Femtosecond Laser Fabrication of Microporous Membranes for Biological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
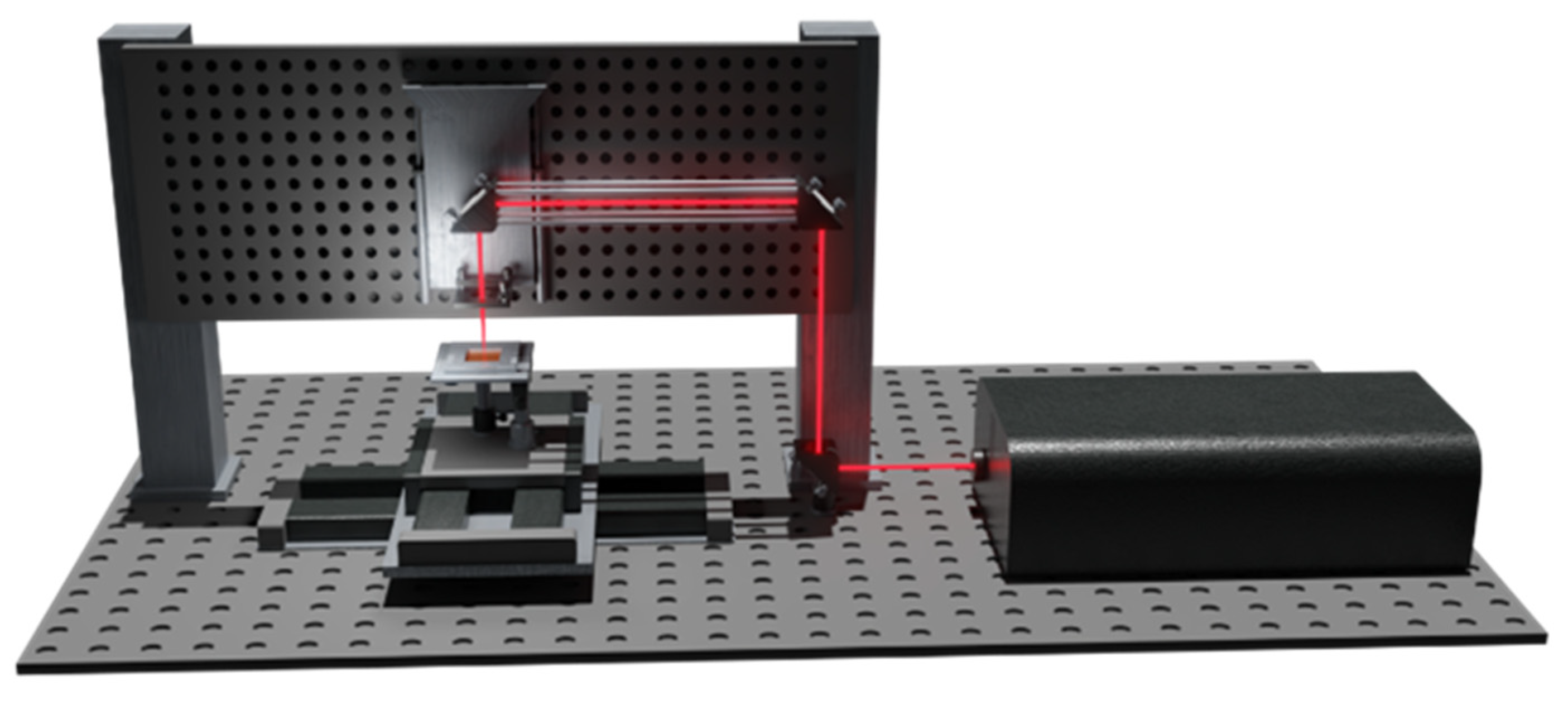
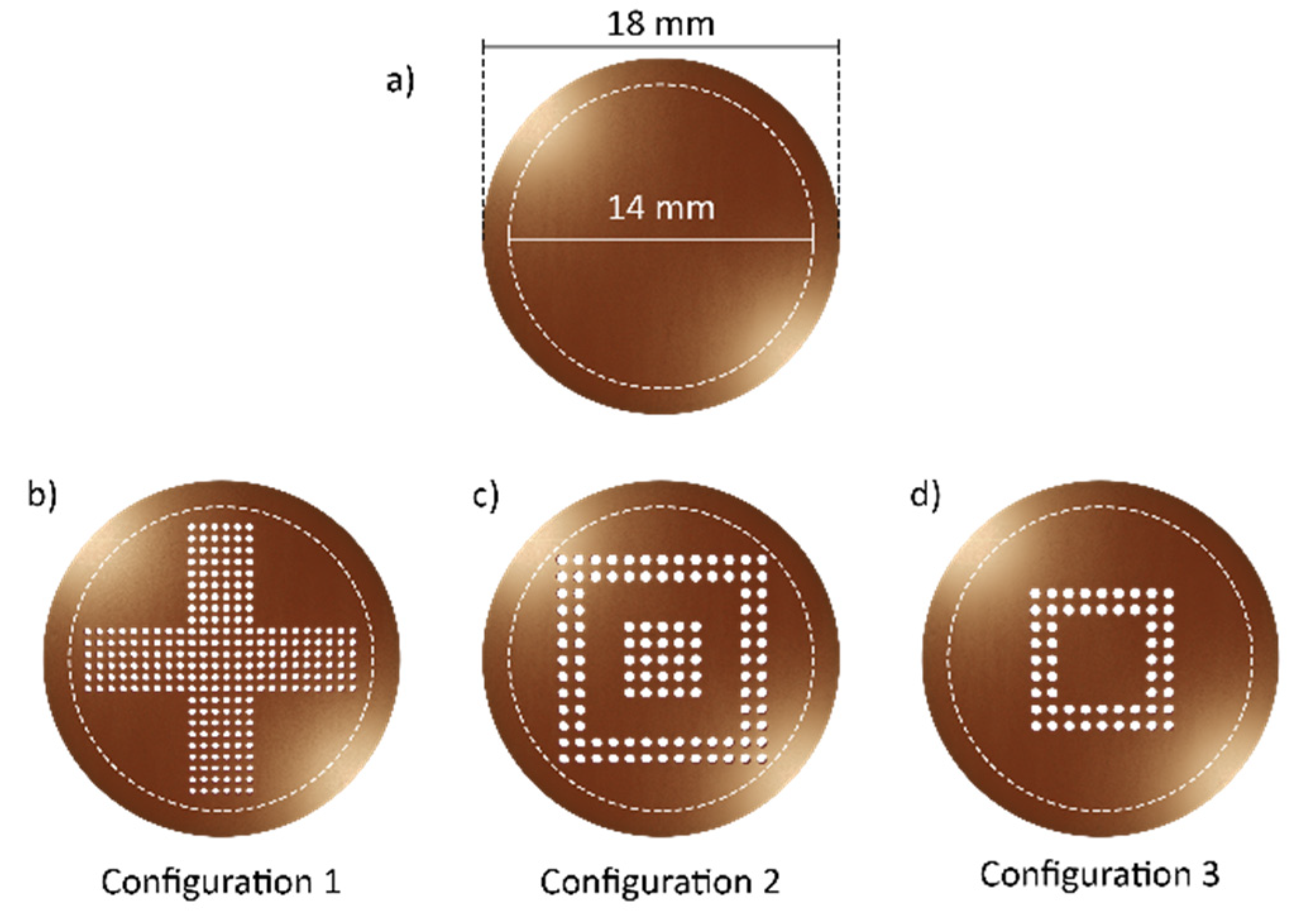
2.2. Micro-Membrane Design and Fabrication
2.3. Cell Culture, Sample Preparation and Cell Adhesion Experiments
3. Results
3.1. Fs-Laser Fabrication of Multilayers and Polymeric Membranes
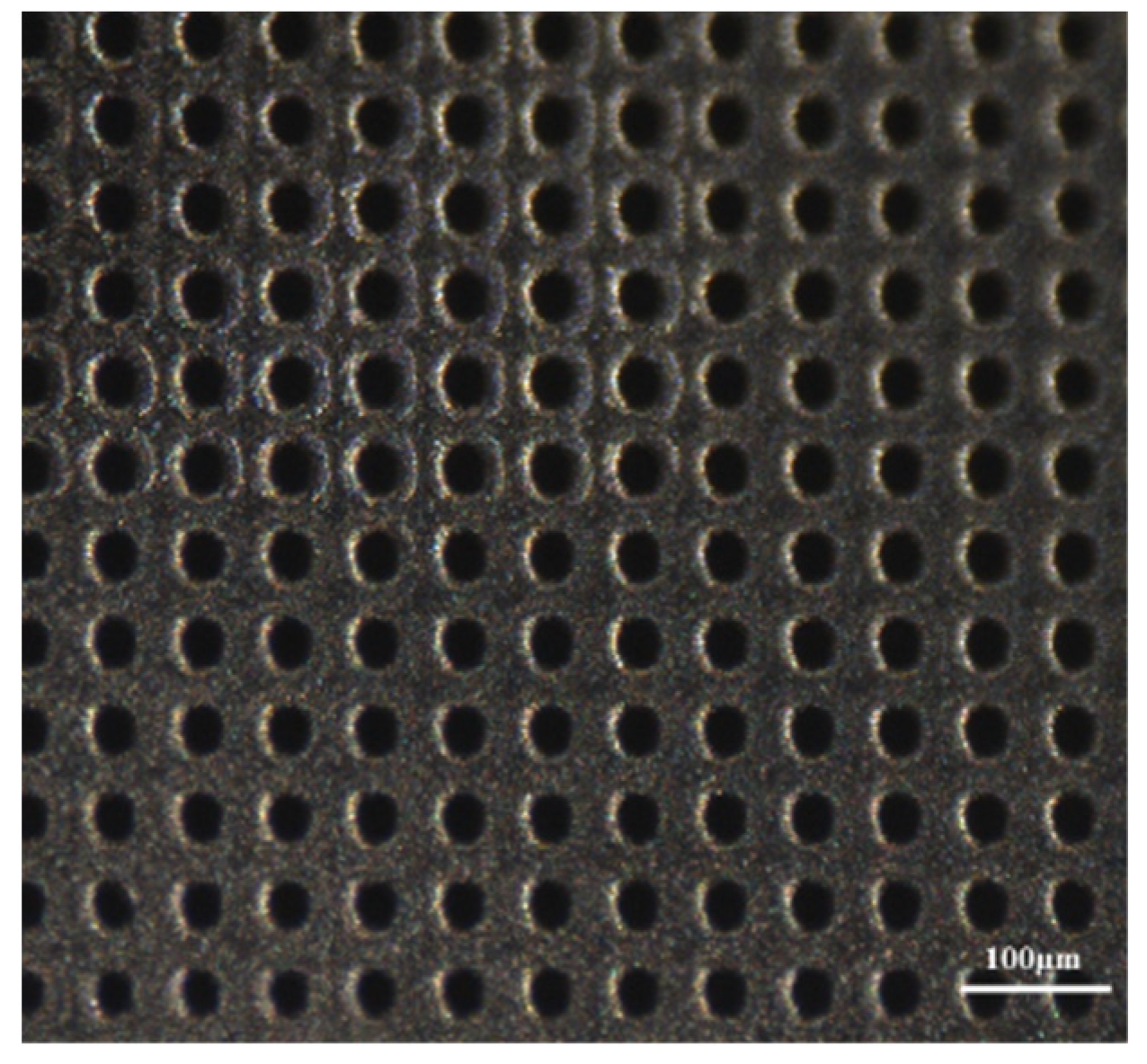
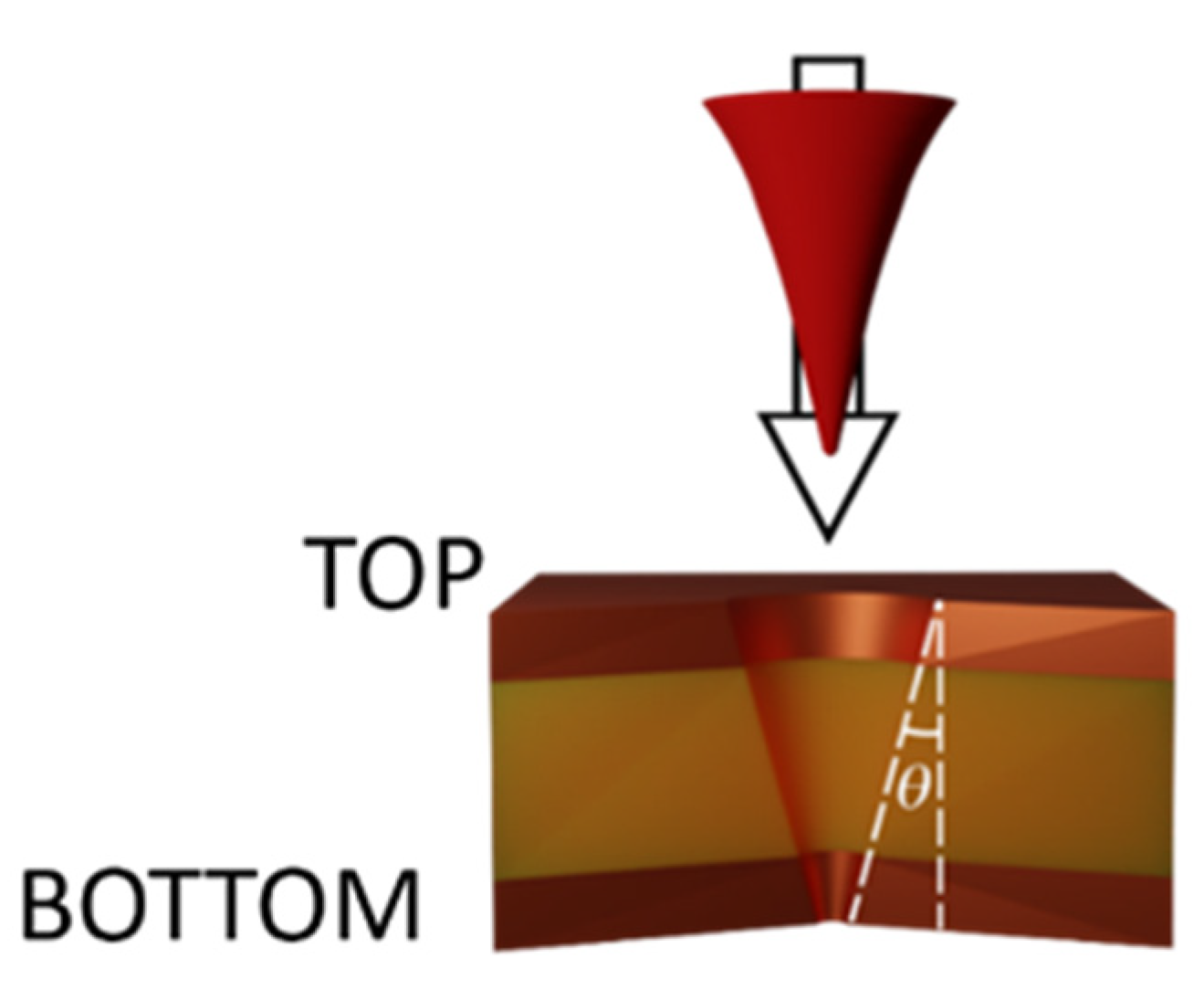
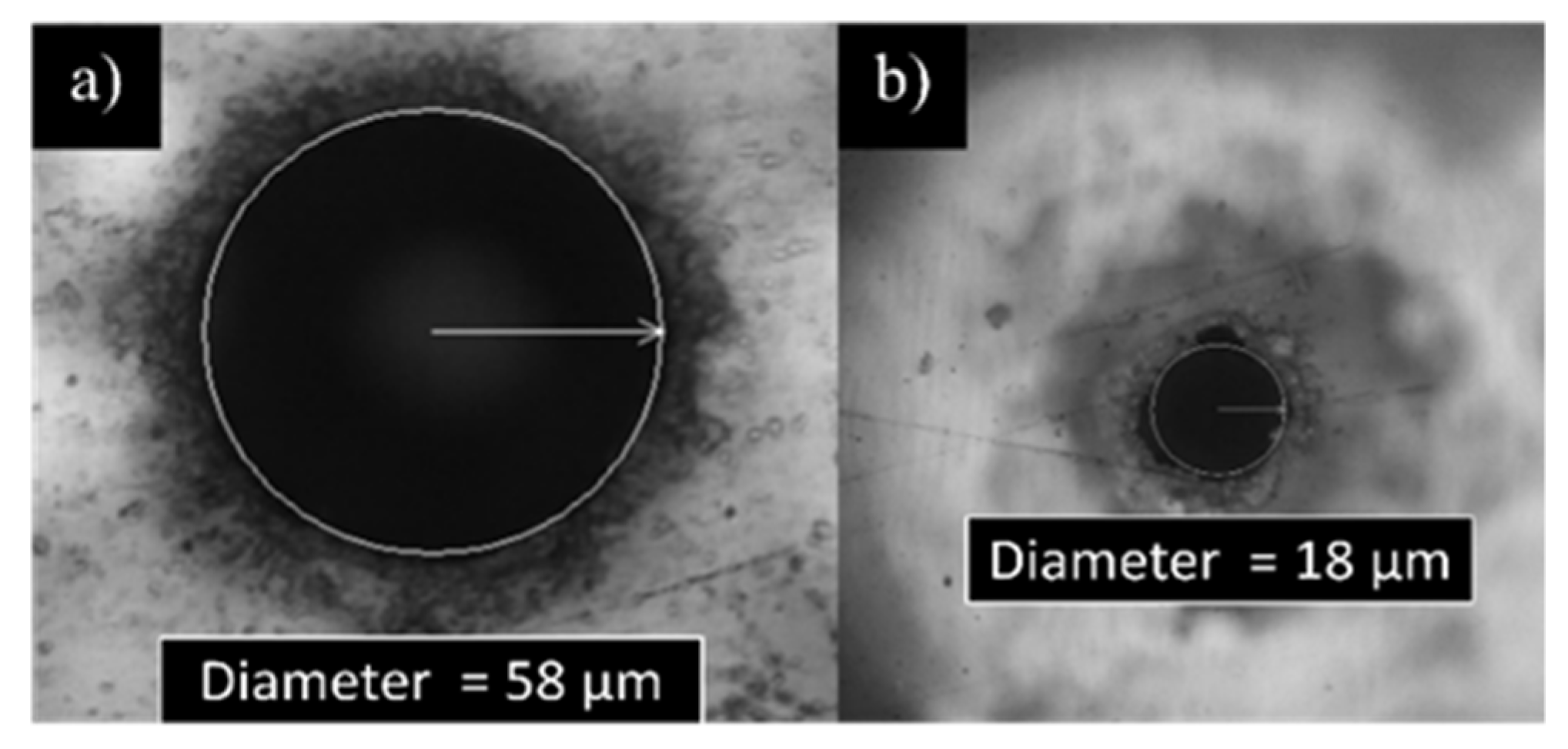
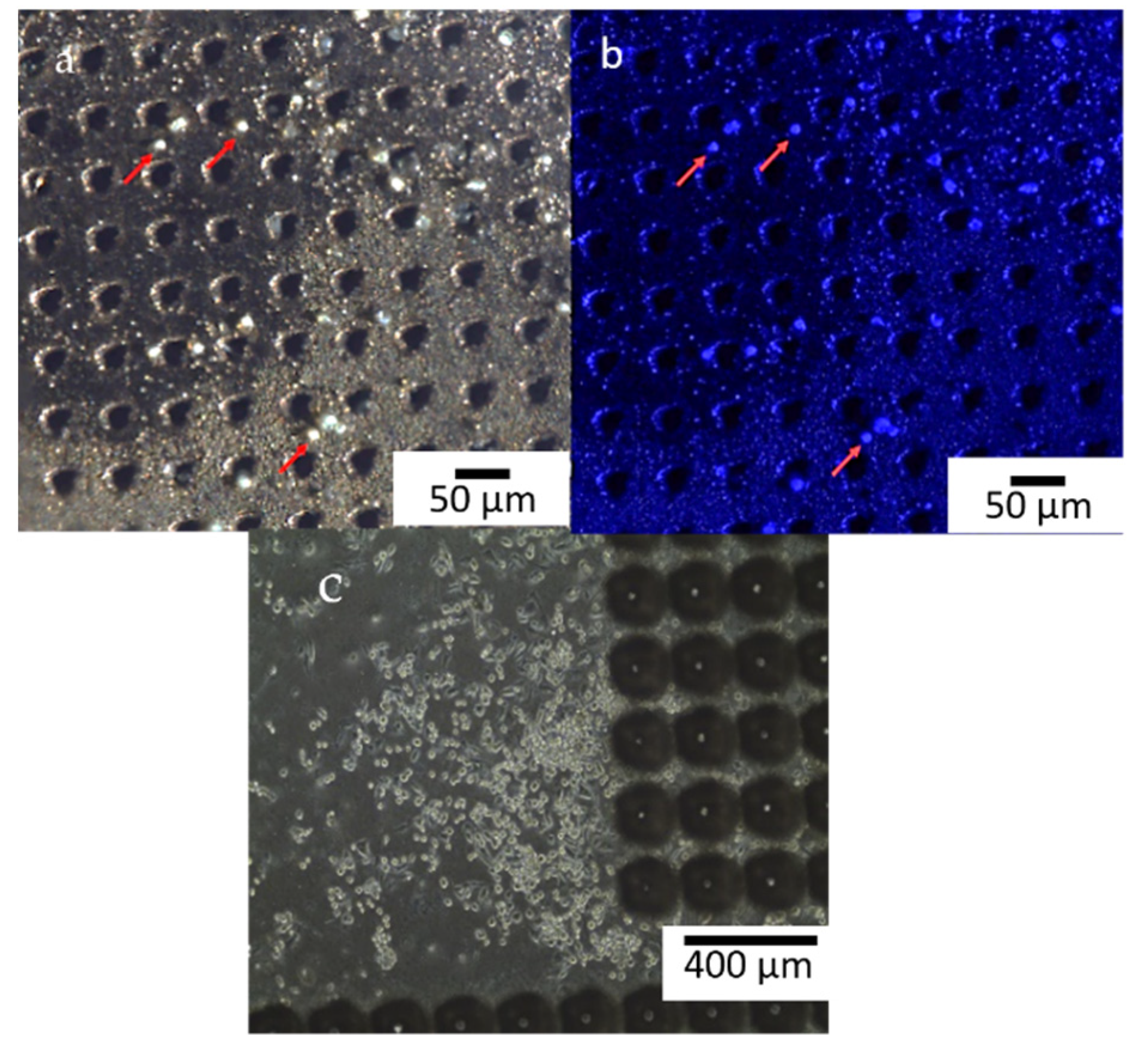
3.1.1. Laser Fabrication of CKC Membrane
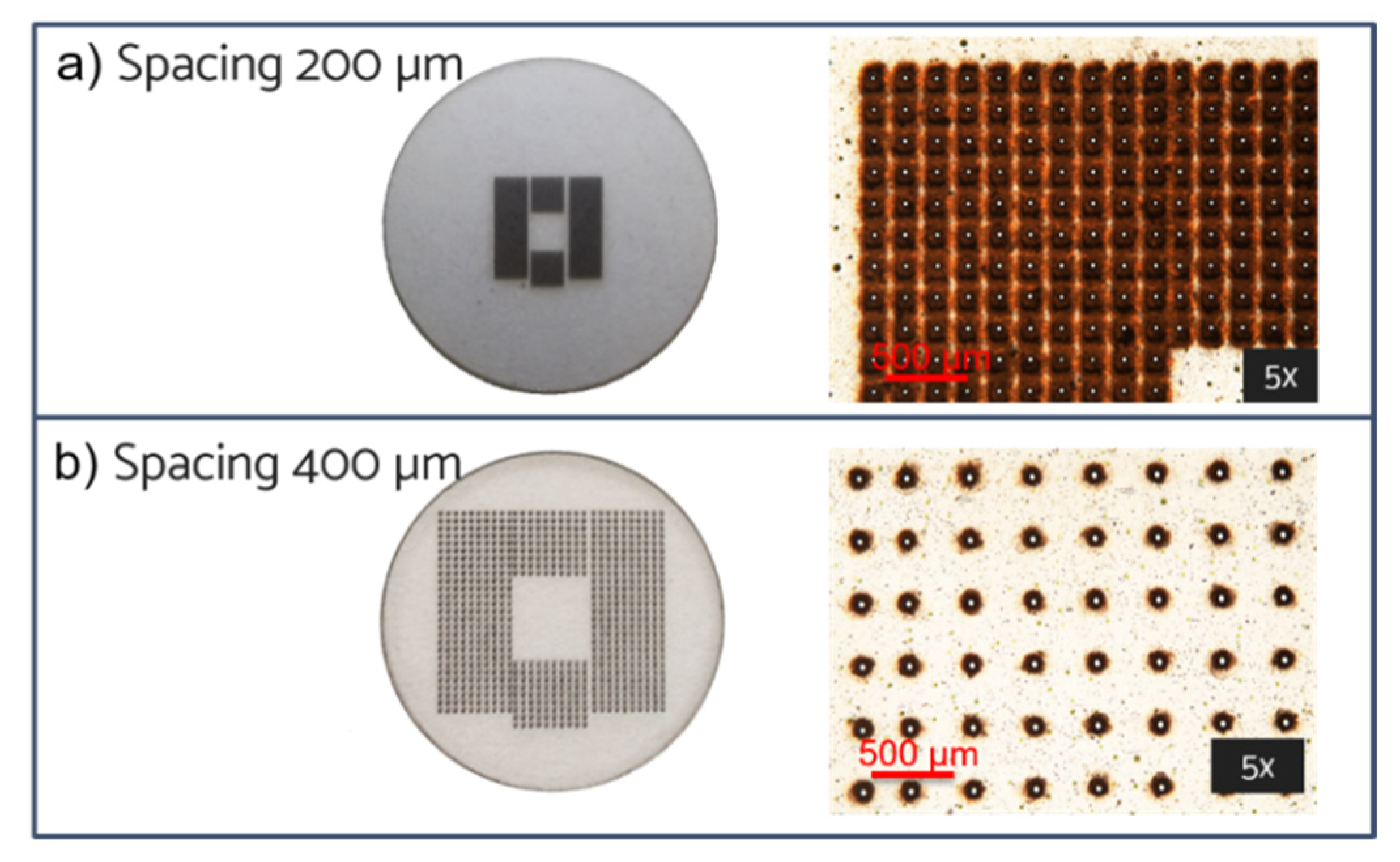
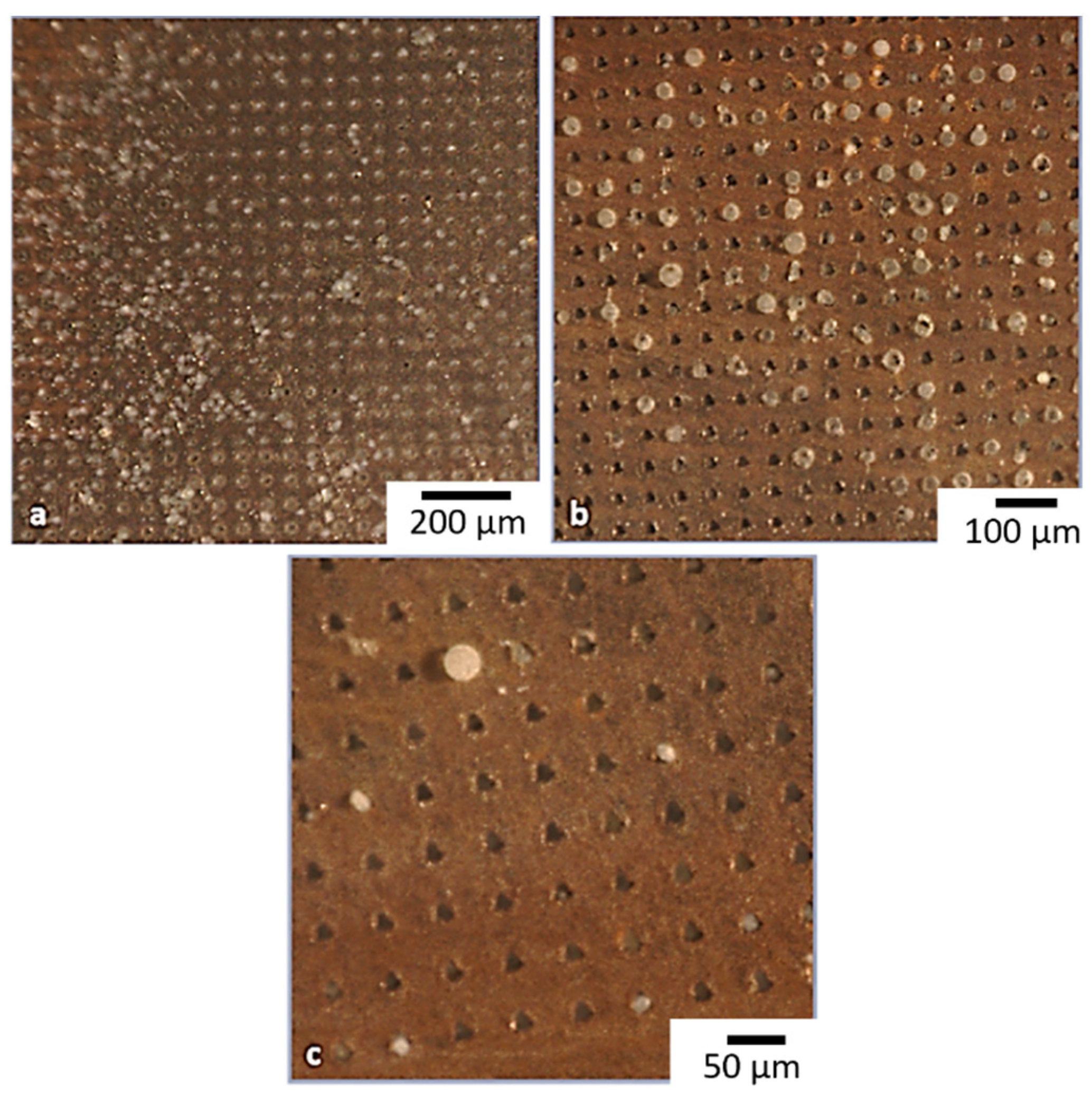
3.1.2. Laser Fabrication of CA Membrane
3.2. Biological Assays
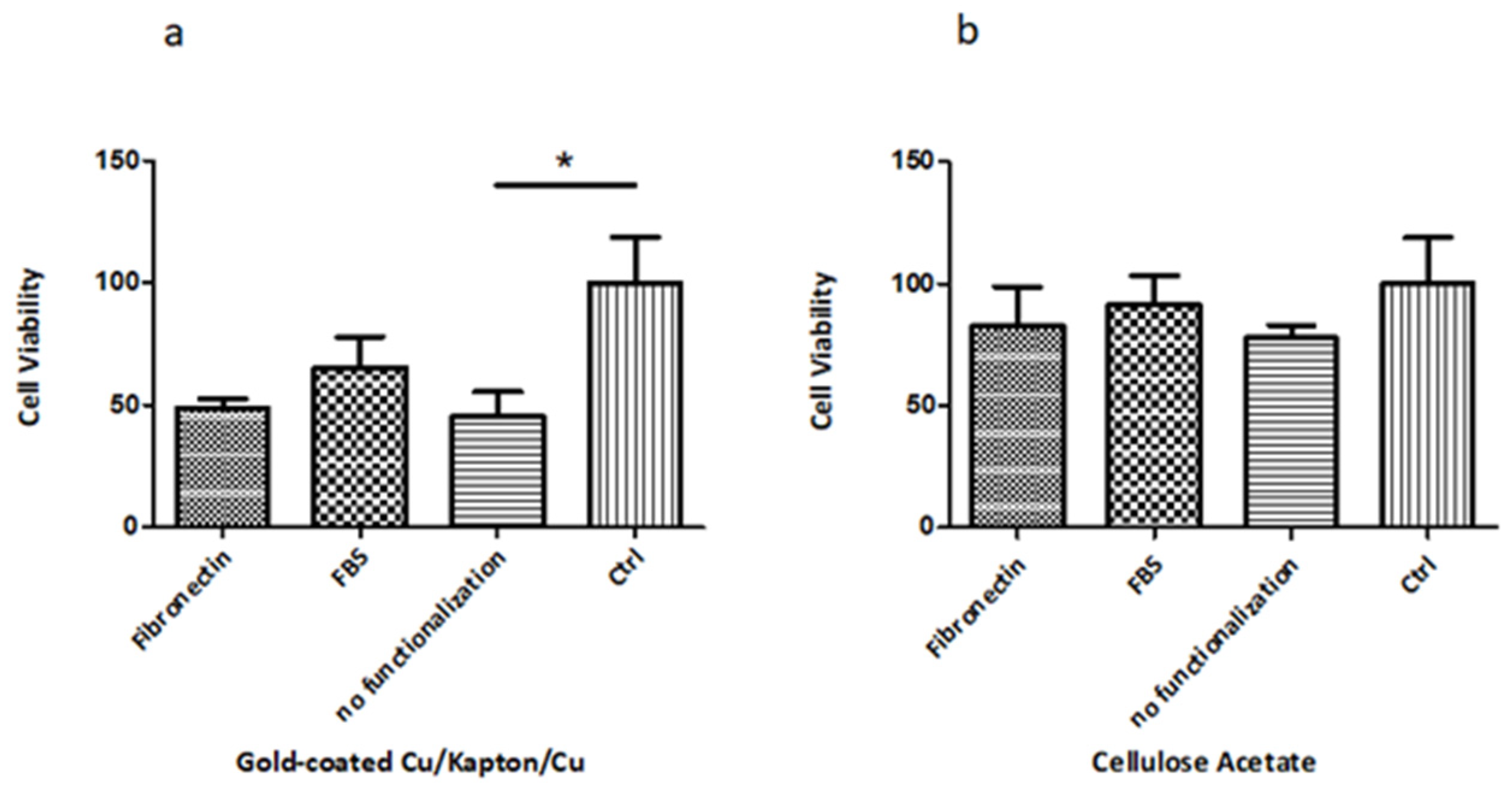
3.2.1. Biocompatibility Tests
3.2.2. Cell Adhesion Experiments
3.2.3. Cell Migration Proof-of-Concept
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zoupanou, S.; Chiriacò, M.S.; Tarantini, I.; Ferrara, F. Innovative 3d Microfluidic Tools for On-Chip Fluids and Particles Manipulation: From Design to Experimental Validation. Micromachines 2021, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Pacella, M.; Liu, Y.; Zhao, L. Surface Engineering and the Application of Laser-Based Processes to Stents—A Review of the Latest Development. Bioact. Mater. 2022, 10, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A Review on Membrane Fabrication: Structure, Properties and Performance Relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Sheridan, S.D.; Gil, S.; Wilgo, M.; Pitt, A. Microporous Membrane Growth Substrates for Embryonic Stem Cell Culture and Differentiation. Methods Cell Biol. 2008, 86, 29–57. [Google Scholar] [PubMed]
- Al Bahrawy, M.; Ghaffar, K.; Gamal, A.; El-Sayed, K.; Iacono, V. Effect of Inflammation on Gingival Mesenchymal Stem/Progenitor Cells’ Proliferation and Migration through Microperforated Membranes: An in Vitro Study. Stem Cells Int. 2020, 2020, 5373418. [Google Scholar] [CrossRef] [Green Version]
- Drioli, E.; Giorno, L. Membrane Contactors and Integrated Membrane Operations. In Comprehensive Membrane Science and Engineering; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 1–257. [Google Scholar]
- Primiceri, E.; Chiriacò, M.S.; Dioguardi, F.; Monteduro, A.G.; D’Amone, E.; Rinaldi, R.; Giannelli, G.; Maruccio, G. Automatic Transwell Assay by an EIS Cell Chip to Monitor Cell Migration. Lab Chip 2011, 11, 4081–4086. [Google Scholar] [CrossRef]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the Chick Embryo Chorioallantoic Membrane (CAM) as a Platform for Anti-Metastatic Drug Testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Saunders, K.B.; D’Amore, P.A. An in Vitro Model for Cell-Cell Interactions. In Vitro Cell. Dev. Biol.-Anim. 1992, 28, 521. [Google Scholar] [CrossRef]
- Carter, R.N.; Casillo, S.M.; Mazzocchi, A.R.; Desormeaux, J.P.S.; Roussie, J.A.; Gaborski, T.R. Ultrathin Transparent Membranes for Cellular Barrier and Co-Culture Models. Biofabrication 2017, 9, 015019. [Google Scholar] [CrossRef] [Green Version]
- Apel, P. Track Etching Technique in Membrane Technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Fleischer, R.L.; Price, P.B.; Symes, E.M. Novel Filter for Biological Materials. Science 1964, 143, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schutze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells: A New Method for the Immunomorphological and Molecular Characterization of Circulating Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, G.; Wu, J.; Xia, H. Preparation of Microporous Silicone Rubber Membrane with Tunable Pore Size via Solvent Evaporation-Induced Phase Separation. ACS Appl. Mater. Interfaces 2013, 5, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Li, D.J.; Pham, L.K.; Wong, B.G.; Hui, E.E. Microfabrication of High-Resolution Porous Membranes for Cell Culture. J. Membr. Sci. 2014, 15, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Castro, J.A.; Li, K.; Meunier, A.; Juncker, D.; Veres, T. Fabrication of Large-Area Polymer Microfilter Membranes and Their Application for Particle and Cell Enrichment. Lab Chip 2017, 17, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.H.; Lepak, L.A.; Hussain, R.J.; Shain, W.; Shuler, M.L. An Endothelial and Astrocyte Co-Culture Model of the Blood-Brain Barrier Utilizing an Ultra-Thin, Nanofabricated Silicon Nitride Membrane. Lab Chip 2005, 5, 74–85. [Google Scholar] [CrossRef]
- Lim, L.S.; Hu, M.; Huang, M.C.; Cheong, W.C.; Gan, A.T.L.; Looi, X.L.; Leong, S.M.; Koay, E.S.C.; Li, M.H. Microsieve Lab-Chip Device for Rapid Enumeration and Fluorescence in Situ Hybridization of Circulating Tumor Cells. Lab Chip 2012, 12, 4388–4396. [Google Scholar] [CrossRef]
- Zhu, B.; Duke, M.; Dumée, L.F.; Merenda, A.; des Ligneris, E.; Kong, L.; Hodgson, P.D.; Gray, S. Short Review on Porous Metal Membranes—Fabrication, Commercial Products, and Applications. Membranes 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.Y.; Wang, B.; Li, K. High Performance Stainless Steel-Ceramic Composite Hollow Fibres for Microfiltration. J. Membr. Sci. 2017, 541, 425–433. [Google Scholar] [CrossRef]
- Mott Introduces Sterilising Grade All-Metal Filtration Membrane. Filtr. + Sep. 2013, 50, 18. [CrossRef]
- Volpe, A.; Gaudiuso, C.; Di Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A. Direct Femtosecond Laser Fabrication of Superhydrophobic Aluminum Alloy Surfaces with Anti-Icing Properties. Coatings 2020, 10, 587. [Google Scholar] [CrossRef]
- Volpe, A.; Krishnan, U.; Chiriacò, M.S.; Primiceri, E.; Ancona, A.; Ferrara, F. A Smart Procedure for the Femtosecond Laser-Based Fabrication of a Polymeric Lab-on-a-Chip for Capturing Tumor Cell. Engineering 2021, 7, 1434–1440. [Google Scholar] [CrossRef]
- Volpe, A.; Covella, S.; Gaudiuso, C.; Ancona, A. Improving the Laser Texture Strategy to Get Superhydrophobic Aluminum Alloy Surfaces. Coatings 2021, 11, 369. [Google Scholar] [CrossRef]
- Sima, F.; Sugioka, K.; Vázquez, R.M.; Osellame, R.; Kelemen, L. Review Article Three-Dimensional Femtosecond Laser Processing for Lab-on-a-Chip Applications. Nanophotonics 2018, 7, 613–634. [Google Scholar] [CrossRef]
- Volpe, A.; Paiè, P.; Ancona, A.; Osellame, R. Polymeric Fully Inertial Lab-on-a-Chip with Enhanced-Throughput Sorting Capabilities. Microfluid. Nanofluid. 2019, 23, 37. [Google Scholar] [CrossRef]
- Zum Gahr, K.H.; Wahl, R.; Wauthier, K. Experimental Study of the Effect of Microtexturing on Oil Lubricated Ceramic/Steel Friction Pairs. Wear 2009, 267, 1241–1251. [Google Scholar] [CrossRef]
- Yalikun, Y.; Tanaka, N.; Hosokawa, Y.; Iino, T.; Tanaka, Y. Ultrathin Glass Filter Fabricated by Femtosecond Laser Processing for High-Throughput Microparticle Filtering. Appl. Phys. Express 2016, 9, 066702. [Google Scholar] [CrossRef]
- Yalikun, Y.; Tanaka, N.; Hosokawa, Y.; Iino, T.; Tanaka, Y. Embryonic Body Culturing in an All-Glass Microfluidic Device with Laser-Processed 4 Μm Thick Ultra-Thin Glass Sheet Filter. Biomed. Microdevices 2017, 19, 85. [Google Scholar] [CrossRef]
- Smeby, L.C.; Widerøe, T.E.; Balstad, T.; Jørstad, S. Biocompatibility Aspects Cellophane, Cellulose Acetate, Polyacrylonitrile, Polysulfone and Polycarbonate Hemodialyzers. Blood Purif. 1985, 4, 93–101. [Google Scholar] [CrossRef]
- Osellame, R.; Cerullo, G.; Ramponi, R. (Eds.) Femtosecond Laser Micromachining: Photonic and Microfluidic Devices in Transparent Materials; Springer: Berlin/Heidelberg, Germany, 2012; Volume 123, ISBN 9783642233654. [Google Scholar]
- Weber, R.; Graf, T.; Berger, P.; Onuseit, V.; Wiedenmann, M.; Freitag, C.; Feuer, A. Heat Accumulation during Pulsed Laser Materials Processing. Opt. Express 2014, 22, 11312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, F.; Michalowski, A.; Kiedrowski, T.; Nolte, S. Heat Accumulation in Ultra-Short Pulsed Scanning Laser Ablation of Metals. Opt. Express 2015, 23, 1035. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Trotta, G.; Krishnan, U.; Ancona, A. Prediction Model of the Depth of the Femtosecond Laser Micro-Milling of PMMA. Opt. Laser Technol. 2019, 120, 105713. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Liu, S.-T.; Lin, W.-R.; Lin, C.-K.; Huang, S.-M. The Mechanisms Underlying the Cytotoxic Effects of Copper Via Differentiated Embryonic Chondrocyte Gene 1. Int. J. Mol. Sci. 2019, 20, 5225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnal, N.; de Alaniz, M.J.T.; Marra, C.A. Cytotoxic Effects of Copper Overload on Human-Derived Lung and Liver Cells in Culture. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Trotta, G.; Volpe, A.; Ancona, A.; Fassi, I. Flexible Micro Manufacturing Platform for the Fabrication of PMMA Microfluidic Devices. J. Manuf. Processes 2018, 35, 107–117. [Google Scholar] [CrossRef]
- Li, L.; Low, D.K.Y.; Ghoreshi, M.; Crookall, J.R. Hole Taper Characterisation and Control in Laser Percussion Drilling. CIRP Ann.-Manuf. Technol. 2002, 51, 153–156. [Google Scholar] [CrossRef]
- Kuang, Z.; Perrie, W.; Leach, J.; Sharp, M.; Edwardson, S.P.; Padgett, M.; Dearden, G.; Watkins, K.G. High Throughput Diffractive Multi-Beam Femtosecond Laser Processing Using a Spatial Light Modulator. Appl. Surf. Sci. 2008, 255, 2284–2289. [Google Scholar] [CrossRef]
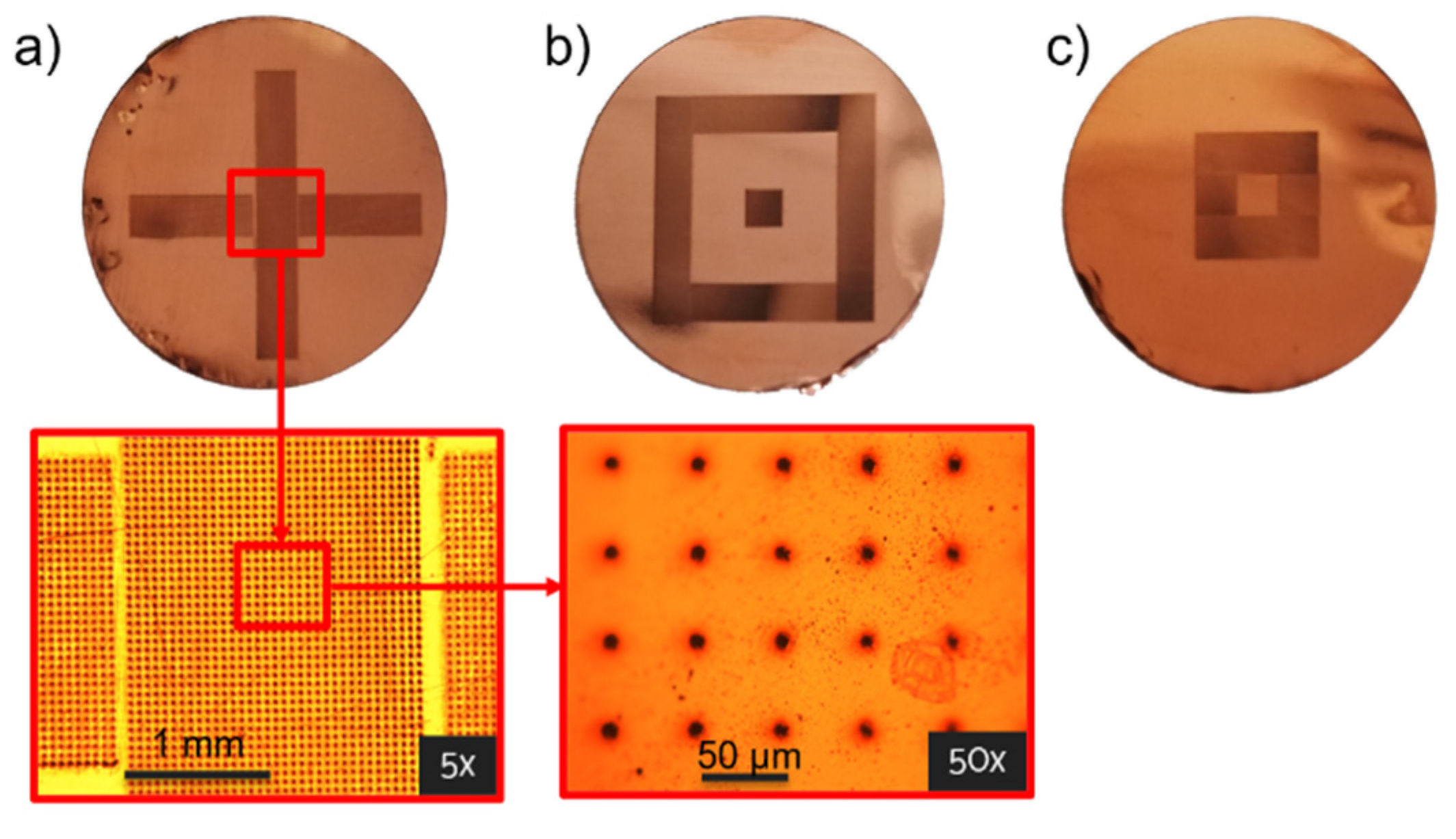
| Cu-Kapton-Cu (CKC) | CA | |
|---|---|---|
| Wavelength (nm) | 1030 | 1030 |
| Pulse duration (fs) | 200 | 200 |
| Power (mW) | 250 | 30 |
| Repetition Rate (kHz) | 50 | 0.05 |
| Pulse Energy (µJ) | 5 | 1 103 |
| Fluence | 22.6 | 45.3 × 102 |
| Shots per hole | 2500 | 30 |
| Configuration 1 | Configuration 2 | Configuration 3 | |
|---|---|---|---|
| Pores number | 15,925 | 20,825 | 9800 |
| Pore-pore distance (µm) | 50 | 50 | 50 |
| Pore exit diameter (µm) | 8 ± 1 | 8 ± 1 | 8 ± 1 |
| Pore density (pores/mm2) | 103 | 135 | 63 |
| Fabrication time (min) | 40 | 52 | 25 |
| Configuration 1 | Configuration 2 | Configuration 3 | |
|---|---|---|---|
| Pores number | 4440 | 5806 | 2732 |
| Pore-pore distance (µm) | 200–400 | 200–400 | 200–400 |
| Pore exit diameter (µm) | 18 ± 2 | 18 ± 2 | 18 ± 2 |
| Pore density (pores/mm2) | 30 | 38 | 18 |
| Fabrication time (min) | 81 | 106 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, A.; Conte Capodacqua, F.M.; Garzarelli, V.; Primiceri, E.; Chiriacò, M.S.; Gaudiuso, C.; Ferrara, F.; Ancona, A. Femtosecond Laser Fabrication of Microporous Membranes for Biological Applications. Micromachines 2022, 13, 1371. https://doi.org/10.3390/mi13091371
Volpe A, Conte Capodacqua FM, Garzarelli V, Primiceri E, Chiriacò MS, Gaudiuso C, Ferrara F, Ancona A. Femtosecond Laser Fabrication of Microporous Membranes for Biological Applications. Micromachines. 2022; 13(9):1371. https://doi.org/10.3390/mi13091371
Chicago/Turabian StyleVolpe, Annalisa, Filippo Maria Conte Capodacqua, Valeria Garzarelli, Elisabetta Primiceri, Maria Serena Chiriacò, Caterina Gaudiuso, Francesco Ferrara, and Antonio Ancona. 2022. "Femtosecond Laser Fabrication of Microporous Membranes for Biological Applications" Micromachines 13, no. 9: 1371. https://doi.org/10.3390/mi13091371
APA StyleVolpe, A., Conte Capodacqua, F. M., Garzarelli, V., Primiceri, E., Chiriacò, M. S., Gaudiuso, C., Ferrara, F., & Ancona, A. (2022). Femtosecond Laser Fabrication of Microporous Membranes for Biological Applications. Micromachines, 13(9), 1371. https://doi.org/10.3390/mi13091371










