Design and Synthesis of Cobalt-Based Hollow Nanoparticles through the Liquid Metal Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
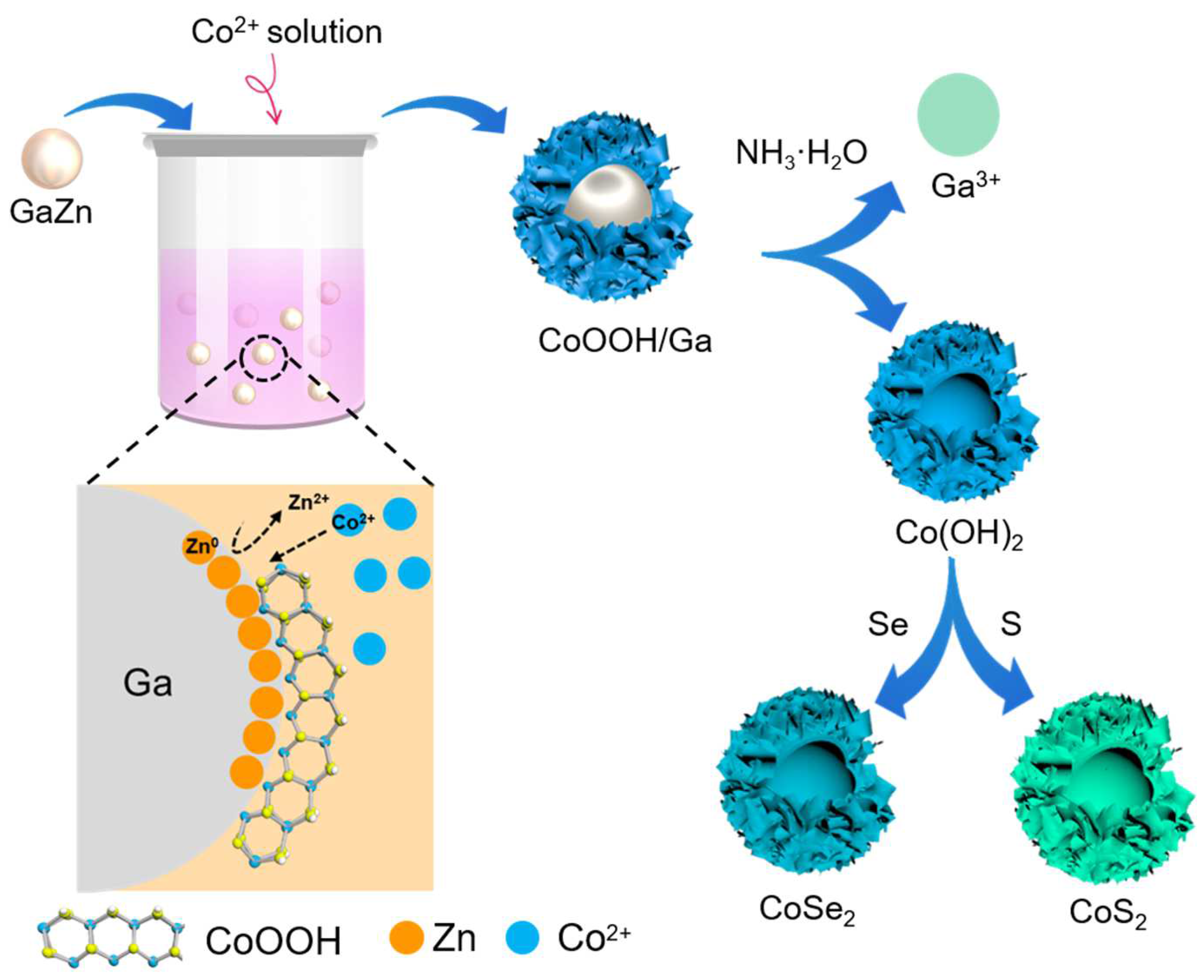
2.2. Preparation of Hollow CoSe2 Nanospheres
2.3. Electrochemical Measurements
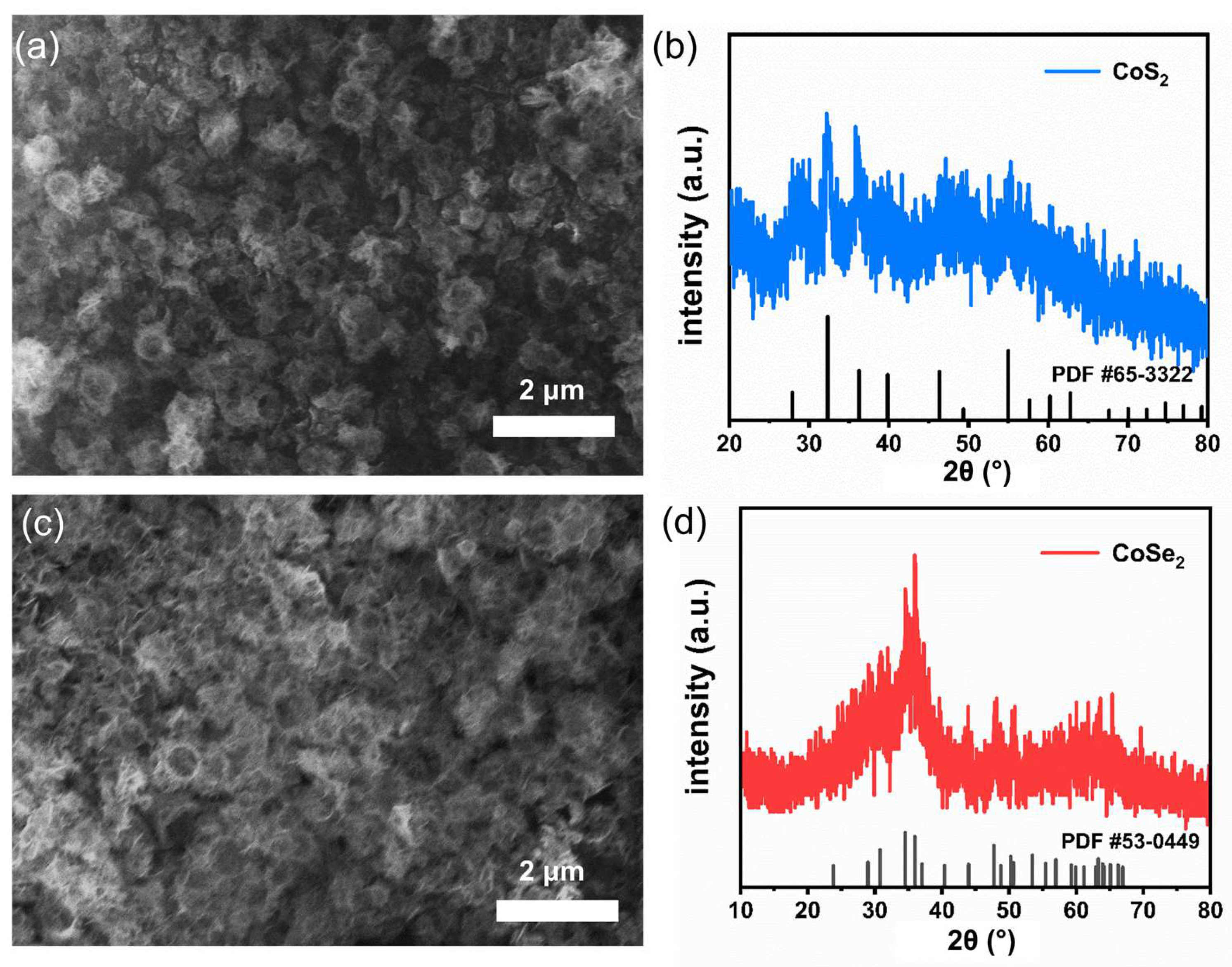
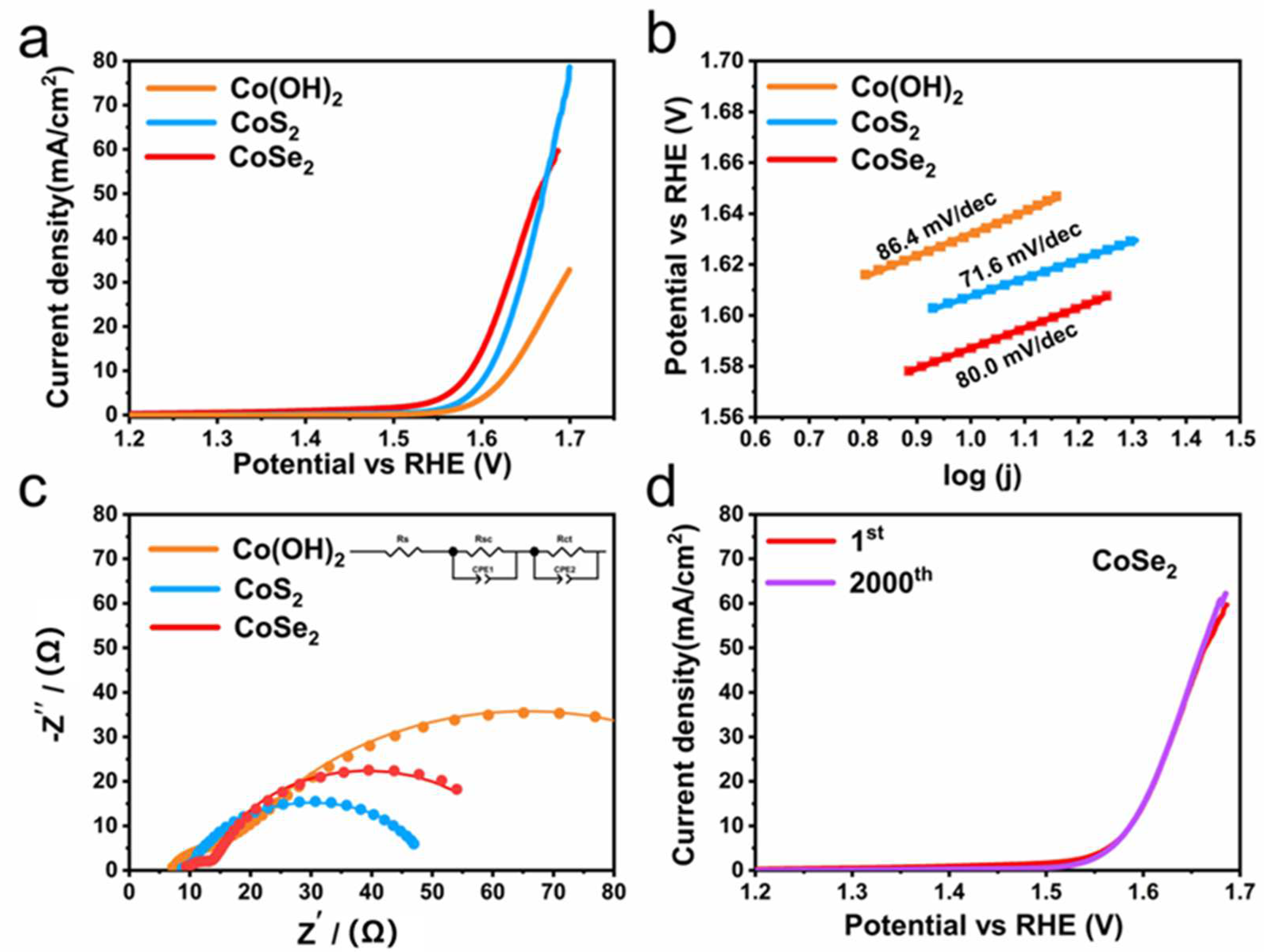
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Qian, X.; Zhu, C.; Jiang, X.; Shao, L.; Hou, L. Template synthesis of CoSe2/Co3Se4 nanotubes: Tuning of their crystal structures for photovoltaics and hydrogen evolution in alkaline medium. J. Mater. Chem. A 2017, 5, 4513–4526. [Google Scholar] [CrossRef]
- Feng, S.; Yang, L.; Zhang, Z.; Li, Q.; Xu, D. Au-decorated CoOOH nanoplate hierarchical hollow structure for plasmon-enhanced electrocatalytic water oxidation. ACS Appl. Energy Mater. 2019, 3, 943–950. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Tang, F.; Zheng, X.; Cheng, W.; Che, W.; Hu, F.; Jiang, Y.; Liu, Q.; Wei, S. Strongly electrophilic heteroatoms confined in atomic CoOOH nanosheets realizing efficient electrocatalytic water oxidation. J. Mater. Chem. A 2018, 6, 3202–3210. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, C.; Wang, Z.; Han, C.B.; Zhang, Q.; Ke, X.; Liu, J.; Wang, H. Seamlessly conductive Co(OH)2 tailored atomically dispersed Pt electrocatalyst with a hierarchical nanostructure for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2020, 13, 3082–3092. [Google Scholar] [CrossRef]
- Zhou, L.; Zhuang, Z.; Zhao, H.; Lin, M.; Zhao, D.; Mai, L. Intricate Hollow Structures: Controlled Synthesis and Applications in Energy Storage and Conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef]
- Kim, E.H.; Reddy, D.A.; Lee, H.; Jeong, S.; Kumar, D.P.; Song, J.K.; Lim, M.; Kim, T.K. Hollow CoSe2 nanocages derived from metal–organic frameworks as efficient non-precious metal co-catalysts for photocatalytic hydrogen production. Catal. Sci. Technol. 2019, 9, 4702–4710. [Google Scholar] [CrossRef]
- Dai, C.; Tian, X.; Nie, Y.; Tian, C.; Yang, C.; Zhou, Z.; Li, Y.; Gao, X. Successful synthesis of 3D CoSe2 hollow microspheres with high surface roughness and its excellent performance in catalytic hydrogen evolution reaction. Chem. Eng. J. 2017, 321, 105–112. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Fan, L.-Z. MOF-derived CoSe2 microspheres with hollow interiors as high-performance electrocatalysts for the enhanced oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 15310–15314. [Google Scholar] [CrossRef]
- Liang, L.; Li, J.; Zhu, M.; Li, Y.; Chou, S.; Li, W. Cobalt chalcogenides/cobalt phosphides/cobaltates with hierarchical nanostructures for anode materials of lithium-ion batteries: Improving the lithiation environment. Small 2019, 17, e1903418. [Google Scholar] [CrossRef]
- Yang, S.H.; Park, G.D.; Kim, J.K.; Kang, Y.C. New strategy to synthesize optimal cobalt diselenide@hollow mesoporous carbon nanospheres for highly efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 424, 130341. [Google Scholar] [CrossRef]
- Xiong, S.; Zeng, H.C. Serial ionic exchange for the synthesis of multishelled copper sulfide hollow spheres. Angew. Chem. Int. Ed. Engl. 2012, 51, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, M.; Song, H.; Noonan, O.; Zhang, J.; Yang, Y.; Zhou, L.; Yu, C. Self-Organized Mesostructured Hollow Carbon Nanoparticles via a Surfactant-Free Sequential Heterogeneous Nucleation Pathway. Chem. Mater. 2015, 27, 6297–6304. [Google Scholar] [CrossRef]
- Kim, D.; Thissen, P.; Viner, G.; Lee, D.W.; Choi, W.; Chabal, Y.J.; Lee, J.B. Recovery of nonwetting characteristics by surface modification of gallium-based liquid metal droplets using hydrochloric acid vapor. ACS Appl. Mater. Interfaces 2013, 5, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kumar, P.V.; Scott, J.A.; Tang, J.; Ghasemian, M.B.; Mousavi, M.; Han, J.; Esrafilzadeh, D.; Khoshmanesh, K.; Daeneke, T.; et al. Low Temperature Nano Mechano-electrocatalytic CH4 Conversion. ACS Nano 2022, 16, 8684–8693. [Google Scholar] [CrossRef]
- Goff, A.; Aukarasereenont, P.; Nguyen, C.K.; Grant, R.; Syed, N.; Zavabeti, A.; Elbourne, A.; Daeneke, T. An exploration into two-dimensional metal oxides, and other 2D materials, synthesised via liquid metal printing and transfer techniques. Dalton Trans. 2021, 50, 7513–7526. [Google Scholar] [CrossRef]
- Wang, S.; Mao, Q.; Ren, H.; Wang, W.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Liquid Metal Interfacial Growth and Exfoliation to Form Mesoporous Metallic Nanosheets for Alkaline Methanol Electroreforming. ACS Nano 2022, 16, 2978–2987. [Google Scholar] [CrossRef]
- Wang, Y.; Mayyas, M.; Yang, J.; Tang, J.; Ghasemian, M.B.; Han, J.; Elbourne, A.; Daeneke, T.; Kaner, R.B.; Kalantar-Zadeh, K. Self-Deposition of 2D Molybdenum Sulfides on Liquid Metals. Adv. Funct. Mater. 2020, 31, 2005866. [Google Scholar] [CrossRef]
- Zavabeti, A.; Zhang, B.Y.; de Castro, I.A.; Ou, J.Z.; Carey, B.J.; Mohiuddin, M.; Datta, R.; Xu, C.; Mouritz, A.P.; McConville, C.F.; et al. Green Synthesis of Low-Dimensional Aluminum Oxide Hydroxide and Oxide Using Liquid Metal Reaction Media: Ultrahigh Flux Membranes. Adv. Funct. Mater. 2018, 28, 1804054–1804057. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Chang, H.; Rao, W. Galvanic Replacement of Liquid Metal/Reduced Graphene Oxide Frameworks. Adv. Mater. Interfaces 2020, 7, 2000626. [Google Scholar] [CrossRef]
- Aukarasereenont, P.; Goff, A.; Nguyen, C.K.; McConville, C.F.; Elbourne, A.; Zavabeti, A.; Daeneke, T. Liquid metals: An ideal platform for the synthesis of two-dimensional materials. Chem. Soc. Rev. 2022, 51, 1253–1276. [Google Scholar] [CrossRef]
- Ghasemian, M.B.; Mayyas, M.; Idrus-Saidi, S.A.; Jamal, M.A.; Yang, J.; Mofarah, S.S.; Adabifiroozjaei, E.; Tang, J.; Syed, N.; O’Mullane, A.P.; et al. Self-Limiting Galvanic Growth of MnO2 Monolayers on a Liquid Metal—Applied to Photocatalysis. Adv. Funct. Mater. 2019, 29, 1901649. [Google Scholar] [CrossRef]
- Ren, L.; Cheng, N.; Man, X.; Qi, D.; Liu, Y.; Xu, G.; Cui, D.; Liu, N.; Zhong, J.; Peleckis, G.; et al. General Programmable Growth of Hybrid Core-Shell Nanostructures with Liquid Metal Nanodroplets. Adv. Mater. 2021, 33, e2008024. [Google Scholar] [CrossRef] [PubMed]
- Falchevskaya, A.S.; Prilepskii, A.Y.; Tsvetikova, S.A.; Koshel, E.I.; Vinogradov, V.V. Facile Synthesis of a Library of Hollow Metallic Particles through the Galvanic Replacement of Liquid Gallium. Chem. Mater. 2021, 33, 1571–1580. [Google Scholar] [CrossRef]
- Zavabeti, A.; Ou, J.Z.; Carey, B.J.; Syed, N.; Orrell-Trigg, R.; Mayes, E.L.H.; Xu, C.; Kavehei, O.; O’Mullane, A.P.; Kaner, R.B.; et al. A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides. Science 2017, 358, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kronawitter, C.X.; Yeh, Y.-W.; Yang, X.; Zhao, P.; Yao, N.; Koel, B.E. Activity of pure and transition metal-modified CoOOH for the oxygen evolution reaction in an alkaline medium. J. Mater. Chem. A 2017, 5, 842–850. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chang, Y.-C.; Lee, J.-F.; Pao, C.-W.; Huang, H.-C.; Wang, C.-H. Operando Identification of Hydrangea-like and Amorphous Cobalt Oxyhydroxide Supported by Thin-Layer Copper for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2021, 9, 12300–12310. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hung, S.F.; Chen, H.Y.; Chan, T.S.; Chen, H.M.; Liu, B. In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36–39. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Yao, T.; He, J.; Jiang, S.; Sun, Z.; Liu, Q.; Cheng, W.; Hu, F.; Jiang, Y.; et al. CoOOH Nanosheets with High Mass Activity for Water Oxidation. Angew. Chem. Int. Ed. Engl. 2015, 54, 8722–8727. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Li, Z.; Liu, Y.; Wu, X.; Ren, L. Design and Synthesis of Cobalt-Based Hollow Nanoparticles through the Liquid Metal Template. Micromachines 2022, 13, 1292. https://doi.org/10.3390/mi13081292
Ji Y, Li Z, Liu Y, Wu X, Ren L. Design and Synthesis of Cobalt-Based Hollow Nanoparticles through the Liquid Metal Template. Micromachines. 2022; 13(8):1292. https://doi.org/10.3390/mi13081292
Chicago/Turabian StyleJi, Yuan, Zhenlong Li, Yundan Liu, Xianghua Wu, and Long Ren. 2022. "Design and Synthesis of Cobalt-Based Hollow Nanoparticles through the Liquid Metal Template" Micromachines 13, no. 8: 1292. https://doi.org/10.3390/mi13081292
APA StyleJi, Y., Li, Z., Liu, Y., Wu, X., & Ren, L. (2022). Design and Synthesis of Cobalt-Based Hollow Nanoparticles through the Liquid Metal Template. Micromachines, 13(8), 1292. https://doi.org/10.3390/mi13081292





