Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging
Abstract
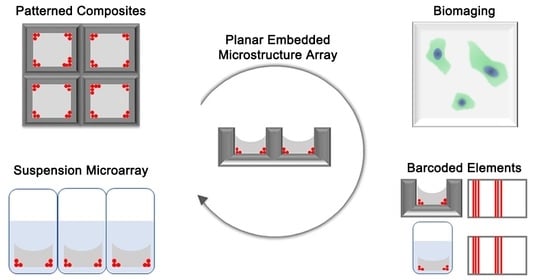
1. Introduction
2. Materials and Methods
2.1. Materials
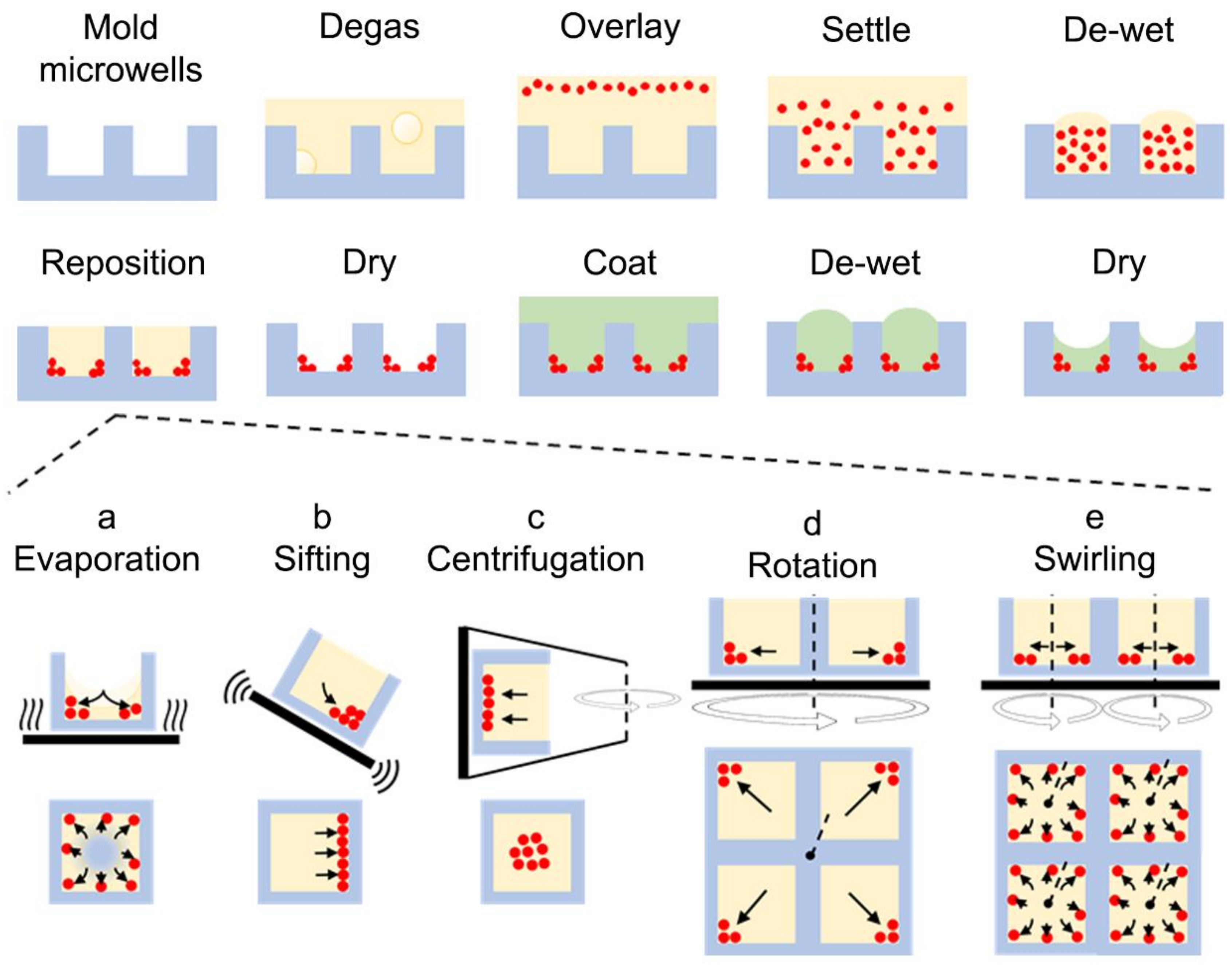
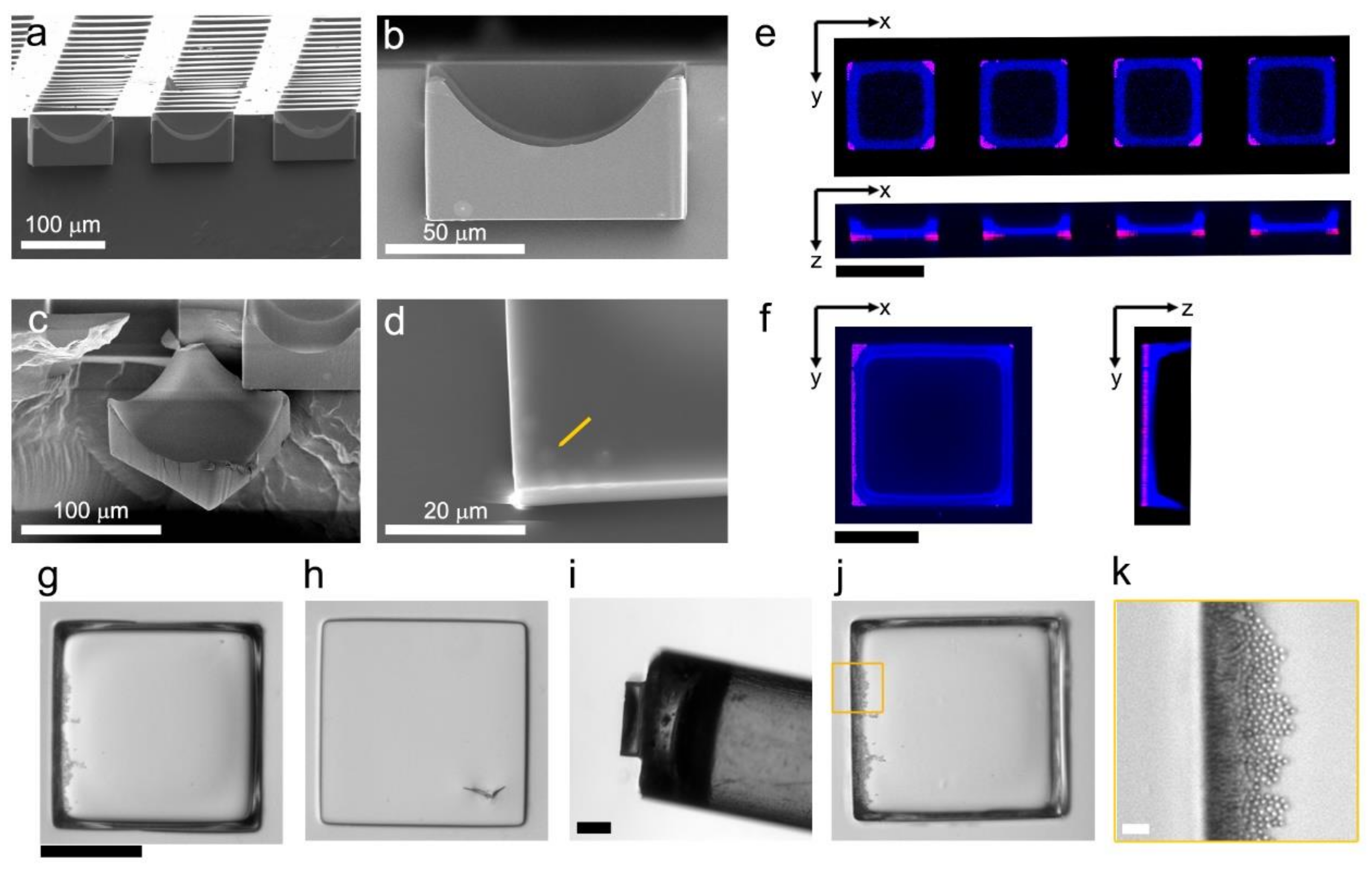
2.2. Microfabrication
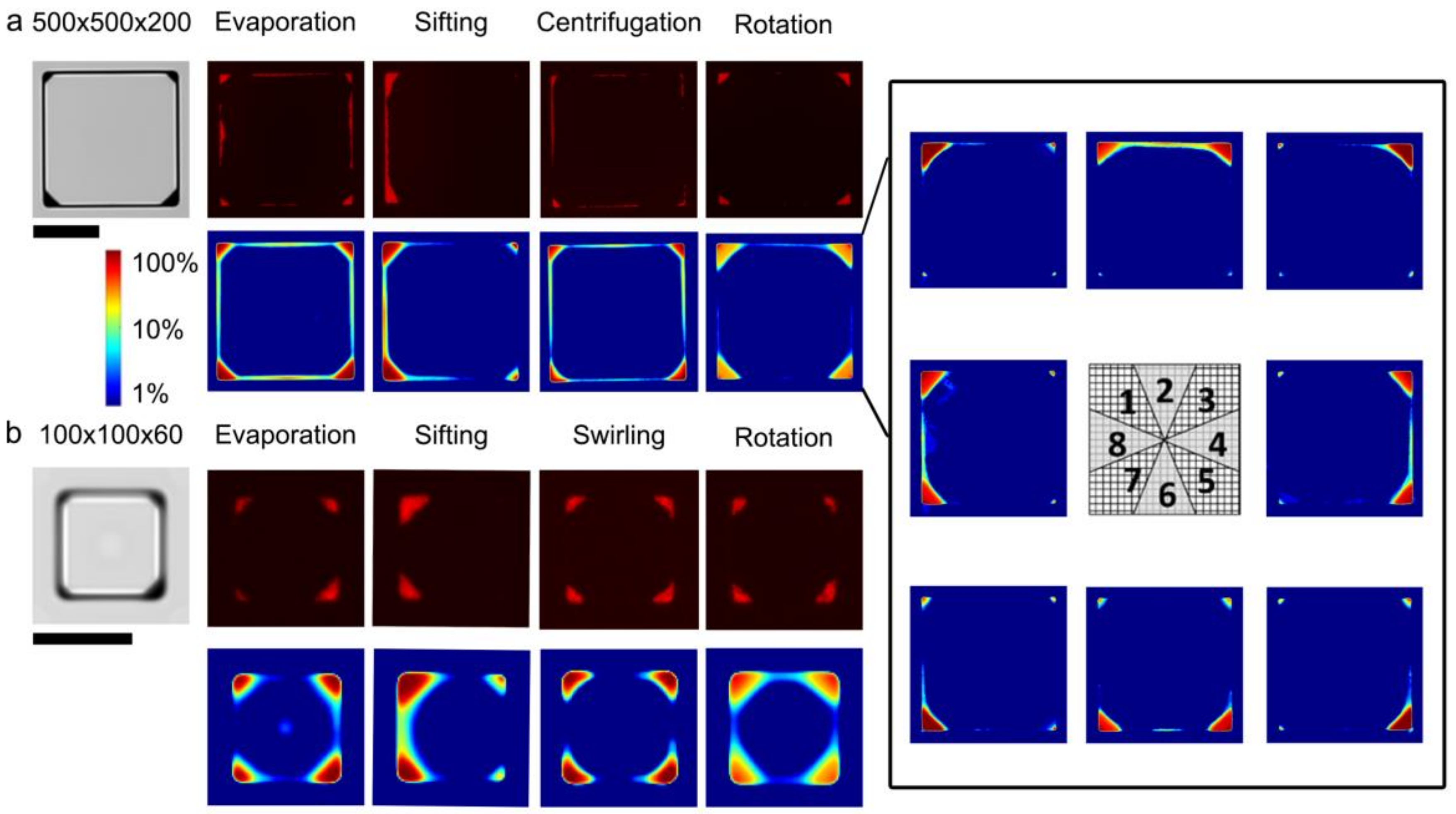
2.3. Microstructure Characterization
2.4. Latent-HIV Assay
2.5. Barcoding
2.6. Statistical Analysis
3. Results and Discussion
3.1. Carrier-Fluid-Mediated Deposition of Microparticles
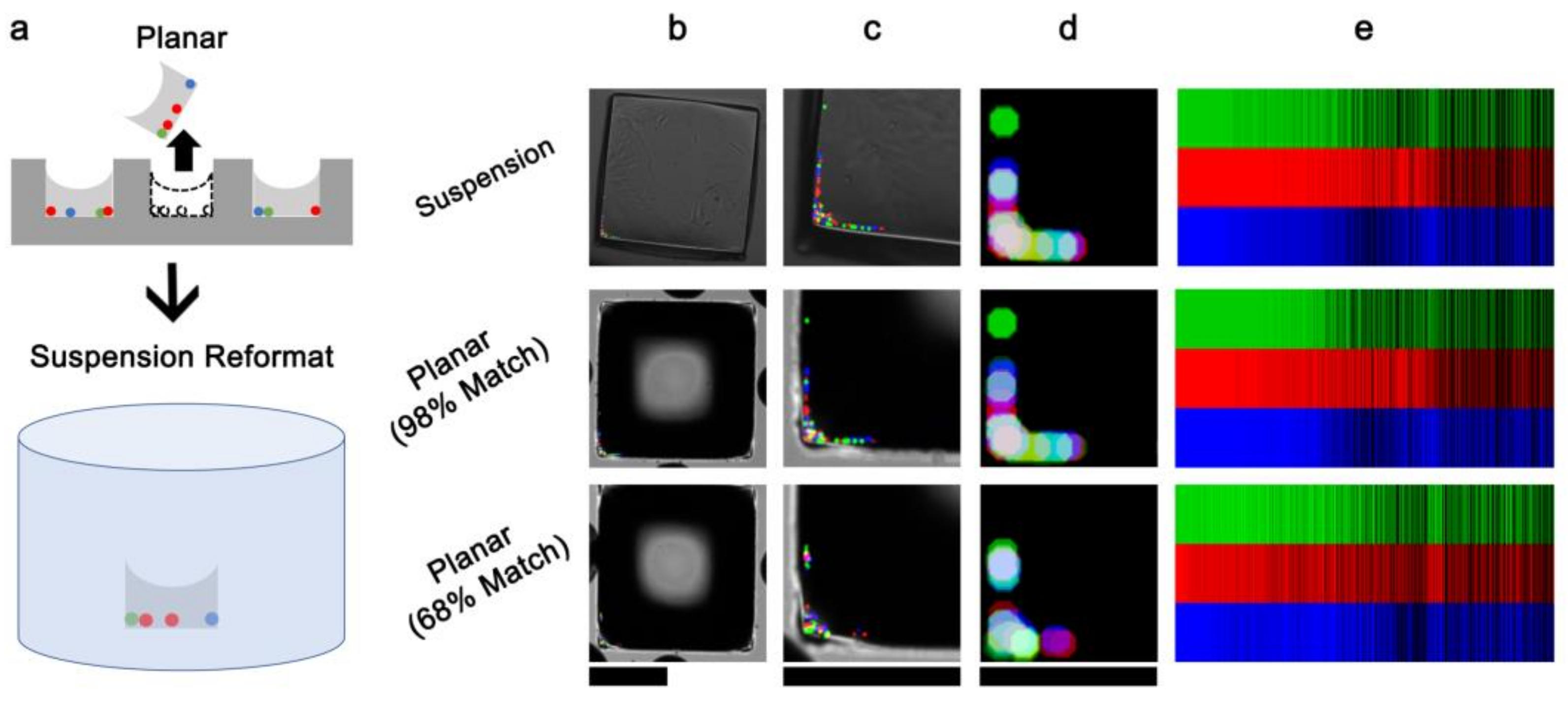
3.2. Embedded Microstructure Arrays for Magnetic Conversion from Planar to Suspension Formats
3.3. EMAs as Microcarriers for Bioimaging
3.4. Stochastic Barcoding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Chen, J.J.; Mu, L.; Xue, Q.; Wu, Y.; Wu, P.-H.; Li, J.; Vortmeyer, A.O.; Miller-Jensen, K.; Wirtz, D.; et al. High-Throughput Secretomic Analysis of Single Cells to Assess Functional Cellular Heterogeneity. Anal. Chem. 2013, 85, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Y.; Zhang, K.; Xiao, Y.; Lu, J.; Fan, R. Multiplexed, Sequential Secretion Analysis of the Same Single Cells Reveals Distinct Effector Response Dynamics Dependent on the Initial Basal State. Adv. Sci. 2019, 6, 1801361. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.T.; Wang, Y.; Young, G.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Micropallet Arrays for the Separation of Single, Adherent Cells. Anal. Chem. 2007, 79, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Llanos, B.; Wang, Y.; Sims, C.E.; Allbritton, N.L. A Technology of a Different Sort: Microraft Arrays. Lab A Chip 2021, 21, 3204–3218. [Google Scholar] [CrossRef]
- Levy, S.E.; Myers, R.M. Advancements in Next-Generation Sequencing. Annu. Rev. Genom. Hum. Genet. 2016, 17, 95–115. [Google Scholar] [CrossRef]
- Parsa, S.F.; Vafajoo, A.; Rostami, A.; Salarian, R.; Rabiee, M.; Rabiee, N.; Rabiee, G.; Tahriri, M.; Yadegari, A.; Vashaee, D.; et al. Early Diagnosis of Disease Using Microbead Array Technology: A Review. Anal. Chim. Acta 2018, 1032, 1–17. [Google Scholar] [CrossRef]
- Vafajoo, A.; Rostami, A.; Foroutan Parsa, S.; Salarian, R.; Rabiee, N.; Rabiee, G.; Rabiee, M.; Tahriri, M.; Vashaee, D.; Tayebi, L.; et al. Multiplexed Microarrays Based on Optically Encoded Microbeads. Biomed. Microdevices 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Leng, Y.; Sun, K.; Chen, X.; Li, W. Suspension Arrays Based on Nanoparticle-Encoded Microspheres for High-Throughput Multiplexed Detection. Chem. Soc. Rev. 2015, 44, 5552–5595. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.; Chen, Z.; Fan, R. Multiplexed PCR-Free Detection of MicroRNAs in Single Cancer Cells Using a DNA-Barcoded Microtrough Array Chip. Micromachines 2019, 10, 215. [Google Scholar] [CrossRef]
- Ma, H.; Horiuchi, K.Y. Chemical Microarray: A New Tool for Drug Screening and Discovery. Drug Discov. Today 2006, 11, 661. [Google Scholar] [CrossRef]
- Han, S.W.; Jang, E.; Koh, W.G. Microfluidic-Based Multiplex Immunoassay System Integrated with an Array of QD-Encoded Microbeads. Sens. Actuators B Chem. 2015, 209, 242–251. [Google Scholar] [CrossRef]
- Pimpin, A.; Srituravanich, W. Review on Micro- and Nanolithography Techniques and Their Applications. Eng. J. 2011, 16, 37–56. [Google Scholar] [CrossRef]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-Printed Biosensor Arrays for Medical Diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Phillips, C.; Xu, W.; Pai, J.-H.J.-H.; Dhopeshwarkar, R.; Sims, C.E.; Allbritton, N.L. Micromolded Arrays for Separation of Adherent Cells. Lab A Chip 2010, 10, 2917–2924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gach, P.C.; Wang, Y.; Phillips, C.; Sims, C.E.; Allbritton, N.L. Isolation and Manipulation of Living Adherent Cells by Micromolded Magnetic Rafts. Biomicrofluidics 2011, 5, 32002–3200212. [Google Scholar] [CrossRef]
- DiSalvo, M.; Harris, D.M.; Kantesaria, S.; Peña, A.N.; Allbritton-King, J.D.; Cole, J.H.; Allbritton, N.L. Characterization of Tensioned PDMS Membranes for Imaging Cytometry on Microraft Arrays. Anal. Chem. 2018, 90, 4792–4800. [Google Scholar] [CrossRef]
- DiSalvo, M.; Smiddy, N.M.; Allbritton, N.L. Automated Sensing and Splitting of Stem Cell Colonies on Microraft Arrays. APL Bioeng. 2019, 3, 036106. [Google Scholar] [CrossRef]
- Labelle, C.A.; Zhang, R.J.; Armistead, P.M.; Allbritton, N.L. Assay and Isolation of Single Proliferating CD4+ Lymphocytes Using an Automated Microraft Array Platform. IEEE Trans. Biomed. Eng. 2020, 67, 2166–2175. [Google Scholar] [CrossRef]
- Attayek, P.J.; Hunsucker, S.A.; Wang, Y.; Sims, C.E.; Armistead, P.M.; Allbritton, N.L. Array-Based Platform to Select, Release, and Capture Epstein-Barr Virus-Infected Cells Based on Intercellular Adhesion. Anal. Chem. 2015, 87, 12281–12289. [Google Scholar] [CrossRef]
- Attayek, P.J.; Waugh, J.P.; Hunsucker, S.A.; Grayeski, P.J.; Sims, C.E.; Armistead, P.M.; Allbritton, N.L. Automated Microraft Platform to Identify and Collect Non-Adherent Cells Successfully Gene-Edited with CRISPR-Cas9. Biosens. Bioelectron. 2017, 91, 175–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mattes, D.; Haynor, D.R.; Vesselle, H.; Lewellyn, T.K.; Eubank, W. Nonrigid Multimodality Image Registration. In Proceedings of the Proc.SPIE, San Diego, CA, United States, 3 July 2001; Volume 4322. [Google Scholar]
- Guizar-Sicairos, M.; Thurman, S.T.; Fienup, J.R. Efficient Subpixel Image Registration Algorithms. Opt. Lett. 2008, 33, 156. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, C.N.P.G.; Prendergast, L.A.; Staudte, R.G. Robust Analogs to the Coefficient of Variation. J. Appl. Stat. 2020, 49, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jackman, R.J.; Duffy, D.C.; Ostuni, E.; Willmore, N.D.; Whitesides, G.M. Fabricating Large Arrays of Microwells with Arbitrary Dimensions and Filling Them Using Discontinuous Dewetting. Anal. Chem. 1998, 70, 2280–2287. [Google Scholar] [CrossRef]
- Oberdick, S.D.; Zabow, G. Patterned Surface Energy in Elastomeric Molds as a Generalized Approach to Polymer Particle Fabrication. ACS Appl. Polym. Mater. 2020, 2, 846–852. [Google Scholar] [CrossRef]
- Štarha, P.; Smola, D.; Tuček, J.; Trávníček, Z. Efficient Synthesis of a Maghemite/Gold Hybrid Nanoparticle System as a Magnetic Carrier for the Transport of Platinum-Based Metallotherapeutics. Int. J. Mol. Sci. 2015, 16, 2034–2051. [Google Scholar] [CrossRef]
- Cortés-Llanos, B.; Serrano, A.; Muñoz-Noval, A.; Urones-Garrote, E.; del Campo, A.; Marco, J.F.; Ayuso-Sacido, A.; Pérez, L. Thermal Route for the Synthesis of Maghemite/Hematite Core/Shell Nanowires. J. Phys. Chem. C 2017, 121, 23158–23165. [Google Scholar] [CrossRef]
- Cortés-Llanos, B.; Ocampo, S.M.; de la Cueva, L.; Calvo, G.F.; Belmonte-Beitia, J.; Pérez, L.; Salas, G.; Ayuso-Sacido, Á. Influence of Coating and Size of Magnetic Nanoparticles on Cellular Uptake for In Vitro MRI. Nanomaterials 2021, 11, 2888. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiSalvo, M.; Cortés-Llanos, B.; LaBelle, C.A.; Murdoch, D.M.; Allbritton, N.L. Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging. Micromachines 2022, 13, 1392. https://doi.org/10.3390/mi13091392
DiSalvo M, Cortés-Llanos B, LaBelle CA, Murdoch DM, Allbritton NL. Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging. Micromachines. 2022; 13(9):1392. https://doi.org/10.3390/mi13091392
Chicago/Turabian StyleDiSalvo, Matthew, Belén Cortés-Llanos, Cody A. LaBelle, David M. Murdoch, and Nancy L. Allbritton. 2022. "Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging" Micromachines 13, no. 9: 1392. https://doi.org/10.3390/mi13091392
APA StyleDiSalvo, M., Cortés-Llanos, B., LaBelle, C. A., Murdoch, D. M., & Allbritton, N. L. (2022). Scalable Additive Construction of Arrayed Microstructures with Encoded Properties for Bioimaging. Micromachines, 13(9), 1392. https://doi.org/10.3390/mi13091392