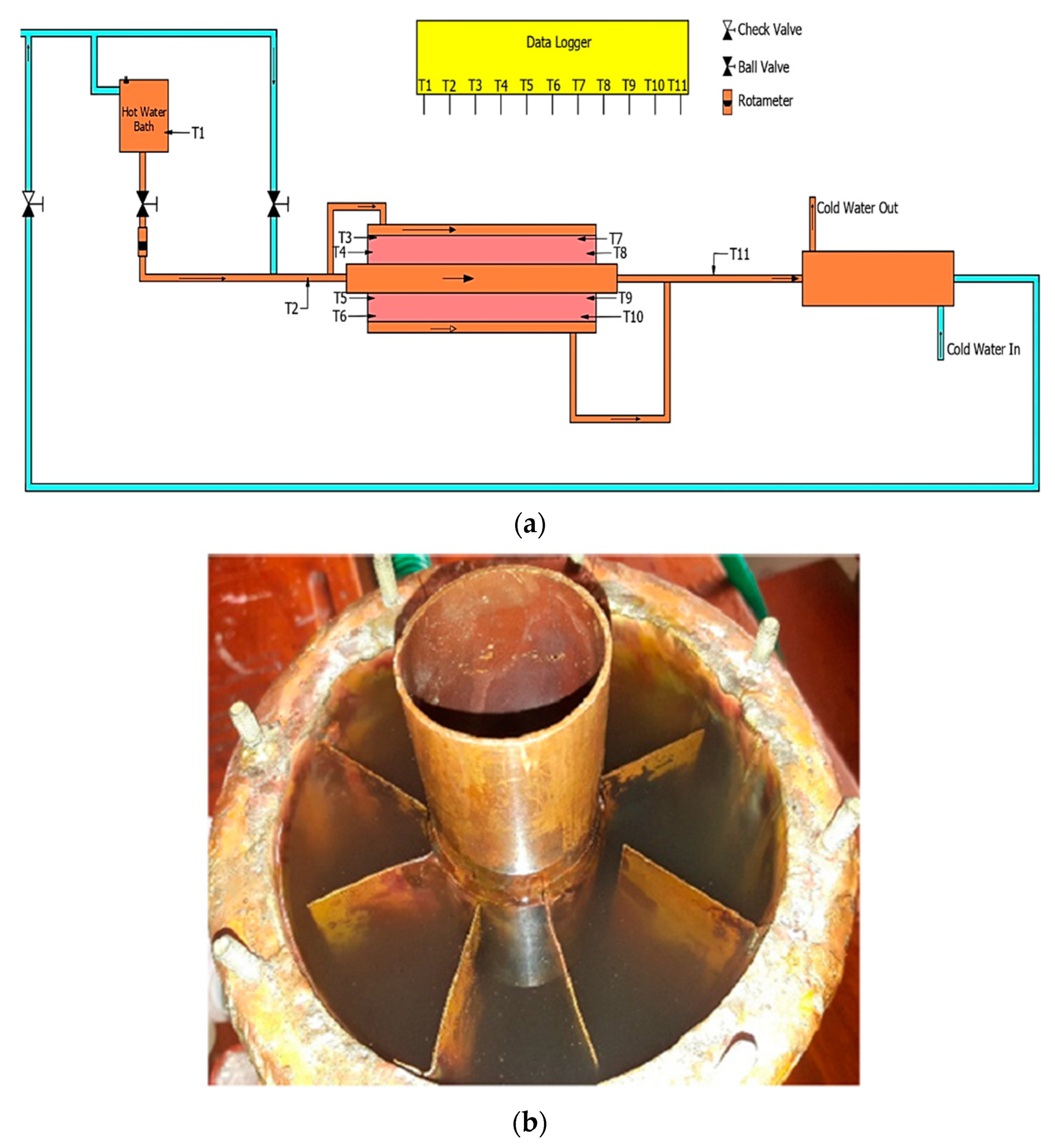
2. Materials and Methods
Composite Preparation
3. Results and Discussion
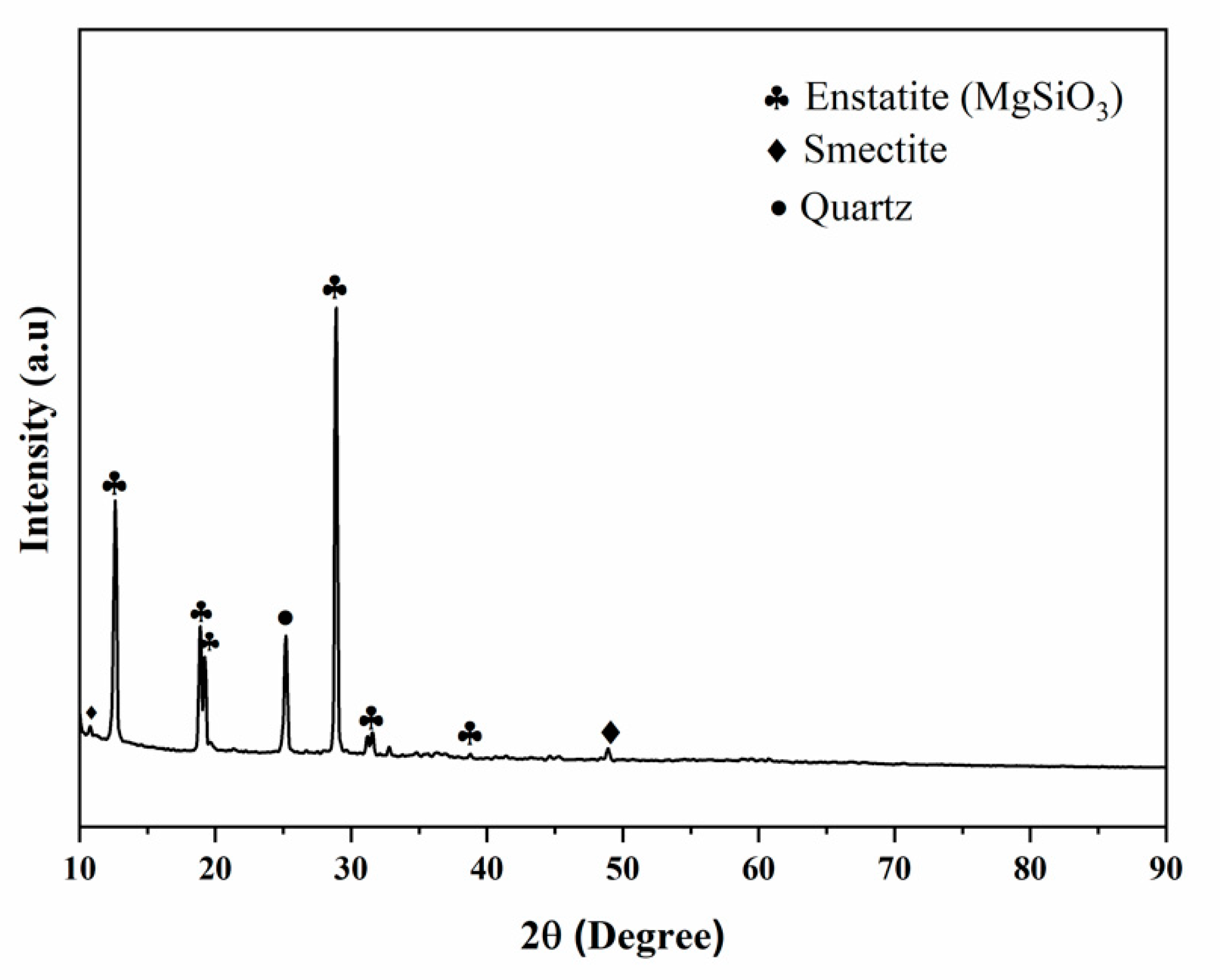
3.1. XRD Analysis
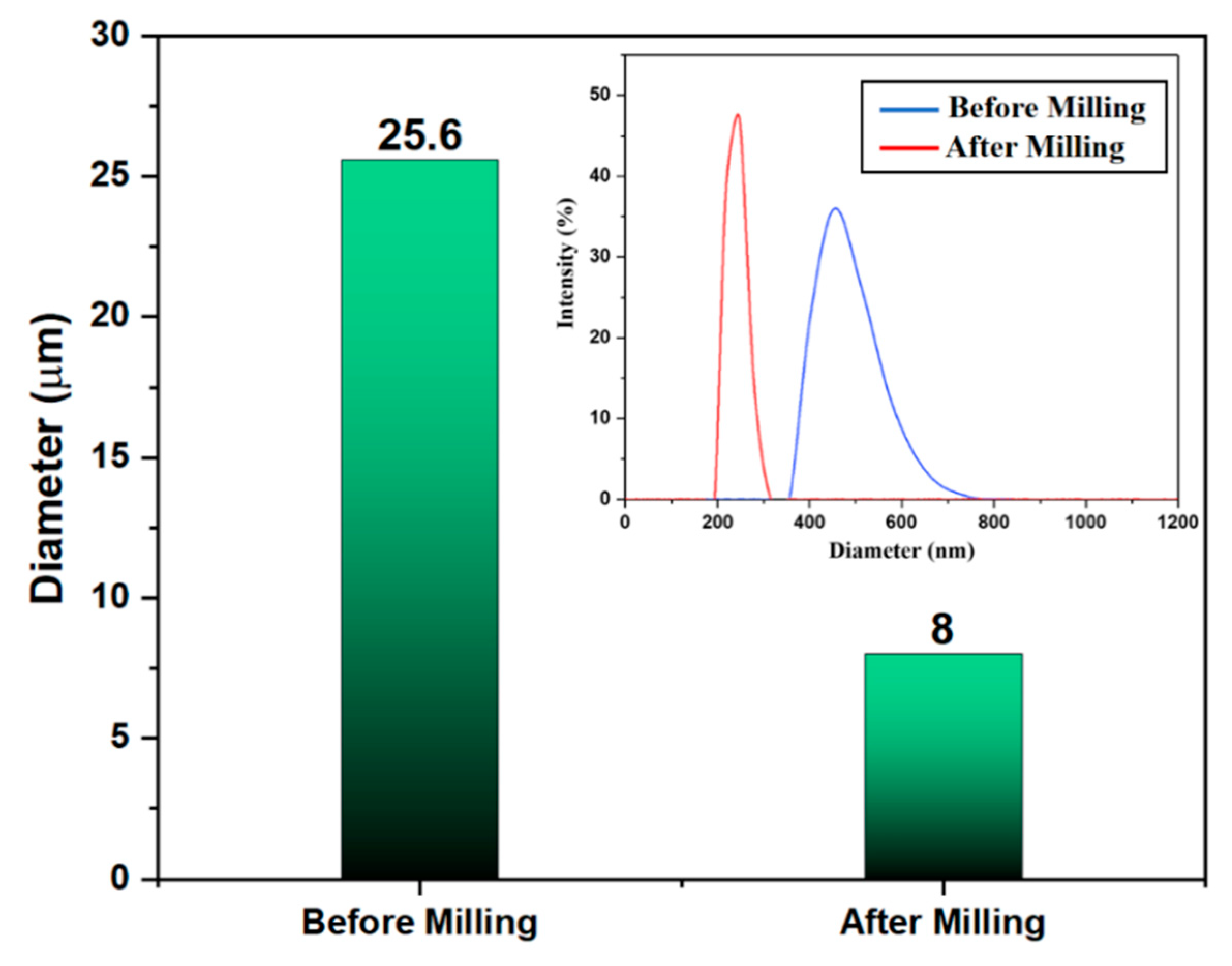
3.2. Particle Size Analysis
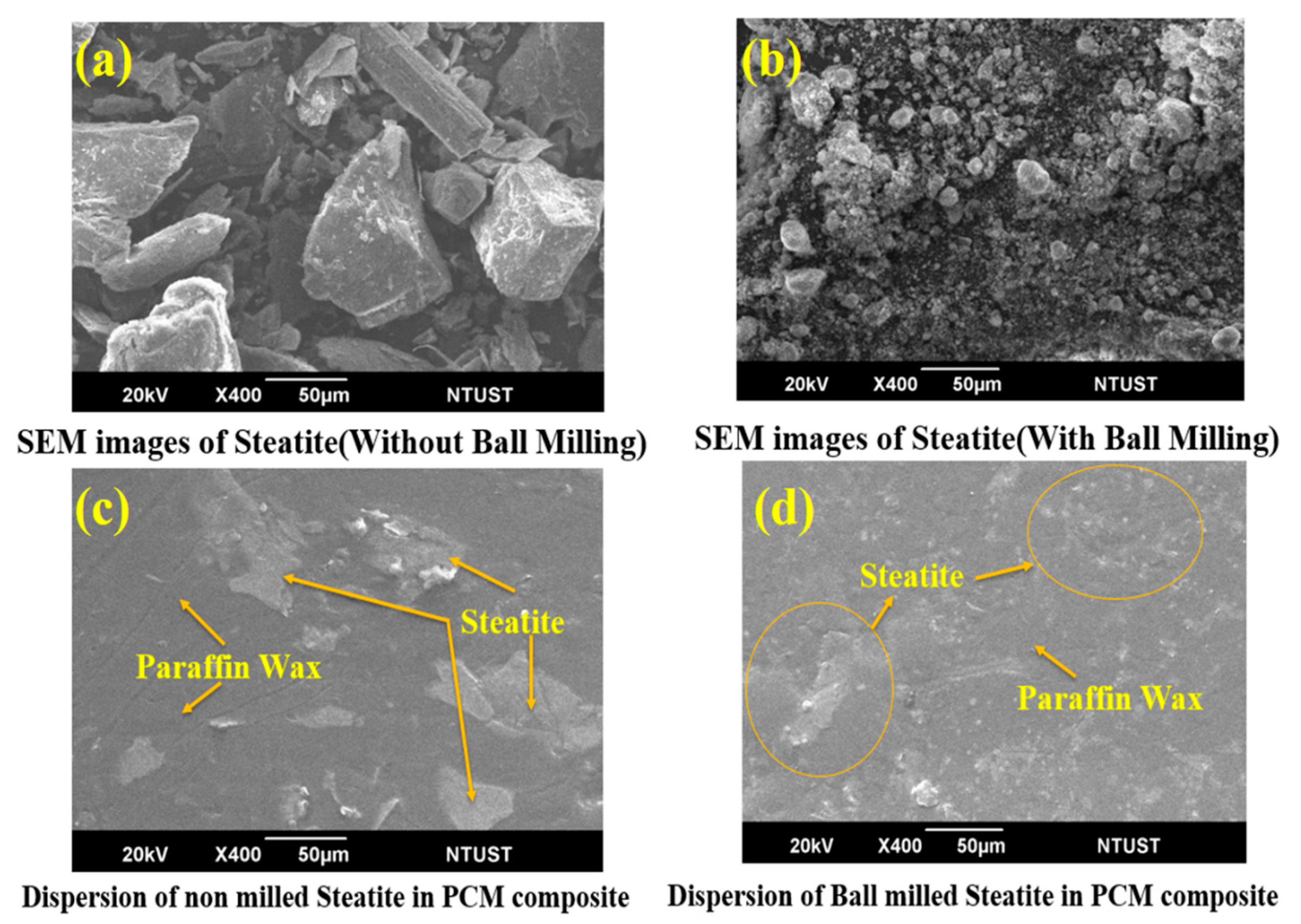
3.3. Microstructural Study
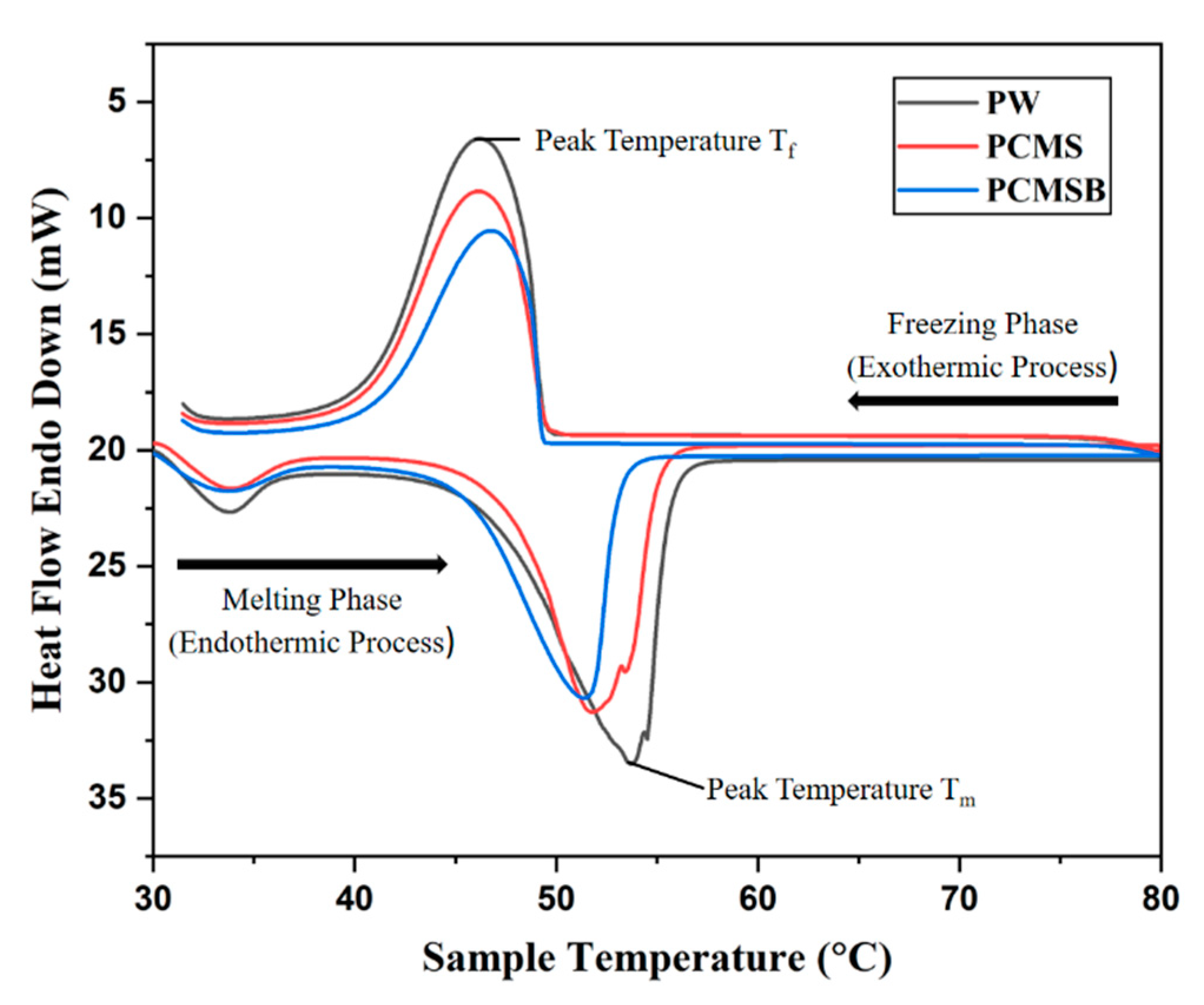
3.4. DSC Analysis
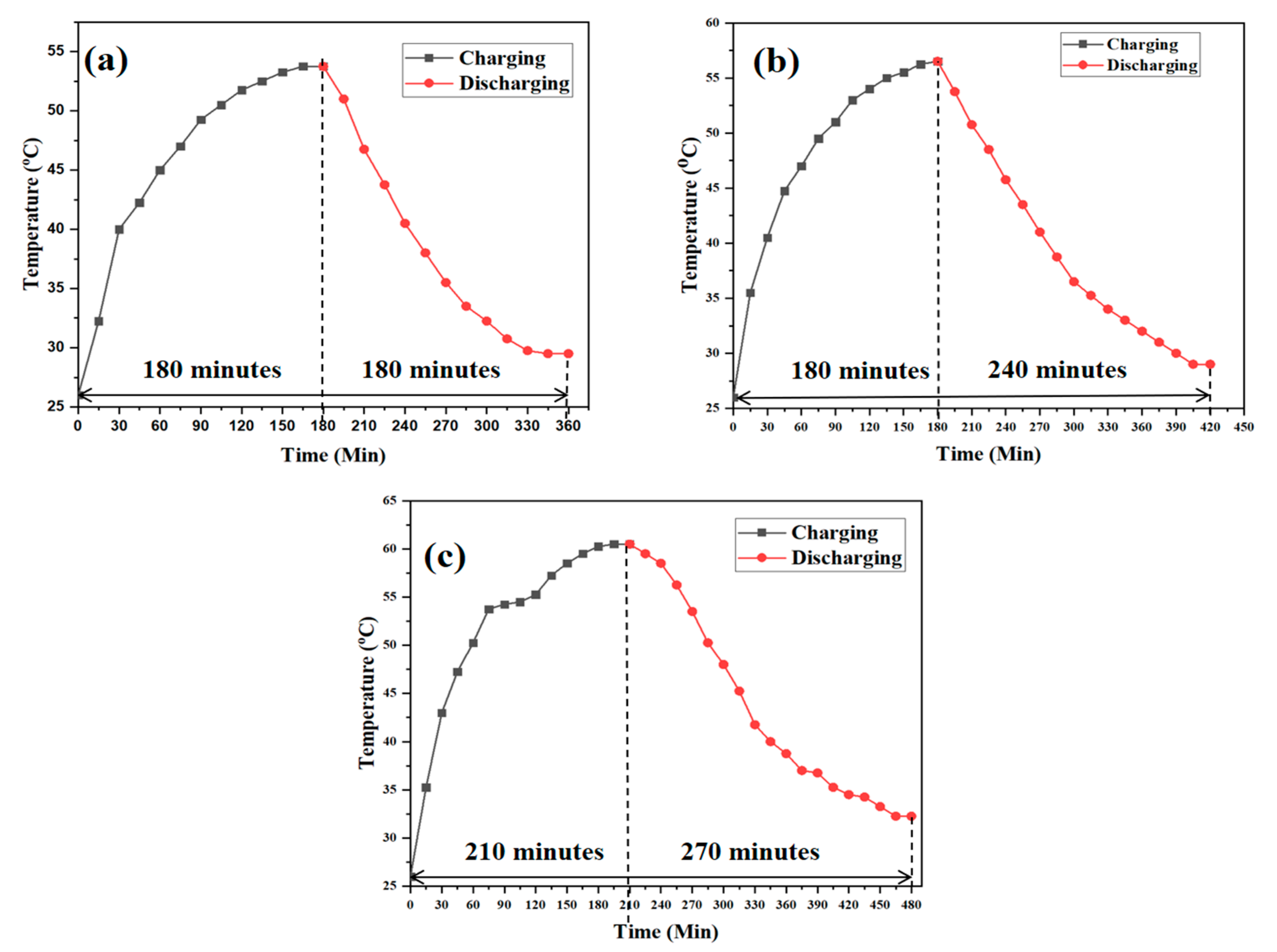
3.5. Performance during Charging and Discharging
4. Conclusions
- Incorporating steatite powder with paraffin wax enhanced the heat transfer of the energy storage system, consequently increasing the rate of charging and discharging.
- The usage of the ball-milled steatite composite increased the discharging time at 50% and the micro-size steatite composite at 33.33%. The thermal conductivity value of the milled steatite-based PCM composite was 7.7% higher than PW.
- The overall increase in the discharge time showed the capability of the PCM material to store the available thermal energy and discharge it as needed by the incorporated system.
- PCM reinforcement with ball-milled steatite in solar water heaters enhances the storage of heat energy and provides hot water availability for a longer period at a higher temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dincer, I.; Rosen, M.A. Exergy, environment, and sustainable development. Exergy 2020, 12, 61–89. [Google Scholar] [CrossRef]
- Sharif, M.K.A.; Al-Abidi, A.A.; Mat, S.; Sopian, K.; Ruslan, M.H.; Sulaiman, M.Y.; Rosli, M.A.M. Review of the application of phase change material for heating and domestic hot water systems. Renew. Sustain. Energy Rev. 2015, 42, 557–568. [Google Scholar] [CrossRef]
- Saranprabhu, M.; Rajan, K. Magnesium oxide nanoparticles dispersed solar salt with improved solid phase thermal conductivity and specific heat for latent heat thermal energy storage. Renew. Energy 2019, 141, 451–459. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Exergy analyses of thermal energy storage systems. Exergy 2020, 12, 167–210. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z. An experimental investigation of discharge/solidification cycle of paraffin in novel shell and tube with longitudinal fins based latent heat storage system. Energy Convers. Manag. 2017, 154, 157–167. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.-N.; Zhou, F. Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Convers. Manag. 2020, 214, 112876. [Google Scholar] [CrossRef]
- Sivapalan, B.; Chandran, M.N.; Manikandan, S.; Saranprabhu, M.; Pavithra, S.; Rajan, K. Paraffin wax–water nanoemulsion: A superior thermal energy storage medium providing higher rate of thermal energy storage per unit heat exchanger volume than water and paraffin wax. Energy Convers. Manag. 2018, 162, 109–117. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Vakhshouri, A.R. Paraffin as Phase Change Material. Paraffin Overv. 2020, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Pandey, A.; Selvaraj, J.A.; Rahim, N.A.; Islam, M.; Tyagi, V. Two side serpentine flow based photovoltaic-thermal-phase change materials (PVT-PCM) system: Energy, exergy and economic analysis. Renew. Energy 2018, 136, 1320–1336. [Google Scholar] [CrossRef]
- Abdulrahman, R.S.; Ibrahim, F.A.; Dakhil, S.F. Development of paraffin wax as phase change material based latent heat storage in heat exchanger. Appl. Therm. Eng. 2018, 150, 193–199. [Google Scholar] [CrossRef]
- Karami, R.; Kamkari, B. Experimental investigation of the effect of perforated fins on thermal performance enhancement of vertical shell and tube latent heat energy storage systems. Energy Convers. Manag. 2020, 210, 112679. [Google Scholar] [CrossRef]
- Barbi, S.; Barbieri, F.; Marinelli, S.; Rimini, B.; Merchiori, S.; Larwa, B.; Bottarelli, M.; Montorsi, M. Phase change material-sand mixtures for distributed latent heat thermal energy storage: Interaction and performance analysis. Renew. Energy 2021, 169, 1066–1076. [Google Scholar] [CrossRef]
- Şahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials—A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Al-Abidi, A.A.; Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Experimental study of melting and solidification of PCM in a triplex tube heat exchanger with fins. Energy Build. 2014, 68, 33–41. [Google Scholar] [CrossRef]
- Agyenim, F.; Eames, P.; Smyth, M. Heat transfer enhancement in medium temperature thermal energy storage system using a multitube heat transfer array. Renew. Energy 2010, 35, 198–207. [Google Scholar] [CrossRef]
- Santosh, R.; Kumaresan, G.; Paranthaman, V.; Swaminathan, M.R.; Velraj, R. Comparative investigation on heat transfer enhancement of surface-roughened and nano-dispersed phase change material for thermal energy storage. Int. J. Energy Res. 2021, 45, 15992–16005. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, P. Experimental and numerical investigation of a tube-in-tank latent thermal energy storage unit using composite PCM. Appl. Energy 2017, 190, 524–539. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M.; Maghrebi, M.-J.; Ghazikhani, M. Experimental study of using both ZnO/ water nanofluid and phase change material (PCM) in photovoltaic thermal systems. Sol. Energy Mater. Sol. Cells 2017, 161, 62–69. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Fahad, M.; Shahzad, A.; Ali, S.; Shah, K.H. Qualitative and quantitative analysis of steatite using calibration-free laser-induced breakdown spectroscopy in conjunction with x-ray fluorescence spectroscopy and Fourier-transform infrared spectroscopy. Appl. Opt. 2021, 60, 5110–5116. [Google Scholar] [CrossRef]
- Jidhesh, P.; Arjunan, T.; Rathnaraj, J.D. Experimental investigation on heat transfer characteristics of phase change composite for thermal energy storage system. Mater. Today Proc. 2020, 42, 618–625. [Google Scholar] [CrossRef]
- Rahmalina, D.; Rahman, R.A. Ismail Improving the phase transition characteristic and latent heat storage efficiency by forming polymer-based shape-stabilized PCM for active latent storage system. Case Stud. Therm. Eng. 2022, 31, 101840. [Google Scholar] [CrossRef]
- Gavalas, G.R. Comparison of effective conductivities calculated by the effective medium approximation and the self consistent approximation for core-shell particulate composites. AIP Adv. 2017, 7, 095222. [Google Scholar] [CrossRef]
- Singh, R.; Sadeghi, S.; Shabani, B. Thermal Conductivity Enhancement of Phase Change Materials for Low-Temperature Thermal Energy Storage Applications. Energies 2018, 12, 75. [Google Scholar] [CrossRef]
- Trigui, A. Performance enhancement of a thermal energy storage system using shape-stabilized LDPE/hexadecane/SEBS composite PCMs by copper oxide addition. RSC Adv. 2022, 12, 21990–22003. [Google Scholar] [CrossRef]
| Properties | Paraffin Wax | Steatite |
|---|---|---|
| Density (g/cm3) | 0.861 | 2.7 |
| Specific Heat (KJ/kg K) | 2.8 | 0.921 |
| Thermal conductivity (W/mK) | 0.214 | 2.9 |
| Melting Point (°C) | 56 | 1600 |
| Name | Composition |
|---|---|
| PW | Paraffin Wax |
| PCMS | Paraffin Wax/Steatite Composite |
| PCMSB | Paraffin Wax/Ball-milled Steatite Composite |
| Thermocouple Probe | Probe Location |
|---|---|
| T3, T6 T7, T10 | 150 mm from either side of the test section |
| T4, T5,T8, T9 | 50 mm from either side of the test section |
| Condition | Fluid Flow Rate |
|---|---|
| Charging | Hot fluid flow rate = 0.0055 kg/s |
| Hot fluid temperature = 60 °C | |
| Discharging | Cold fluid flow rate = 0.0033 kg/s |
| Cold fluid temperature = 30 °C |
| Sample | The Melting Process | The Solidification Process | ||||
|---|---|---|---|---|---|---|
| Tm (℃) | Tpeak-m (℃) | Hm (J⋅g−1) | Tm (℃) | Tpeak-m (℃) | Hm (J⋅g−1) | |
| PW | 47.2 | 53.7 | 163.5 | 49.3 | 46.3 | 165.2 |
| PCMS | 46.6 | 51.4 | 133.8 | 48.9 | 46.0 | 134.5 |
| PCMSB | 45.4 | 50.7 | 135.2 | 46.2 | 44.8 | 132.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannaiyan, S.; Huang, S.-J.; Rathnaraj, D.; Srinivasan, S.A. Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material. Micromachines 2022, 13, 1456. https://doi.org/10.3390/mi13091456
Kannaiyan S, Huang S-J, Rathnaraj D, Srinivasan SA. Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material. Micromachines. 2022; 13(9):1456. https://doi.org/10.3390/mi13091456
Chicago/Turabian StyleKannaiyan, Sathiyalingam, Song-Jeng Huang, David Rathnaraj, and S. A. Srinivasan. 2022. "Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material" Micromachines 13, no. 9: 1456. https://doi.org/10.3390/mi13091456
APA StyleKannaiyan, S., Huang, S.-J., Rathnaraj, D., & Srinivasan, S. A. (2022). Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material. Micromachines, 13(9), 1456. https://doi.org/10.3390/mi13091456











