Automated Denudation of Oocytes
Abstract
1. Introduction
2. System Overview
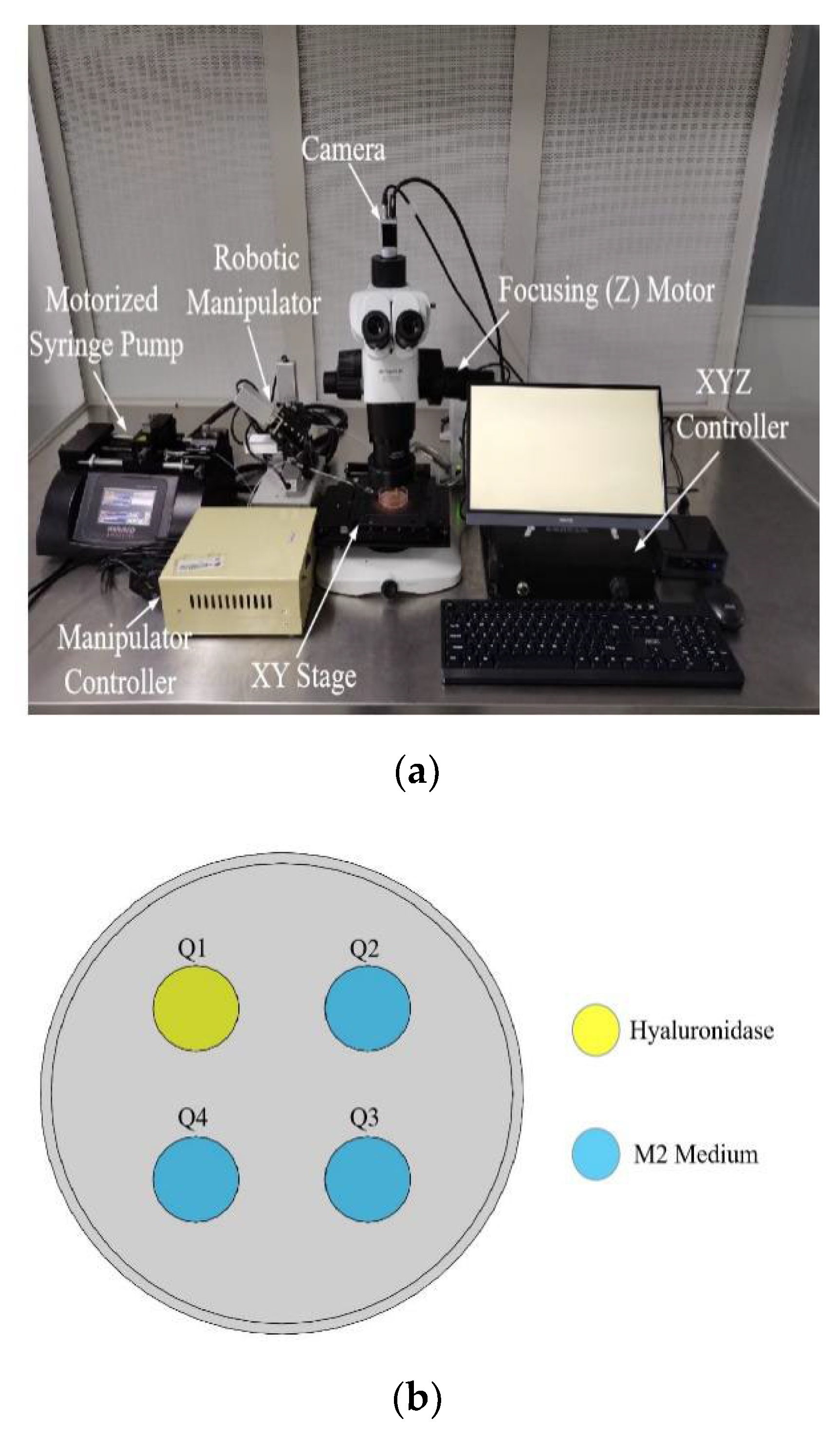
2.1. System Setup
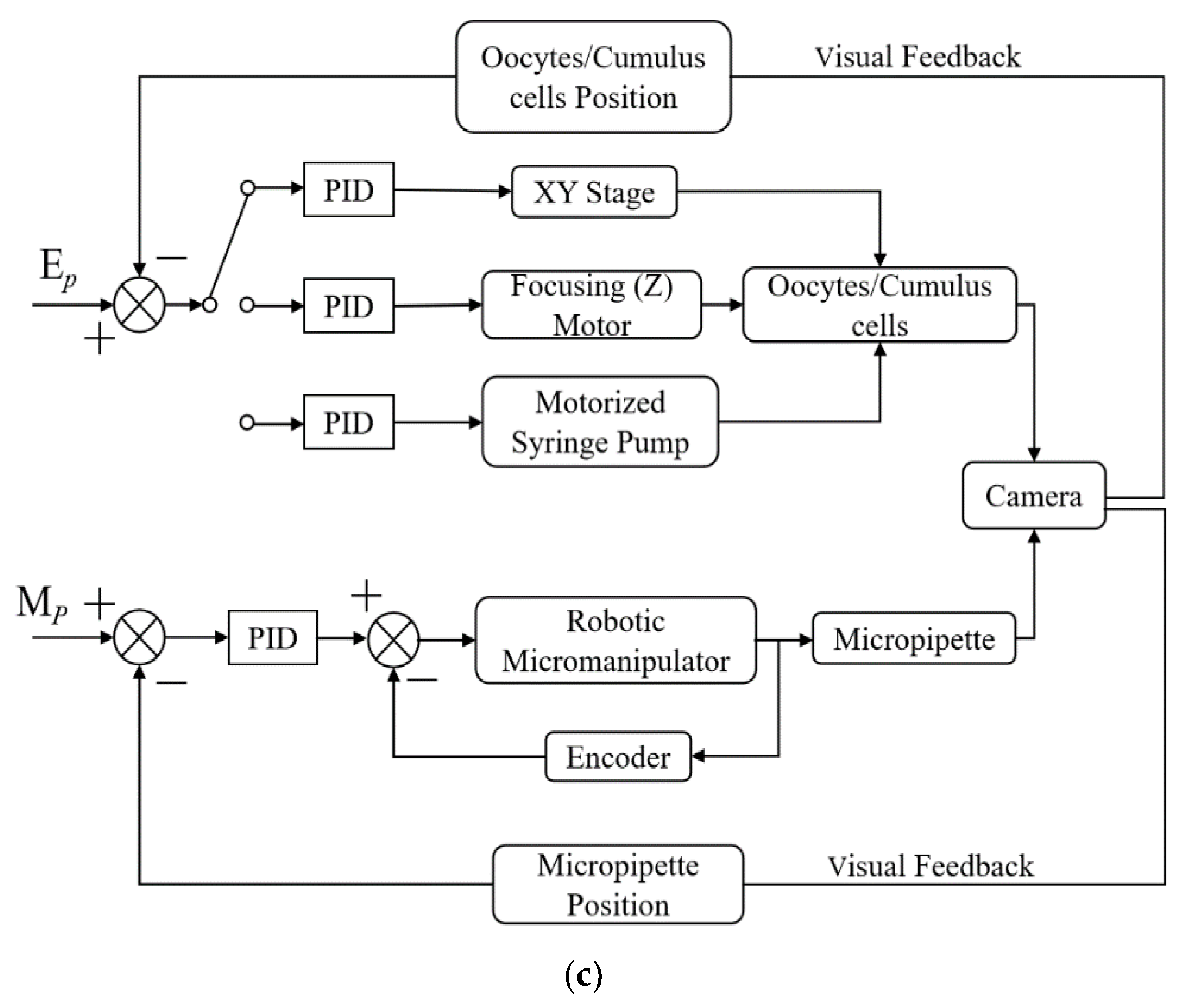
2.2. System Operation
3. Key Methods
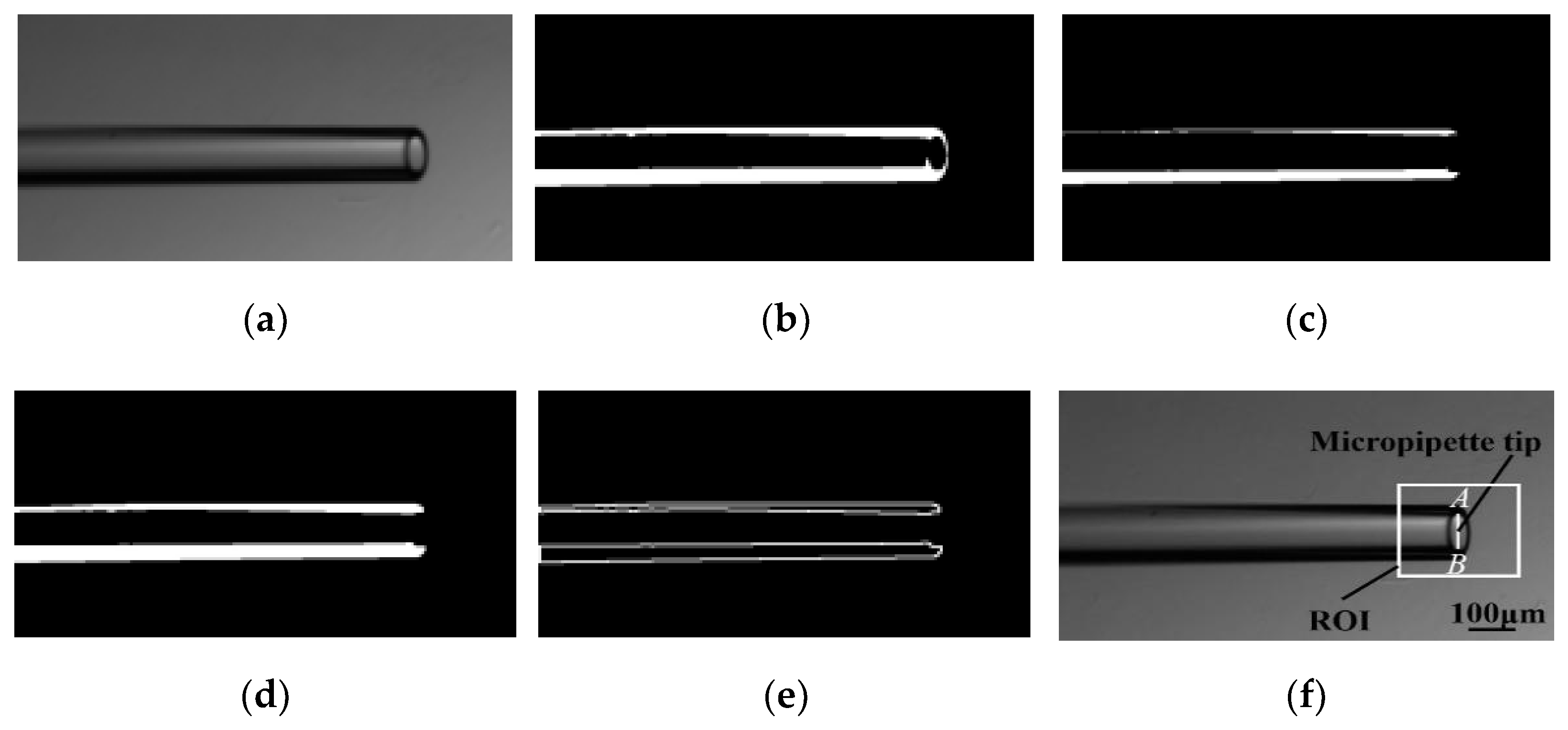
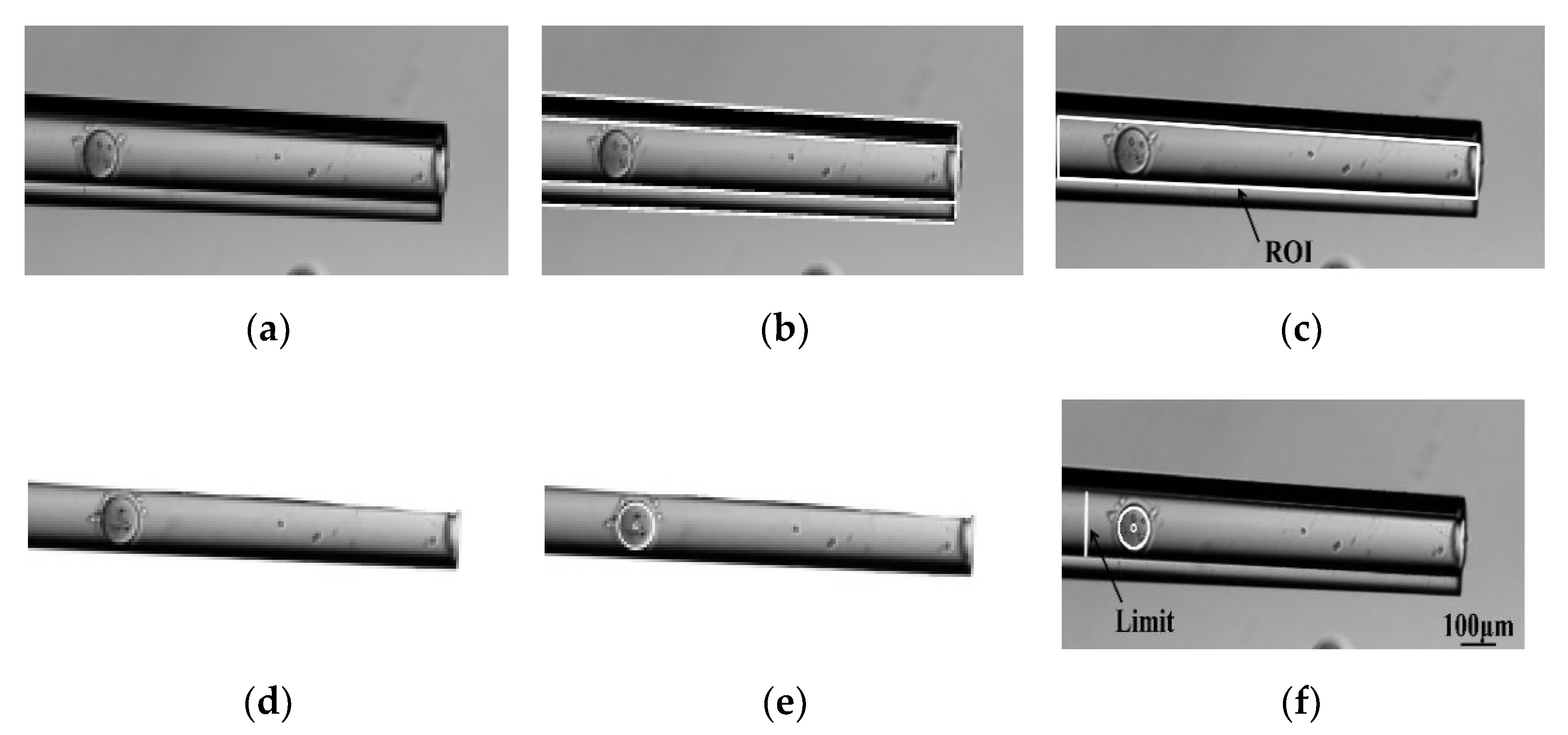
3.1. Contact Detection and Micropipette Tip Identification
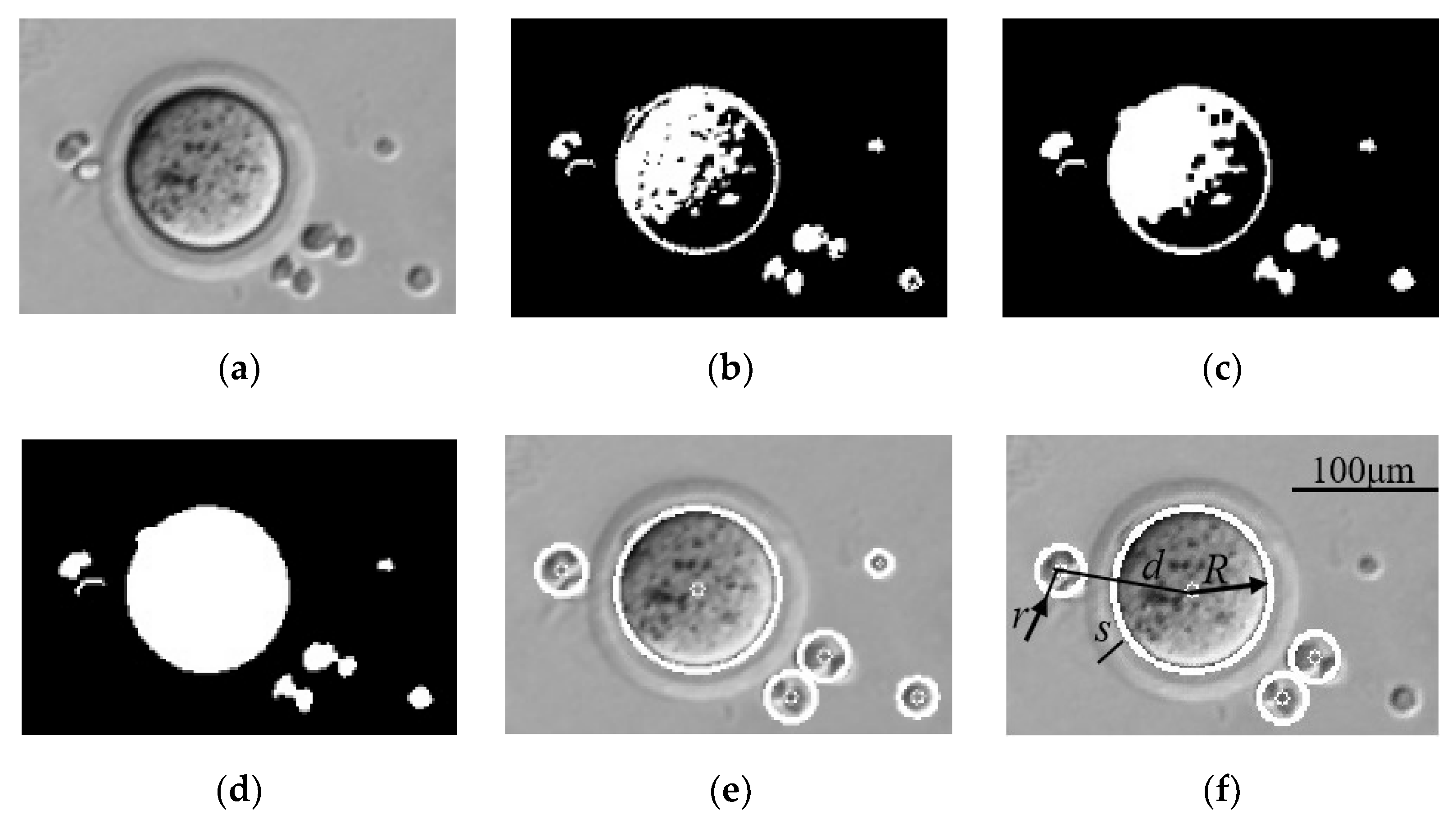
3.2. Oocyte and Cumulus Cell Recognition
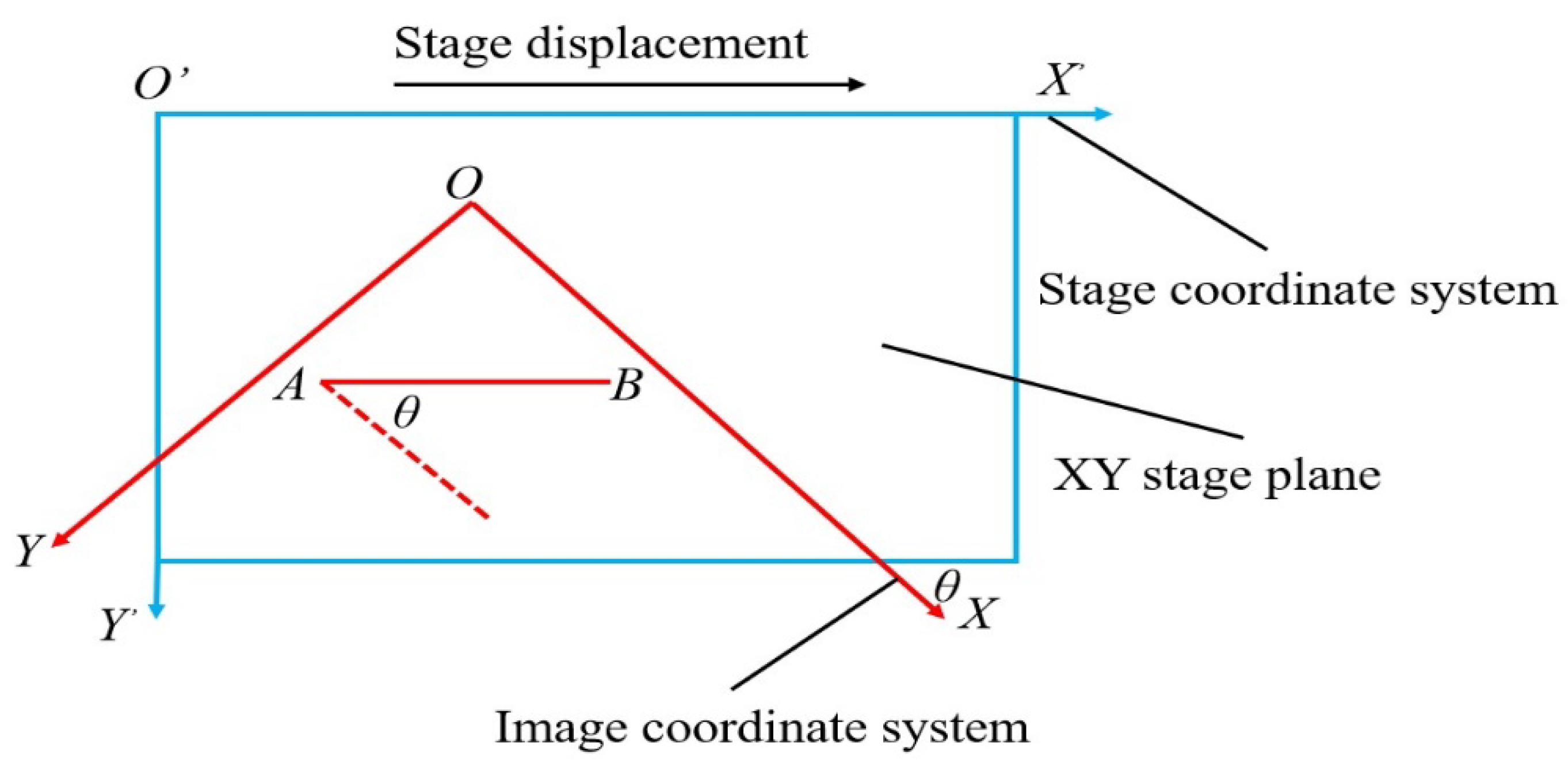
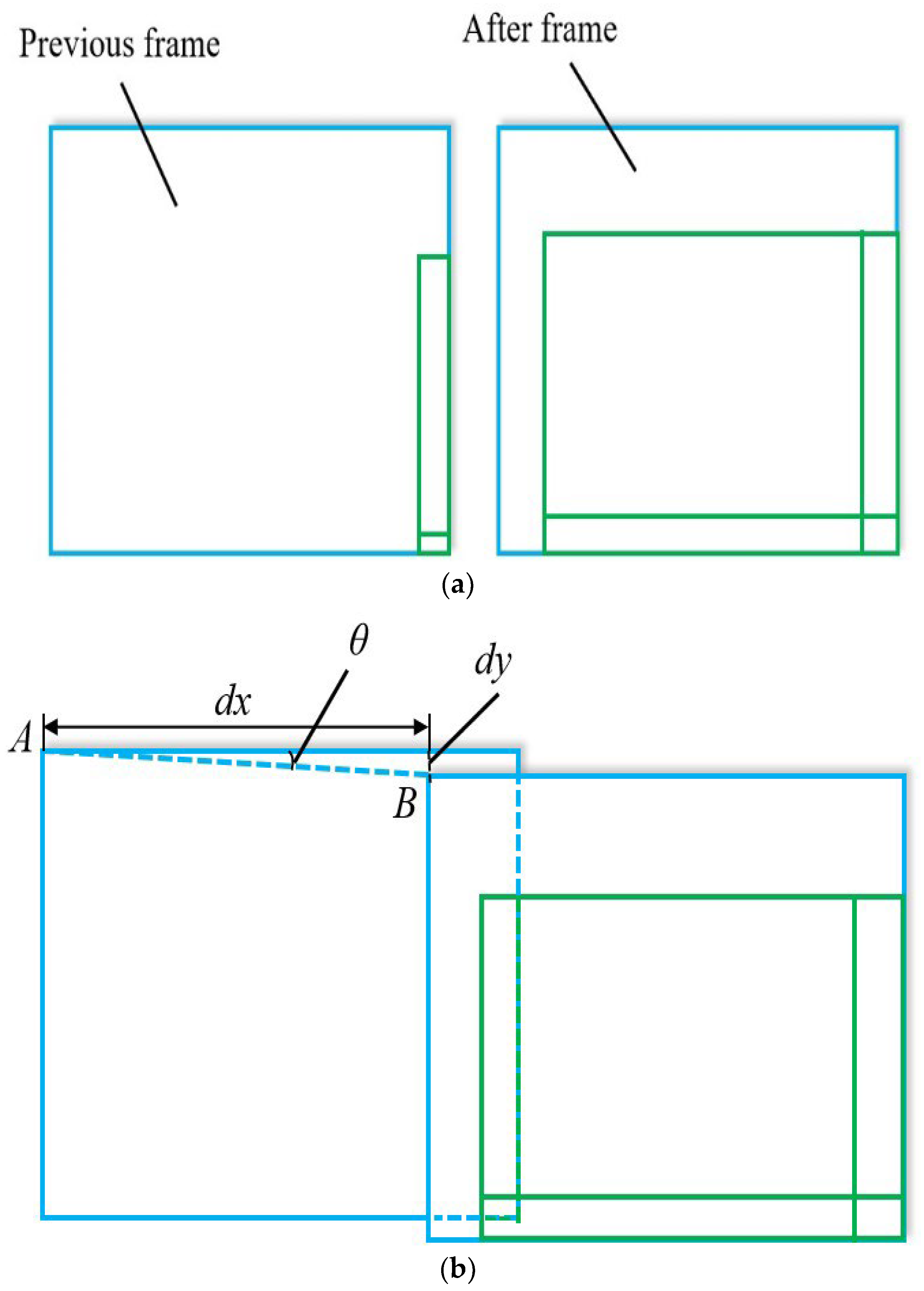
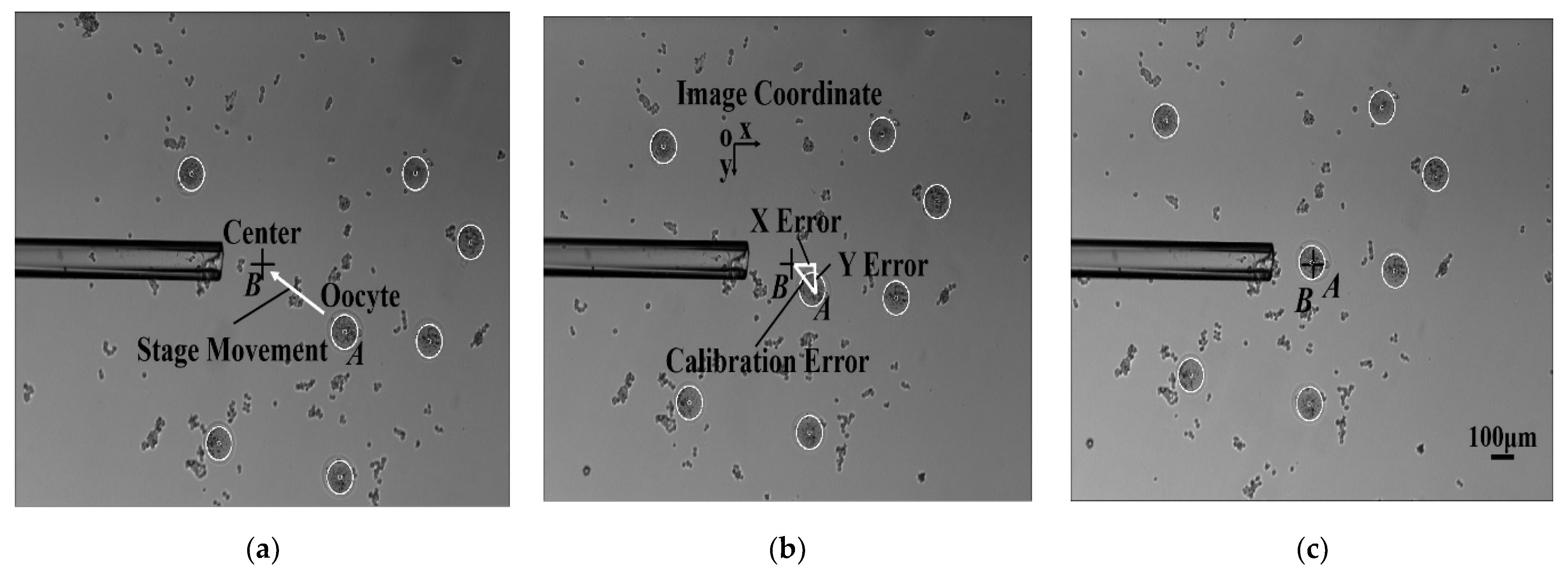
3.3. Calibration with XY Stage
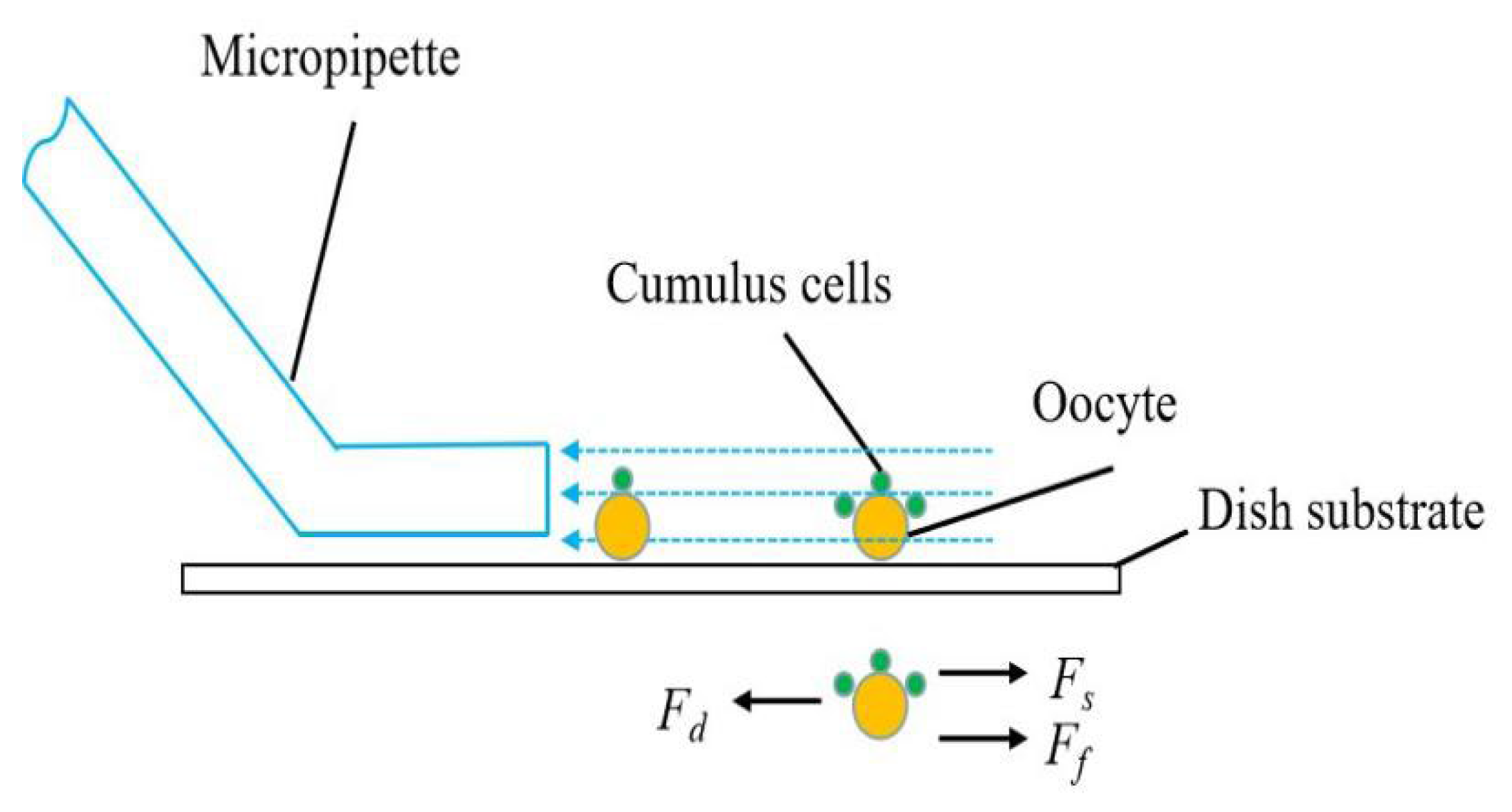
3.4. Fluid Flow Control
4. Results and Discussion
4.1. Visual Recognition
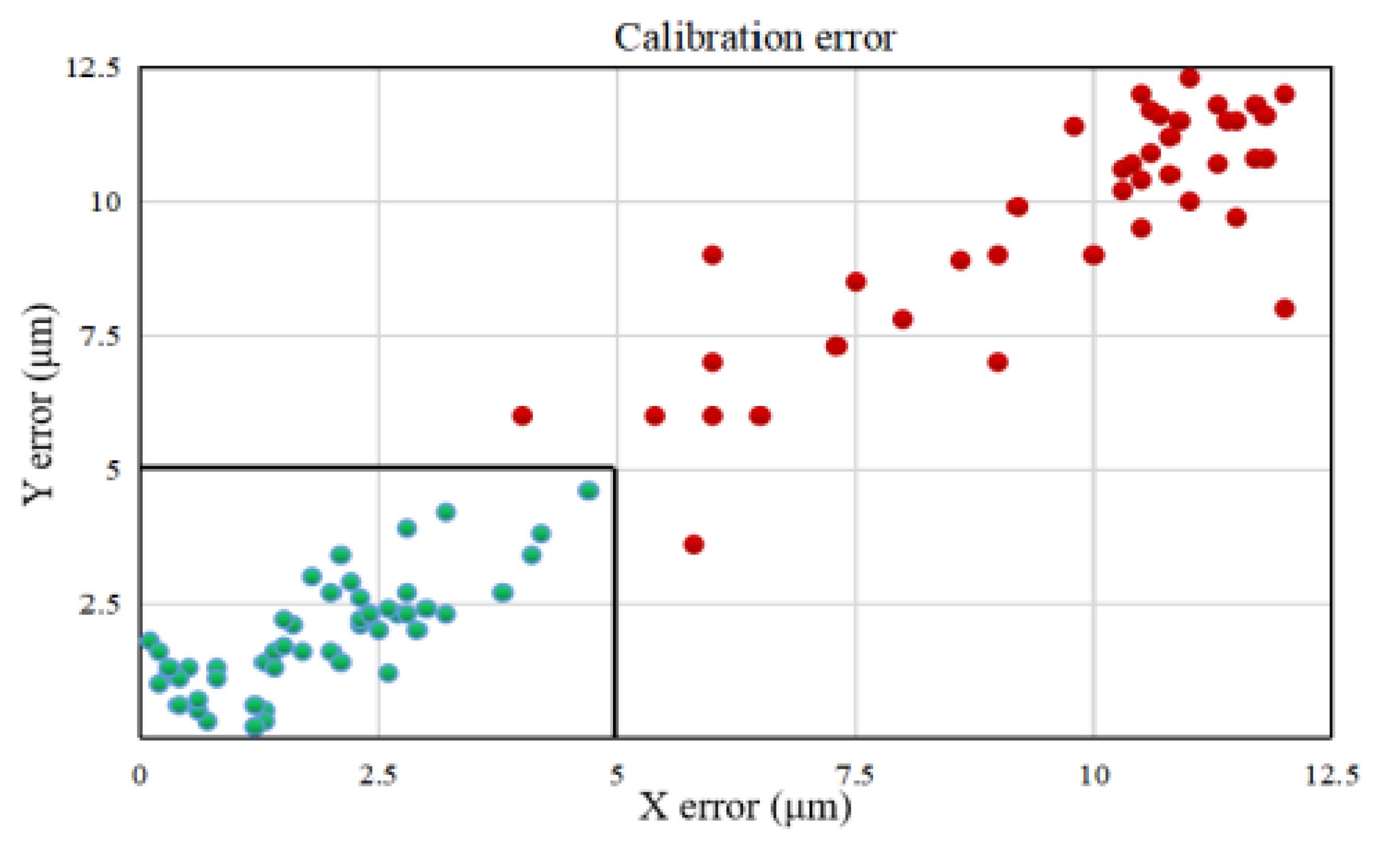
4.2. Automated Calibration
4.3. Yield Rate and Denudation Efficiency
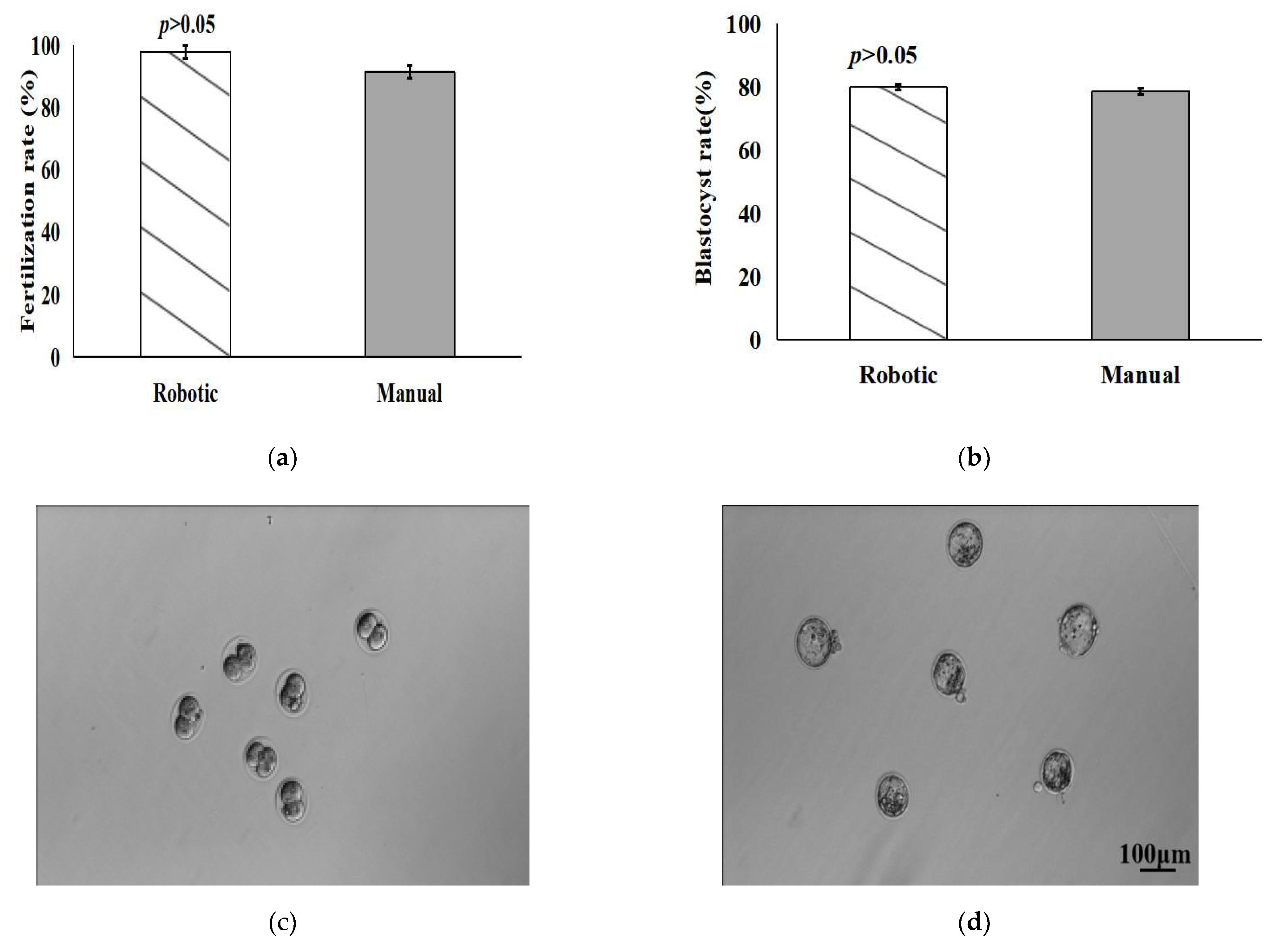
4.4. Fertilization and Developmental Potential
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ombelet, W.; Cooke, I.; Dyer, S.; Serour, G.; Devroey, P. Infertility and the provision of fertility medical services in developing countries. Hum. Reprod. Update 2008, 14, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Chen, M.J.; Yi, Y.C.; Guu, H.F.; Ho, E.S. The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. J. Assist. Reprod. Genet. 2003, 20, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Plachot, M.; Belaisch-Allart, J.; Mayenga, J.M.; Chouraqui, A.; Tesquier, L.; Serkine, A.M. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum. Reprod. 2002, 17, 362–369. [Google Scholar] [CrossRef]
- Rienzi, L.; Ubaldi, F.; Anniballo, R.; Cerulo, G.; Greco, E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A. Cumulus cell contribution to cytoplasmic maturation and oocyte developmental competence in vitro. J. Assist. Reprod. Genet. 2001, 18, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The oocyte. Hum. Reprod. 2012, 27, i2–i21. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Ebner, T.; Moser, M.; Sommergruber, M.; Shebl, O.; Tews, G. Incomplete denudation of oocytes prior to ICSI enhances embryo quality and blastocyst development. Hum. Reprod. 2006, 21, 2972–2977. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; Van Landuyt, L.; Van Ranst, H.; Vander Monde, A.; D’Haese, V.; Sterckx, J.; Haentjens, P.; Devroey, P.; Van der Elst, J. Randomized sibling-oocyte study using recombinant human hyaluronidase versus bovine derived Sigma hyaluronidase in ICSI patients. Hum. Reprod. 2008, 23, 1815–1819. [Google Scholar] [CrossRef]
- Van de Velde, H.; Nagy, Z.P.; Joris, H.; De Vos, A.; Van Steirteghem, A.C. Effects of different hyaluronidase concentrations and mechanical procedures for cumulus cellremoval on the outcome of intracytoplasmic sperm injection. Hum. Reprod. 1997, 12, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Van de Veldel, H.; de Vos, A.; Joris, H.; Nagy, Z.P.; van Steirteghem, A.C. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- De Moura, B.R.; Gurgel, M.C.; Machado, S.P.; Marques, P.A.; Rolim, J.R.; de Lima, M.C.; Salgueiro, L.L. Low concentration of hyaluronidase for oocyte denudation can improve fertilization rates and embryo quality. JBRA Assist. Reprod. 2017, 21, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, M.M.; Trounson, A.O. Removal of the cumulus oophorus from the human oocyte for in vitro fertilization. Fertil. Steril. 1985, 43, 263–267. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, X.; Leung, C.; Esfandiari, N.; Casper, R.F.; Sun, Y. Robotic ICSI (Intracytoplasmic Sperm Injection). IEEE Trans. Biomed. Eng. 2011, 58, 2102–2108. [Google Scholar] [PubMed]
- Leung, C.; Lu, Z.; Esfandiari, N.; Casper, R.F.; Sun, Y. Automated Sperm Immobilization for Intracytoplasmic Sperm Injection. IEEE Trans. Biomed. Eng. 2011, 58, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Leung, C.; Lu, Z.; Esfandiari, N.; Casper, R.F.; Sun, Y. Casper and Yu Sun. Controlled aspiration and positioning of biological cells in a micropipette. IEEE Trans. Biomed. Eng. 2012, 59, 1032–1040. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Wen, J.; Pyne, D.; Liu, H.; Ru, C.; Sun, Y. Automated vitrification of embryos: A robotics approach. IEEE Robot. Autom. Mag. 2015, 22, 33–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Wang, X.; Zhao, Q.; Zhou, C.; Tan, M.; Sun, Y. Robotic Pick-And-Place of Multiple Embryos for Vitrification. IEEE Robot. Autom. Lett. 2017, 2, 570–576. [Google Scholar] [CrossRef]
- Zeringue, H.; Beebe, D.; Wheeler, M. A microfluidic method for removal of the zona pellucida from mammalian embryos. Lab Chip 2005, 5, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Zeringue, H.; Rutledge, J.; Beebe, D. Early mammalian embryo development depends on cumulus removal technique. Lab Chip 2005, 5, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Lee, G.Y.; Liu, J.; Kapur, R.; Toth, T.L.; Toner, M. Toth and Mehmet Toner. On-chip oocyte denudation from cumulus–oocyte complexes for assisted reproductive therapy. Lab Chip 2018, 18, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Mokhtare, A.; Xie, P.; Abbaspourrad, A.; Rosenwaks, Z.; Palermo, G. Toward an ICSI chip: Automated microfluidic oocyte denudation module. Hum. Reprod. 2020, 35, 71–72. [Google Scholar]
- Xie, P.; Mokhtare, A.; Davaji, B.; Rosenwaks, Z.; Abbaspourrad, A.; D Palermo, G. An expedited and safe oocyte denudationb system based on soundwaves in a microfluidic chip. Fertil. Steril. 2021, 116, e152–e153. [Google Scholar] [CrossRef]
- Reeder, A.; Monson, R.; Beebe, D.; Lindsey, B.; Rutledge, J. Enhanced bovine embryonic development after microfluidic cumulus cell removal post-fertilization. Reprod. Fertil. Dev. 2005, 17, 228–229. [Google Scholar] [CrossRef]
- Wang, W.H.; Liu, X.Y.; Sun, Y. Contact detection in microrobotic manipulation. Int. J. Robot. Res. 2007, 26, 821–823. [Google Scholar] [CrossRef]
- Ru, C.H.; Liu, J.; Pang, M.; Sun, Y. Controlled ultrasonic micro-dissection of thin tissue sections. Biomed. Microdevices 2014, 4, 567–573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lingua, A.; Marenchino, D.; Nex, F. Performance analysis of the SIFT operator for automatic feature extraction and matching in photogrammetric applications. Sensors 2009, 9, 3745–3766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, M.; Liu, Y.; Cai, W.; Chen, Y.; Yang, K. Remote sensing image matching by integrating affine invariant feature extraction and RANSAC. Comput. Electr. Eng. 2012, 38, 1023–1032. [Google Scholar] [CrossRef]
| Experiment | 1 | 2 | 3 | 4 | 5 | Mean ± SD |
|---|---|---|---|---|---|---|
| Manual yield rate (%) | 85 (17/20) | 87.5 (14/16) | 86.7 (13/15) | 85.7 (12/14) | 89.5 (17/19) | 86.9 ± 1.7 |
| Robotic yield rate (%) | 100 (25/25) | 95.2 (20/21) | 100 (18/18) | 94.1 (16/17) | 95.5 (21/22) | 97.0 ± 2.8 |
| Manual denudation efficiency (%) | 80 (16/20) | 81.3 (13/16) | 80 (12/15) | 85.7 (12/14) | 78.9 (15/19) | 81.2 ± 2.7 |
| Robotic denudation efficiency (%) | 96 (24/25) | 95.2 (20/21) | 94.4 (17/18) | 94.1 (16/17) | 95.5 (21/22) | 95.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, R.; Shan, G.; Dai, C.; Hao, M.; Zhu, J.; Ru, C.; Sun, Y. Automated Denudation of Oocytes. Micromachines 2022, 13, 1301. https://doi.org/10.3390/mi13081301
Zhai R, Shan G, Dai C, Hao M, Zhu J, Ru C, Sun Y. Automated Denudation of Oocytes. Micromachines. 2022; 13(8):1301. https://doi.org/10.3390/mi13081301
Chicago/Turabian StyleZhai, Rongan, Guanqiao Shan, Changsheng Dai, Miao Hao, Junhui Zhu, Changhai Ru, and Yu Sun. 2022. "Automated Denudation of Oocytes" Micromachines 13, no. 8: 1301. https://doi.org/10.3390/mi13081301
APA StyleZhai, R., Shan, G., Dai, C., Hao, M., Zhu, J., Ru, C., & Sun, Y. (2022). Automated Denudation of Oocytes. Micromachines, 13(8), 1301. https://doi.org/10.3390/mi13081301






