Electrodeposition as a Tool for Nanostructuring Magnetic Materials
Abstract
1. Introduction
2. General Description of the Electrodeposition Process
3. Electrodeposition of Nanowires
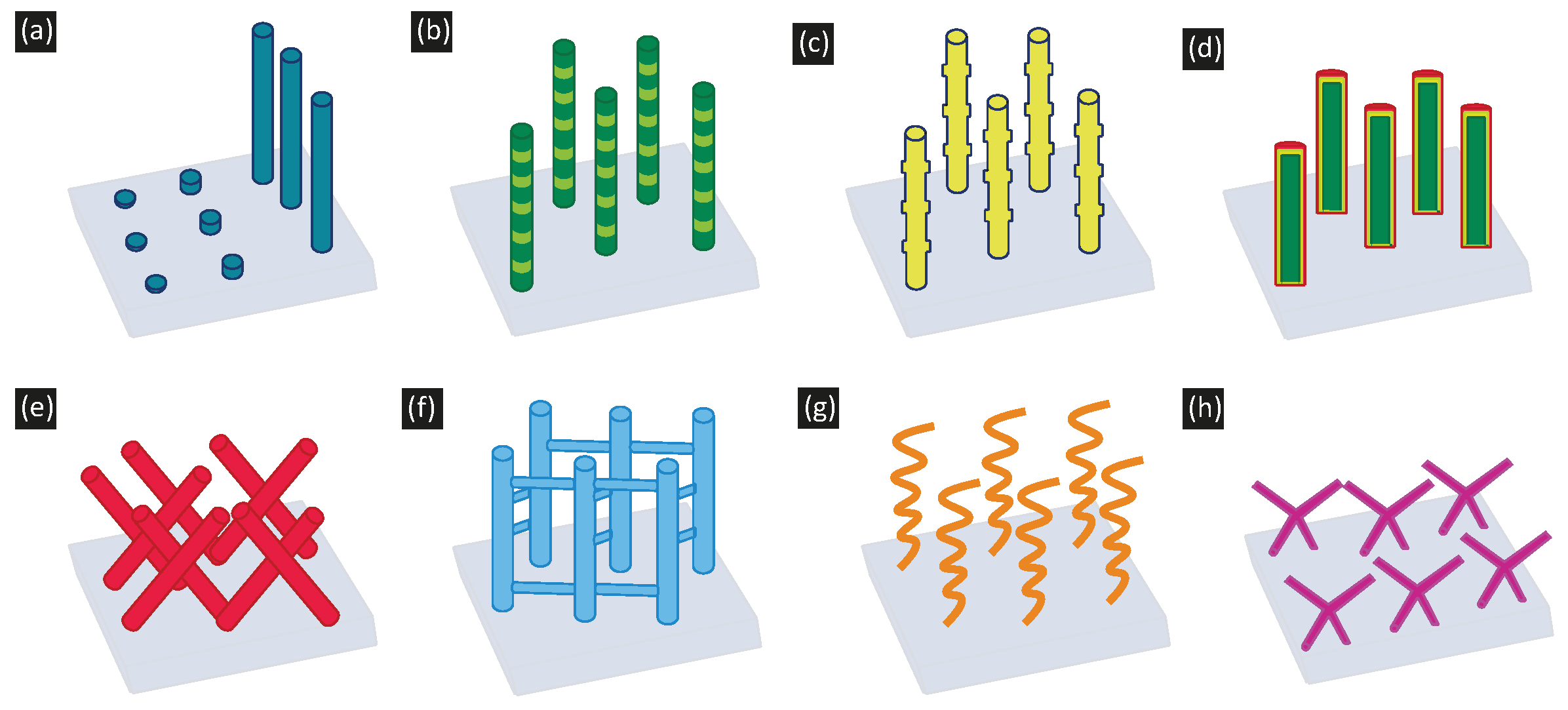
4. Nanostructuring Electrodeposited Nanowires
5. Electrodeposition of Complex Nanostructures
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ruythooren, W.; Attenborough, K.; Beerten, S.; Merken, P.; Fransaer, J.; Beyne, E.; Hoof, C.V.; Boeck, J.D.; Celis, J.P. Electrodeposition for the synthesis of microsystems. J. Micromech. Microeng. 2000, 10, 101–107. [Google Scholar] [CrossRef]
- Péter, L. Electrochemical Methods of Nanostructure Preparation; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Sanz-Hernández, D.; Donnelly, C.; Pérez, L.; Fernández-Pacheco, A. Nanofabrication of three-dimensional magnetic structure. In Nanofabrication: Nanolithography Techniques and Their Applications; de Teresa, J.M., Ed.; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Datta, M. Applications of electrochemical microfabrication: An introduction. IBM J. Res. Develop. 1998, 42, 563–566. [Google Scholar] [CrossRef]
- Datta, M.; Shenoy, R.V.; Jahnes, C.; Andricacos, P.C.; Horkans, J.; Dukovic, J.O.; Romankiw, L.T.; Roeder, J.; Deligianni, H.; Nye, H.; et al. Electrochemical Fabrication of Mechanically Robust PbSn C4 Interconnections. J. Electrochem. Soc. 1995, 142, 3779–3785. [Google Scholar] [CrossRef]
- Liu, P.; Cottrill, A.L.; Kozawa, D.; Koman, V.B.; Parviz, D.; Liu, A.T.; Yang, J.; Tran, T.Q.; Wong, M.H.; Wang, S.; et al. Emerging trends in 2D nanotechnology that are redefining our understanding of Nanocomposites. Nano Today 2018, 21, 18–40. [Google Scholar] [CrossRef]
- Perez, L.; Aroca, C.; Sánchez, P.; Sánchez, M.C. Planar fluxgate sensor with an electrodeposited amorphous core. Sens. Actuators A 2004, 3, 208–211. [Google Scholar] [CrossRef]
- Romankiv, T. A path: From electroplating through lithographic masks in electronics to LIGA in MEMS. Electrochim. Acta 1997, 42, 2985–3005. [Google Scholar] [CrossRef]
- Genolet, G.; Lorenz, H. UV-LIGA: From Development to Commercialization. Micromachines 2014, 5, 486–495. [Google Scholar] [CrossRef]
- Lincot, D. Electrodeposition of semiconductors. Thin Solid Film. 2005, 487, 40–48. [Google Scholar] [CrossRef]
- Ojo, A.A.; Dharmadasa, I.M. Electroplating of Semiconductor Materials for Applications in Large Area Electronics: A Review. Coatings 2018, 8, 262. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Zheng, X.; Yangn, S. A scalable electrodeposition route to the low-cost, versatile and controllable fabrication of perovskite solar cells. Nano Energy 2015, 15, 216–226. [Google Scholar] [CrossRef]
- Deligianni, H.; Ahmed, S.; Romankiw, L.T. The Next Frontier: Electrodeposition for Solar Cell Fabrication. Electrochem. Soc. Interface 2011, 20, 47. [Google Scholar] [CrossRef]
- Prados, A.; Ranchal, R.; Perez, L. Strategies to unblock the n-GaAs surface when electrodepositing Bi from acidic solutions. Electrochim. Acta 2015, 174, 264–272. [Google Scholar] [CrossRef][Green Version]
- Chen, S. Practical Electrochemical Cells. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 33–56. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Bagri, P.; Luo, H.; Popovs, I.; Thapaliya, B.P.; Dehaudt, J.; Dai, S. Trimethyl phosphate based neutral ligand room temperature ionic liquids for electrodeposition of rare earth elements. Electrochem. Commun. 2018, 96, 88–92. [Google Scholar] [CrossRef]
- Xu, X.; Sturm, S.; Zavasnik, J.; Rozman, K.Z. Electrodeposition of a Rare-Earth Iron Alloy from an Ionic-Liquid Electrolyte. ChemElectroChem 2019, 6, 2860. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Zhang, R.; Liu, H.; Liu, Z. Progress on Electrodeposition of Rare Earth Metals and Their Alloys. Electrocatalysis 2021, 12, 628–640. [Google Scholar] [CrossRef]
- Ruiz-Gómez, S.; Ranchal, R.; Abuín, M.; Aragón, A.M.; Velasco, V.; Marín, P.; Mascaraque, A.; Perez, L. Antiferromagnetic FeMn alloys electrodeposited from chloride-based electrolytes. Phys. Chem. Chem. Phys. 2016, 18, 8212. [Google Scholar] [CrossRef]
- Benfedda, B.; Benbrahim, N.; Kadri, A.; Chainet, E.; Charlot, F.; Coindeau, S. Electrodeposition and characterization of manganese-bismuth system from chloride based acidic bath. Electrochim. Acta 2011, 56, 1275–1282. [Google Scholar] [CrossRef]
- Iselt, D.; Gaitzscha, U.; Oswalda, S.; Fahler, S.; Schultz, L.; Schlorb, H. Electrodeposition and characterization of Fe80Ga20 alloy films. Electrochim. Acta 2020, 56, 5178–5183. [Google Scholar] [CrossRef]
- Estrine, E.C.; Hein, M.; Robbins, W.P.; Stadler, B.J.H. Composition and crystallinity in electrochemically deposited magnetostrictive galfenol (FeGa). J. Appl. Phys. 2014, 115, 17A918. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Academic Press Inc.: New York, NY, USA, 1963. [Google Scholar]
- Perez, L.; Attenborough, K.; Boeck, J.D.; Celis, J.; Aroca, C.; Sanchez, P.; Lopez, E.; Sanchez, M. Planar fluxgate sensor with an electrodeposited amorphous core. J. Magn. Magn. Mater. 2002, 242–245, 163–165. [Google Scholar] [CrossRef]
- Llavona, A.; Perez, L.; Sanchez, M.C.; de Manuel, V. Enhancement of anomalous codeposition in the synthesis of Fe-Ni alloys in nanopores. Electrochim. Acta 2013, 106, 392–397. [Google Scholar] [CrossRef]
- Switzer, J.A.; Shumsky, M.G.; Bohannan, E.W. Electrodeposited Ceramic Single Crystals. Science 1999, 284, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct Electrodeposition of Graphene Enabling the One-Step Synthesis of Graphene-Metal Nanocomposite Films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Beck, F. Electrodeposition of polymer coatings. Electrochim. Acta 1988, 33, 839–850. [Google Scholar] [CrossRef]
- Plaza, M.; Abuín, M.; Mascaraque, A.; González-Barrio, M.; Pérez, L. Epitaxial growth of Bi ultra-thin films on GaAs by electrodeposition. Mat. Chem. Phys. 2012, 134, 523–530. [Google Scholar] [CrossRef]
- Banik, A.; Tubbesing, J.Z.; Luo, B.; Zhang, X.; Switzer, J.A. Epitaxial Electrodeposition of Optically Transparent Hole-Conducting CuI on n-Si(111). Chem. Mater. 2021, 33, 3220–3227. [Google Scholar] [CrossRef]
- Gusley, R.; Ezzat, S.; Coffey, K.R.; West, A.C.; Barmak, K. Influence of the Seed Layer and Electrolyte on the Epitaxial Electrodeposition of Co(0001) for the Fabrication of Single Crystal Interconnects. J. Electrochem. Soc. 2020, 167, 162503. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Köckar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Schwarzacher, W.; Lashmore, D. Giant magnetoresistance in electrodeposited films. IEEE Trans. Magn. 1996, 32, 3133–3153. [Google Scholar] [CrossRef]
- Bakonyi, I.; Péter, L. Electrodeposited multilayer films with giant magnetoresistance (GMR): Progress and problems. Prog. Mater. Sci. 2010, 55, 107–245. [Google Scholar] [CrossRef]
- Goldman, L.M.; Ross, C.A.; Ohashi, W.; Wu, D.; Spaepen, F. New dual-bath technique for electrodeposition of short repeat length multilayers. App. Phys. Lett. 1989, 55, 2182. [Google Scholar] [CrossRef]
- Attenborough, K.; Boeve, H.; Boeck, J.D.; Borghs, G.; Celis, J.P. Electrodeposited spin valves on n-type GaAs. Appl. Phys. Lett. 1999, 74, 2206–2208. [Google Scholar] [CrossRef]
- Alper, M.; Attenborough, K.; Hart, R.; Lane, S.J.; Lashmore, D.S.; Younes, C.; Schwarzacher, W. Giant magnetoresistance in electrodeposited superlattices. Appl. Phys. Lett. 1993, 63, 2144–2146. [Google Scholar] [CrossRef]
- Prida, V.M.; Vega, V.; Garcia, J.; Iglesias, L.; Hernando, B.; Minguez-Bacho, I. Electrochemical methods for template-assisted synthesis of nanostructured materials. In Magnetic Nano- and Microwires: Design, Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Stano, M.; Fruchart, O. Chapter 3—Magnetic Nanowires and Nanotubes. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 155–267. [Google Scholar]
- Piraux, L. Magnetic Nanowires. Appl. Sci. 2020, 10, 1832. [Google Scholar] [CrossRef]
- Fernández-González, C.; Guzmán-Mínguez, J.C.; Guedeja-Marrón, A.; García-Martín, E.; Foerster, M.; Niño, M.A.; Aballe, L.; Quesada, A.; Pérez, L.; Ruiz-Gómez, S. Scaling Up the Production of Electrodeposited Nanowires: A Roadmap Towards Applications. Nanomaterials 2021, 11, 1657. [Google Scholar] [CrossRef]
- Whitney, T.M.; Jiang, J.S.; Searson, P.; Chien, C.L. Fabrication and magnetic properties of arrays of metallic nanowires. Science 1993, 261, 1316–1319. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Toimil-Molares, M.E. Characterization and properties of micro- and nanowires of controlled size, composition, and geometry fabricated by electrodeposition and ion-track technology. Beilstein J. Nanotechnol. 2012, 3, 860–883. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 1–22. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7596. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 12, 2227–2265. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, S.; Dohler, D.; Trapp, B.; Stano, M.; Fruchart, O.; Bachmann, J. Preparation and physical properties of soft magnetic nickel-cobalt three-segmented nanowires. J. Appl. Phys. 2018, 124, 163907. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Rubino, A.; Pagnanelli, F. Electrodeposition of cobalt nanowires into alumina templates generated by one-step anodization. Electrochim. Acta 2018, 259, 711–722. [Google Scholar] [CrossRef]
- Masuda, H.; Satoh, M. Fabrication of Gold Nanodot Array using Anodic Porous Alumina as an Evaporation Mask. Jap. J. Appl. Phys. 1996, 35, L126. [Google Scholar] [CrossRef]
- Surawathanawises, K.; Cheng, X. Nanoporous anodic aluminum oxide with a long-range order and tunable cell sizes by phosphoric acid anodization on pre-patterned substrates. Electrochim. Acta 2015, 117, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kustandi, T.S.; Loh, W.W.; Gao, H.; Low, H.Y. Wafer-Scale Near-Perfect Ordered Porous Alumina on Substrates by Step and Flash Imprint Lithography. ACS Nano 2010, 4, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, M.; Navas, D.; Hernández-Vélez, M.; Baldonedo, J.; Vázquez, M.; Asenjo, A. Nanoporous alumina membrane prepared by nanoindentation and anodic oxidation. Surf. Sci. 2009, 603, 3155–3159. [Google Scholar] [CrossRef]
- Chen, B.; Lu, K.; Tian, Z. Gradient and alternating diameter nanopore templates by focused ion beam guided anodization. Electrochim. Acta 2010, 56, 435–440. [Google Scholar] [CrossRef]
- Vega, V.; Montero-Moreno, J.M.; García, J.; Prida, V.M.; Rahimi, W.; Waleczek, M.; Bae, C.; Zierold, R.; Nielsch, K. Long-Range Hexagonal Arrangement of TiO2 Nanotubes by Soft Lithography-Guided Anodization. Electrochim. Acta 2016, 203, 51–58. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Ross, C.A.; Gösele, U.; Nielsch, K. Wafer-Scale Ni imprint stamps for porous alumina membranes based on interference lithography. Small 2006, 2, 978. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Carvalho-Santos, V.; Altbir, D.; Chubykalo-Fesenko, O. Detailed examination of domain wall types, their widths and critical diameters in cylindrical magnetic nanowires. J. Magn. Magn. Mater. 2022, 542, 168495. [Google Scholar] [CrossRef]
- Wartelle, A.; Trapp, B.; Staňo, M.; Thirion, C.; Bochmann, S.; Bachmann, J.; Foerster, M.; Aballe, L.; Menteş, T.O.; Locatelli, A.; et al. Bloch-point-mediated topological transformations of magnetic domain walls in cylindrical nanowires. Phys. Rev. B 2019, 99, 024433. [Google Scholar] [CrossRef]
- Bochmann, S.; Fernandez-Pacheco, A.; Mavković, M.; Neff, A.; Siefermann, K.R.; Spiecker, E.; Cowburn, R.P.; Bachmann, J. Systematic tuning of segmented magnetic nanowires into three-dimensional arrays of bits. RSC Adv. 2017, 7, 37627–37635. [Google Scholar] [CrossRef]
- Vázquez, M.; Vivas, L.G. Magnetization reversal in Co-base nanowire arrays. Phys. Status Solidi B 2011, 248, 2368–2381. [Google Scholar] [CrossRef]
- Andersen, I.M.; Wolf, D.; Rodriguez, L.A.; Lubk, A.; Oliveros, D.; Bran, C.; Niermann, T.; Rößler, U.K.; Vazquez, M.; Gatel, C.; et al. Field tunable three-dimensional magnetic nanotextures in cobalt-nickel nanowires. Phys. Rev. Res. 2021, 3, 033085. [Google Scholar] [CrossRef]
- Lupu, N.; Lostun, M.; Chiriac, H. Surface magnetization processes in soft magnetic nanowires. J. Appl. Phys. 2010, 107, 9E315. [Google Scholar] [CrossRef]
- Ramulu, T.; Venu, R.; Anandakumar, S.; Rani, V.S.; Yoon, S.; Kim, C. Structure, growth and magnetic property of hard magnetic CoPtP nanowires synthesized by electrochemical deposition. Thin Solid Film. 2012, 520, 5508–5511. [Google Scholar] [CrossRef]
- Reddy, S.M.; Park, J.J.; Na, S.M.; Maqableh, M.M.; Flatau, A.B.; Stadler, B.J.H. Electrochemical Synthesis of Magnetostrictive Fe-Ga/Cu Multilayered Nanowire Arrays with Tailored Magnetic Response. Adv. Func. Mater. 2011, 21, 4677–4683. [Google Scholar] [CrossRef]
- Varga, M.; Galdun, L.; Kunca, B.; Vega, V.; García, J.; Prida, V.; Barriga-Castro, E.; Luna, C.; Diko, P.; Saksl, K.; et al. FORC and TFORC analysis of electrodeposited magnetic shape memory nanowires array. J. Alloys Compd. 2022, 897, 163211. [Google Scholar] [CrossRef]
- Ruiz-Gómez, S.; Fernández-González, C.; Guedeja-Marrón, A.; Serrano, A.; González-Barrio, M.A.; Varela, M.; Mascaraque, A.; Pérez, L. Highly Bi-doped electrodeposited Cu nanowires for spintronics applications. J. Magn. Magn. Mater 2022, 545, 168645. [Google Scholar] [CrossRef]
- Llavona, A.; Prados, A.; Velasco, V.; Crespo, P.; Sanchez, M.C.; Perez, L. Electrochemical synthesis and magnetic properties of goethite single crystal nanowires. CrystEngComm 2013, 15, 4905. [Google Scholar] [CrossRef]
- Fert, A.; Piraux, L. Magnetic nanowires. J. Magn. Magn. Mater. 1999, 200, 338–358. [Google Scholar] [CrossRef]
- Rosa, W.; Vivas, L.; Pirota, K.; Asenjo, A.; Vázquez, M. Influence of aspect ratio and anisotropy distribution in ordered CoNi nanowire arrays. J. Magn. Magn. Mater. 2012, 324, 3679–3682. [Google Scholar] [CrossRef]
- Raposo, V.; Zazo, M.; Flores, A.G.; Garcia, J.; Vega, V.; Iniguez, J.; Prida, V.M. Ferromagnetic resonance in low interacting permalloy nanowire arrays. J. Appl. Phys. 2016, 119, 143903. [Google Scholar] [CrossRef]
- Guzman-Mínguez, J.C.; Ruiz-Gomez, S.; Vicente-Arche, L.M.; Granados-Miralles, C.; Fernández-González, C.; Mompeán, F.; García-Hernández, M.; Erohkin, S.; Berkov, D.; Mishra, D.; et al. FeCo Nanowire-Strontium Ferrite Powder Composites for Permanent Magnets with High-Energy Products. ACS Appl. Nano Mater. 2020, 3, 9842–9851. [Google Scholar] [CrossRef]
- Alfadhel, A.; Li, B.; Zaher, A.; Yassine, O.; Kosel, J. A magnetic nanocomposite for biomimetic flow sensing. Lab Chip 2014, 14, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Alnassar, M.Y.; Ivanov, Y.P.; Kosel, J. Flexible Magnetoelectric Nanocomposites with Tunable Properties. Adv. Electron. Mater. 2016, 2, 1600081. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Alfadhel, A.; Alnassar, M.; Perez, J.E.; Vazquez, M.; Chuvilin, A.; Kosel, J. Tunable magnetic nanowires for biomedical and harsh environment applications. Sci. Rep. 2016, 6, 24189. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Peixoto, L.; Magalhães, R.; Navas, D.; Moraes, S.; Redondo, C.; Morales, R.; Araújo, J.P.; Sousa, C.T. Magnetic nanostructures for emerging biomedical applications. Appl. Phys. Rev. 2020, 7, 011310. [Google Scholar] [CrossRef]
- Marcano, N.; Sangiao, S.; Plaza, M.; Perez, L.; Fernández Pacheco, A.; Córdoba, R.; Sánchez, M.C.; Morellón, L.; Ibarra, M.R.; Teresa, J.M.D. Weak-antilocalization signatures in the magnetotransport properties of individual electrodeposited Bi nanowires. App. Phys. Lett. 2010, 96, 082110. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Chuvilin, A.; Lopatin, S.; Mohammed, H.; Kosel, J. Direct Observation of Current-Induced Motion of a 3D Vortex Domain Wall in Cylindrical Nanowires. ACS Appl. Mater. Interfaces 2017, 9, 16741–16744. [Google Scholar] [CrossRef] [PubMed]
- Proenca, M.P.; Muñoz, M.; Villaverde, I.; Migliorini, A.; Raposo, V.; Lopez-Diaz, L.; Martinez, E.; Prieto, J.L. Deterministic and time resolved thermo-magnetic switching in a nickel nanowire. Sci. Rep. 2019, 9, 17339. [Google Scholar] [CrossRef] [PubMed]
- Costas, A.; Florica, C.; Matei, E.; Toimil-Molares, M.E.; Stavarache, I.; Kuncser, A.; Kuncser, V.; Enculescu, I. Magnetism and magnetoresistance of single Ni-Cu alloy nanowires. Beilstein J. Nanotechnol. 2018, 9, 2345–2355. [Google Scholar] [CrossRef]
- Schöbitz, M.; Riz, A.D.; Martin, S.; Bochmann, S.; Thirion, C.; Vogel, J.; Foerster, M.; Aballe, L.; Mente, T.O.; Locatelli, A.; et al. Fast domain walls governed by Oersted fields in cylindrical magnetic nanowire. Phys. Rev. Lett. 2019, 123, 217201. [Google Scholar] [CrossRef]
- Schöbitz, M.; Finizio, S.; De Riz, A.; Hurst, J.; Thirion, C.; Gusakova, D.; Toussaint, J.C.; Bachmann, J.; Raabe, J.; Fruchart, O. Time-resolved imaging of Oersted field induced magnetization dynamics in cylindrical magnetic nanowires. Appl. Phys. Lett. 2021, 118, 172411. [Google Scholar] [CrossRef]
- Moreno, J.A.; Khan, M.A.; Ivanov, Y.P.; Lopatin, S.; Holguín-Lerma, J.A.; Marinaro, G.; Ooi, B.S.; Idriss, H.; Kosel, J. Growth of Ordered Iron Oxide Nanowires for Photo-electrochemical Water Oxidation. ACS Appl. Energy Mater. 2019, 12, 8473–8480. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; Rodilla, B.L.; Calaresu, I.; Arché-Núnez, A.; González-Mayorga, A.; Scaini, D.; Pérez, L.; Camarero, J.; Miranda, R.; López-Dolado, E.; et al. Interfacing Neurons with Nanostructured Electrodes Modulates Synaptic Circuit Features. Adv. Biosyst. 2020, 4, 2000117. [Google Scholar] [CrossRef]
- Domínguez-Bajo, A.; Rosa, J.M.; González-Mayorga, A.; Rodilla, B.L.; Arché-Núnez, A.; Benayas, E.; Ocón, P.; Pérez, L.; Camarero, J.; Miranda, R.; et al. Nanostructured gold electrodes promote neural maturation and network connectivity. Biomaterials 2021, 279, 121186. [Google Scholar] [CrossRef]
- Perez, J.E.; Bajaber, B.; Alsharif, N.; Martínez-Banderas, A.I.; Patel, N.; Sharip, A.; Fabrizio, E.D.; Merzaban, J.; Kosel, J. Modulated nanowire scaffold for highly efficient differentiation of mesenchymal stem cells. J. Nanobiotechnol. 2022, 20, 282. [Google Scholar] [CrossRef]
- Parkin, S.; Yang, S.H. Memory on the racetrack. Nat. Nanotechnol. 2005, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Parkin, S.S.P.; Hayashi, M.; Thomas, L. Magnetic domain-wall racetrack memory. Science 2008, 320, 194. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Chuvilin, A.; Lopatin, S.; Kosel, J. Modulated Magnetic Nanowires for Controlling Domain Wall Motion: Toward 3D Magnetic Memories. ACS Nano 2016, 10, 5326–5332. [Google Scholar] [CrossRef] [PubMed]
- García Fernández, J.; Vega Martínez, V.; Thomas, A.; De la Prida Pidal, V.M.; Nielsch, K. Two-Step Magnetization Reversal FORC Fingerprint of Coupled Bi-Segmented Ni/Co Magnetic Nanowire Arrays. Nanomaterials 2018, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Susano, M.; Proenca, M.P.; Moraes, S.; Sousa, C.T.; Araújo, J.P. Tuning the magnetic properties of multisegmented Ni/Cu electrodeposited nanowires with controllable Ni lengths. Nanotechnology 2016, 27, 335301. [Google Scholar] [CrossRef]
- Chen, M.; Chien, C.L.; Searson, P.C. Potential Modulated Multilayer Deposition of Multisegment Cu/Ni Nanowires with Tunable Magnetic Properties. Chem. Mater. 2006, 18, 1595–1601. [Google Scholar] [CrossRef]
- Moraes, S.; Navas, D.; Béron, F.; Proenca, M.P.; Pirota, K.R.; Sousa, C.T.; Araújo, J.P. The Role of Cu Length on the Magnetic Behaviour of Fe/Cu Multi-Segmented Nanowires. Nanomaterials 2018, 8, 490. [Google Scholar] [CrossRef]
- Liu, K.; Nagodawithana, K.; Searson, P.C.; Chien, C.L. Perpendicular giant magnetoresistance of multilayered Co/Cu nanowires. Phys. Rev. B 1995, 51, 7381–7384. [Google Scholar] [CrossRef]
- Reyes, D.; Biziere, N.; Warot-Fonrose, B.; Wade, T.; Gatel, C. Magnetic Configurations in Co/Cu Multilayered Nanowires: Evidence of Structural and Magnetic Interplay. Nano Lett. 2016, 16, 1230–1236. [Google Scholar] [CrossRef]
- Jang, B.; Pellicer, E.; Guerrero, M.; Chen, X.; Choi, H.; Nelson, B.J.; Sort, J.; Pané, S. Fabrication of Segmented Au/Co/Au Nanowires: Insights in the Quality of Co/Au Junctions. ACS Appl. Mater. Interfaces 2014, 6, 14583–14589. [Google Scholar] [CrossRef]
- Um, J.; Zamani Kouhpanji, M.R.; Liu, S.; Nemati Porshokouh, Z.; Sung, S.Y.; Kosel, J.; Stadler, B. Fabrication of Long-Range Ordered Aluminum Oxide and Fe/Au Multilayered Nanowires for 3-D Magnetic Memory. IEEE Trans. Magn. 2020, 56, 1–6. [Google Scholar] [CrossRef]
- Evans, P.R.; Yi, G.; Schwarzacher, W. Current perpendicular to plane giant magnetoresistance of multilayered nanowires electrodeposited in anodic aluminum oxide membranes. Appl. Phys. Lett. 2000, 76, 481. [Google Scholar] [CrossRef]
- Tang, X.T.; Wang, G.C. Superparamagnetic behavior in ultrathin CoNi layers of electrodeposited CoNi/Cu multilayer nanowires. J. Appl. Phys. 2006, 99, 123910. [Google Scholar] [CrossRef]
- Zsurzsa, S.; Pellicer, E.; Sort, J.; Péter, L.; Bakonyi, I. Electron Microscopy Characterization of Electrodeposited Homogeneous and Multilayered Nanowires in the Ni-Co-Cu System. J. Electrochem. Soc. 2018, 165, D536. [Google Scholar] [CrossRef]
- Nuñez, A.; Pérez, L.; Abuín, M.; Araujo, J.P.; Proenca, M.P. Magnetic behaviour of multisegmented FeCoCu/Cu electrodeposited nanowires. J. Phys. D Appl. Phys. 2017, 50, 155003. [Google Scholar] [CrossRef]
- Bran, C.; Berganza, E.; Fernandez-Roldan, J.A.; Palmero, E.M.; Meier, J.; Calle, E.; Jaafar, M.; Foerster, M.; Aballe, L.; Rodriguez, A.F.; et al. Magnetization Ratchet in Cylindrical Nanowires. ACS Nano 2018, 12, 5932–5939. [Google Scholar] [CrossRef]
- Sun, L.; Hao, Y.; Chien, C.L.; Searson, P.C. Tuning the properties of magnetic nanowires. IBM J. Res. Dev. 2005, 49, 79–102. [Google Scholar] [CrossRef]
- Prida, V.M.; García, J.; Iglesias, L.; Vega, V.; Görlitz, D.; Nielsch, K.; Barriga-Castro, E.D.; Mendoza-Reséndez, R.; Ponce, A.; Luna, C. Electroplating and magnetostructural characterization of multisegmented Co54Ni46/Co85Ni15 nanowires from single electrochemical bath in anodic alumina templates. Nanoscale Res. Lett. 2013, 8, 263. [Google Scholar] [CrossRef]
- Ruiz-Gómez, S.; Fernández-González, C.; Martínez, E.; Raposo, V.; Sorrentino, A.; Foerster, M.; Aballe, L.; Mascaraque, A.; Ferrer, S.; Pérez, L. Helical surface magnetization in nanowires: The role of chirality. Nanoscale 2020, 12, 17880–17885. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gómez, S.; Foerster, M.; Aballe, L.; Proenca, M.P.; Lucas, I.; Prieto, J.L.; Mascaraque, A.; de la Figuera, J.; Quesada, A.; Pérez, L. Observation of a topologically protected state in a magnetic domain wall stabilized by a ferromagnetic chemical barrier. Sci. Rep. 2018, 8, 16695. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Gómez, L.; Ruiz-Gómez, S.; Fernández-González, C.; Schöbitz, M.; Mille, N.; Hurst, J.; Tiwari, D.; Riz, A.D.; Andersen, I.; Bachmann, J.; et al. Micromagnetics of magnetic chemical modulations in soft-magnetic cylindrical nanowire. arXiv 2022, arXiv:2205.06705. [Google Scholar]
- Sulka, G.D.; Brzózka, A.; Liu, L. Fabrication of diameter-modulated and ultrathin porous nanowires in anodic aluminum oxide templates. Electrochim. Acta 2011, 56, 4972–4979. [Google Scholar] [CrossRef]
- Rodríguez, L.A.; Bran, C.; Reyes, D.; Berganza, E.; Vázquez, M.; Gatel, C.; Snoeck, E.; Asenjo, A. Quantitative Nanoscale Magnetic Study of Isolated Diameter-Modulated FeCoCu Nanowires. ACS Nano 2016, 10, 9669–9678. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.; Vega, V.; González, S.; Caballero-Flores, R.; García, J.; Prida, V.M. Effect of Sharp Diameter Geometrical Modulation on the Magnetization Reversal of Bi-Segmented FeNi Nanowires. Nanomaterials 2018, 8, 595. [Google Scholar] [CrossRef]
- Berganza, E.; Bran, C.; Jaafar, M.; Vázquez, M.; Asenjo, A. Domain wall pinning in FeCoCu bamboo-like nanowires. Sci. Rep. 2016, 6, 29702. [Google Scholar] [CrossRef]
- De Riz, A.; Trapp, B.; Fernandez-Roldan, J.; Thirion, C.; Toussaint, J.C.; Fruchart, O.; Gusakova, D. Domain wall pinning in a circular cross-section wire with modulated diameter. In Magnetic Nano- and Microwires, 2nd ed.; Vázquez, M., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Sawston, UK, 2020; pp. 427–453. [Google Scholar]
- Cortés-Llanos, B.; Serrano, A.; Muñoz-Noval, A.; Urones-Garrote, E.; del Campo, A.; Marco, J.F.; Ayuso-Sacido, A.; Pérez, L. Thermal Route for the Synthesis of Maghemite/Hematite Core/Shell Nanowires. J. Phys. Chem. C 2017, 121, 23158–23165. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.W.; Li, G.H.; Zhang, L.D. Conversion of a Bi Nanowire Array to an Array of Bi-Bi2O3 Core-Shell Nanowires and Bi2O3 Nanotubes. Small 2006, 2, 548–553. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Marzolo, F.; Rubino, A.; Zanoni, R.; Pagnanelli, F. Optimizing the structure of Ni-Ni(OH)2/NiO core-shell nanowire electrodes for application in pseudocapacitors: The influence of metallic core, Ni(OH)2/NiO ratio and nanowire length. J. Alloy. Compd. 2021, 856, 157718. [Google Scholar] [CrossRef]
- Loh, P.Y.; Liu, C.; Sow, C.H.; Chin, W.S. Coaxial hetero-nanostructures with controllable shell thickness: A pore widening method. RSC Adv. 2014, 4, 8735–8740. [Google Scholar] [CrossRef]
- Fernández-González, C.; Guedeja-Marrón, A.; Arché-Nuñez, A.; Rodilla, B.L.; Corcuera, R.; Lucas, I.; González, M.T.; Varela, M.; de la Presa, P.; Aballe, L.; et al. Electrodeposited magnetic nanowires with radial modulation of composition. Nanomaterials 2022, 12, 2565. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, L.; Ruiz-Gomez, S.; Abuín, M.; Montana, Y.; Carmona, N.; Pérez, L. Multifunctional Core-Shell Co-SiO2 Nanowires via Electrodeposition and Sol-Gel Techniques. RSC Adv. 2015, 5, 97503–97507. [Google Scholar] [CrossRef]
- Khan, U.; Li, W.J.; Adeela, N.; Irfan, M.; Javed, K.; Wan, C.H.; Riaz, S.; Han, X.F. Magnetic response of hybrid ferromagnetic and antiferromagnetic core-shell nanostructures. Nanoscale 2016, 8, 6064–6070. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Da Col, S.; Darques, M.; Fruchart, O.; Cagnon, L. Reduction of magnetostatic interactions in self-organized arrays of nickel nanowires using atomic layer deposition. Appl. Phys. Lett. 2011, 98, 112501. [Google Scholar] [CrossRef]
- García, J.; Méndez, M.; González, S.; Vega, V.; Caballero, R.; Prida, V.M. Electrochemical methods assisted with ALD for the synthesis of nanowires. In Magnetic Nano- and Microwires, 2nd ed.; Vázquez, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 21–60. [Google Scholar]
- García, J.; Manterola, A.M.; Méndez, M.; Fernández-Roldán, J.A.; Vega, V.; González, S.; Prida, V.M. Magnetization Reversal Process and Magnetostatic Interactions in Fe56Co44/SiO2/Fe3O4 Core/Shell Ferromagnetic Nanowires with Non-Magnetic Interlayer. Nanomaterials 2021, 11, 2282. [Google Scholar] [CrossRef]
- Rauber, M.; Alber, I.; Muller, S.; Neumann, R.; Picht, O.; Roth, C.; Schoke, A.; Toimil-Molares, M.E.; Ensinger, W. Highly-Ordered Supportless Three-Dimensional Nanowire Networks with Tunable Complexity and Interwire Connectivity for Device Integration. Nano Lett. 2011, 11, 1304–2310. [Google Scholar] [CrossRef]
- Resende, P.M.; Sanz, R.; Ruiz-de Clavijo, A.; Caballero-Calero, O.; Martin-Gonzalez, M. Cylindrical Three-Dimensional Porous Anodic Alumina Networks. Coatings 2016, 6, 59. [Google Scholar] [CrossRef]
- Garcia, C.; Rosa, W.O.; Garcia, J.; Prida, V.M.; Hernando, B.; López, J.A.; Vargas, P.; Ross, C.A. Magnetization Reversal in Radially Distributed Nanowire Arrays. J. Phys. Chem. C 2018, 122, 5124–5130. [Google Scholar] [CrossRef]
- Martín, J.; Martín-González, M.; Fernández, J.F.; Caballero-Calero, O. Ordered three-dimensional interconnected nanoarchitectures in anodic porous alumina. Nat. Commun. 2014, 5, 5130. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Ruiz-Gómez, S.; Caballero-Calero, O.; Pérez, L.; Martín-González, M. Tailoring Magnetic Anisotropy at Will in 3D Interconnected Nanowire Networks. Phys. Status Solidi. RLL 2019, 13, 1900263. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Navas, D.; Ordoñez-Cencerrado, A.A.; Blanco-Portals, J.; Peiró, F.; Sanz, R.; Martín-González, M. Unveiling the Complex Magnetization Reversal Process in 3D Nickel Nanowire Networks. Adv. Electron. Mater. 2022, 2200342. [Google Scholar] [CrossRef]
- Araujo, E.; Encinas, A.; Velázquez-Galván, Y.; Martínez-Huerta, J.M.; Hamoir, G.; Ferain, E.; Piraux, L. Artificially modified magnetic anisotropy in interconnected nanowire networks. Nanoscale 2015, 7, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Medina, J.; da Camara Santa Clara Gomes, T.; Velázquez Galván, Y.G.; Piraux, L. Large-scale 3-D interconnected Ni nanotube networks with controlled structural and magnetic properties. Sci. Rep. 2018, 8, 14555. [Google Scholar] [CrossRef] [PubMed]
- Dodulenko, I.M.; Volchlkov, I.S.; Turenko, B.A.; Koshelev, I.O.; Podkur, P.L.; Zagorski, D.L.; Kanevskii, V.M. Electrical properties of arrays of intersecting nanowires obtained in the pores of track membranes. Mat. Chem. Phys. 2022, 287, 126285. [Google Scholar]
- da Camara Santa Clara Gomes, T.; Marchal, N.; Abreu Araujo, F.; Velázquez Galván, Y.; de la Torre Medina, J.; Piraux, L. Magneto-transport in flexible 3D Networks made of interconnected nanowires and nanotubes. Nanomaterials 2021, 11, 221. [Google Scholar] [CrossRef]
- da Camara Santa Clara Gomes, T.; Abreu Araujo, F.; Piraux, L. Making flexible spin caloritronic devices with interconnected nanowire networks. Sci. Adv. 2019, 5, eaav2782. [Google Scholar] [CrossRef]
- da Camara Santa Clara Gomes, T.; Marchal, N.; Abreu Araujo, F.; Piraux, L. Spin caloritronics in 3D interconnected nanowire networks. Nanomaterials 2020, 10, 2092. [Google Scholar] [CrossRef]
- Duvail, J.L.; Dubois, S.; Piraux, L.; VaurÚs, A.; Fert, A.; Adam, D.; Champagne, M.; Rousseaux, F.; Decanini, D. Electrodeposition of patterned magnetic nanostructures. J. Appl. Phys. 1998, 84, 6359–6365. [Google Scholar] [CrossRef]
- Cho, K.; Loget, G.; Corn, G.L. Lithographically patterned nanoscale electrodeposition of plasmonic, bimetallic, semiconductor, magnetic and polymer nanoring arrays. J. Phys. Chem. C 2014, 118, 28993–29000. [Google Scholar] [CrossRef]
- Imtaar, M.A.; Yadav, A.; Epping, A.; Becherer, M.; Fabel, B.; Rezgani, J.; Csaba, G.; Bernstein, G.H.; Scarpa, G.; Porod, W.; et al. Nanomagnet Fabrication Using Nanoimprint Lithography and Electrodeposition. IEEE Trans. Nanotechnol. 2013, 12, 547–552. [Google Scholar] [CrossRef]
- Gansel, J.K.; Thiel, M.; Rill, M.S.; Decker, M.; Bade, K.; Saile, V.; von Freymann, G.; Linden, S.; Wegener, M. Gold Helix Photonic Metamaterial as Broadband Circular Polarizer. Science 2009, 325, 1513–1515. [Google Scholar] [CrossRef]
- Williams, G.; Hunt, M.; Boehm, B.; May, A.; Taverne, M.; Ho, D.; Giblin, S.; Read, D.; Rarity, J.; Allenspach, R.; et al. Two-photon lithography for 3D magnetic nanostructure fabrication. Nano Res. 2018, 11, 845–854. [Google Scholar] [CrossRef]
- Askey, J.; Hunt, M.O.; Langbein, W.; Ladak, S. Use of Two-Photon Lithography with a Negative Resist and Processing to Realise Cylindrical Magnetic Nanowires. Nanomaterials 2020, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Taverne, M.; Askey, J.; May, A.; Van Den Berg, A.; Ho, Y.L.D.; Rarity, J.; Ladak, S. Harnessing Multi-Photon Absorption to Produce Three-Dimensional Magnetic Structures at the Nanoscale. Materials 2020, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Gliga, S.; Seniutinas, G.; Weber, A.; David, C. Architectural structures open new dimensions in magnetism: Magnetic buckyballs. Mater. Today 2019, 26, 100–101. [Google Scholar] [CrossRef]
- Zeeshan, M.A.; Grisch, R.; Pellicer, E.; Sivaraman, K.M.; Peyer, K.E.; Sort, J.; Özkale, B.; Sakar, M.S.; Nelson, B.J.; Pané, S. Hybrid Helical Magnetic Microrobots Obtained by 3D Template-Assisted Electrodeposition. Small 2014, 10, 1284–1288. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, S.; Williams, G.; May, A.; Ladak, S.; Barman, A. Ultrafast magnetization dynamics in a nanoscale three-dimensional cobalt tetrapod structure. Nanoscale 2018, 10, 9981–9986. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, O.; Thiaville, A. Magnetism in reduced dimensions. Comptes Rendus Phys. 2005, 6, 921. [Google Scholar] [CrossRef]
- Moreno, J.A.; Bran, C.; Vázquez, M.; Kosel, J. Cylindrical Magnetic Nanowires Applications. IEEE Trans. Magn. 2021, 57, 800317. [Google Scholar] [CrossRef]
- Martínez-Banderas, A.I.; Aires, A.; Plaza-García, S.; Colás, L.; Moreno, J.A.; Ravasi, T.; Merzaban, J.S.; Ramos-Cabrer, P.; Cortajarena, A.L.; Kosel, J. Magnetic core-shell nanowires as MRI contrast agents for cell tracking. J. Nanobiotechnol. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Contreras, M.F.; Sougrat, R.; Zaher, A.; Ravasi, T.; Kosel, J. Non-chemotoxic induction of cancer cell death using magnetic nanowires. Int. J. Nanomed. 2015, 10, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Banderas, A.I.; Aires, A.; Quintanilla, M.; Holguín-Lerma, J.A.; Lozano-Pedraza, C.; Teran, F.J.; Moreno, J.A.; Perez, J.E.; Ooi, B.S.; Ravasi, T.; et al. Iron-Based Core-Shell Nanowires for Combinatorial Drug Delivery and Photothermal and Magnetic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43976–43988. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Hoop, M.; Shamsudhin, N.; Huang, T.; Özkale, B.; Li, Q.; Siringil, E.; Mushtaq, F.; Di Tizio, L.; Nelson, B.J.; et al. Hybrid Magnetoelectric Nanowires for Nanorobotic Applications: Fabrication, Magnetoelectric Coupling, and Magnetically Assisted In Vitro Targeted Drug Delivery. Adv. Mater. 2017, 29, 1605458. [Google Scholar] [CrossRef]
- Maurer, T.; Ott, F.; Chaboussant, G.; Soumare, Y.; Piquemal, J.Y.; Viau, G. Magnetic nanowires as permanent magnet materials. Appl. Phys. Lett. 2007, 91, 172501. [Google Scholar] [CrossRef]
- Niarchos, D.; Giannopoulos, G.; Gjoka, M.; Sarafidis, C.; Psycharis, V.; Rusz, J.; Edström, A.; Eriksson, O.; Toson, P.; Fidler, J.; et al. Toward Rare-Earth-Free Permanent Magnets: A Combinatorial Approach Exploiting the Possibilities of Modeling, Shape Anisotropy in Elongated Nanoparticles, and Combinatorial Thin-Film Approach. JOM 2015, 67, 1318–1328. [Google Scholar] [CrossRef]
- Zamani Kouhpanji, M.R.; Stadler, B.J.H. Magnetic Nanowires for Nanobarcoding and Beyond. Sensors 2021, 21, 4573. [Google Scholar] [CrossRef]
- Ramírez-Villegas, R.; Huynen, I.; Piraux, L.; Encinas, A.; De La Torre Medina, J. Configurable Microwave Filter for Signal Processing Based on Arrays of Bistable Magnetic Nanowires. IEEE Trans. Microw. Theory Technol. 2016, 65, 72–77. [Google Scholar] [CrossRef]
- McGary, P.D.; Tan, L.; Zou, J.; Stadler, B.J.H.; Downey, P.R.; Flatau, A.B. Magnetic nanowires for acoustic sensors (invited). J. Appl. Phys. 2006, 99, 8B310. [Google Scholar] [CrossRef]
- Luo, Z.; Hrabec, A.; Dao, T.P.; Sala, G.; Finicio, S.; Feng, J.; Mayr, S.; Raabe, J.; Gambardella, P.; Heyderman, L. Current-driven magnetic domain logic. Nature 2020, 579, 214. [Google Scholar] [CrossRef]
- Allwood, D.A.; Xiong, G.; Faulkner, C.C.; Atkinson, D.; Petit, D.; Cowburn, R.P. Magnetic Domain-Wall Logic. Science 2005, 309, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Kuncic, Z.; Nakayama, T. Neuromorphic nanowire networks: Principles, progress and future prospects for neuro-inspired information processing. Adv. Phys. X 2021, 6, 1894234. [Google Scholar] [CrossRef]
- Fernandez-Roldan, J.A.; Perez del Real, R.; Bran, C.; Vazquez, M.; Chubykalo-Fesenko, O. Magnetization pinning in modulated nanowires: From topological protection to the corkscrew mechanism. Nanoscale 2018, 10, 5923–5927. [Google Scholar] [CrossRef] [PubMed]
- Hertel, R. Ultrafast domain wall dynamics in magnetic nanotubes and nanowires. J. Phys. Condens. Matter 2016, 328, 483002. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pacheco, A.; Streubel, R.; Fruchart, O.; Hertel, R.; Fischer, P.; Cowburn, R.P. Three-dimensional nanomagnetism. Nat. Commun 2017, 8, 15756. [Google Scholar] [CrossRef] [PubMed]
- Streubel, R.; Fischer, P.; Kronast, F.; Kravchuk, V.P.; Sheka, D.D.; Gaididei, Y.; Schmidt, O.G.; Makarov, D. Magnetism in curved geometries. J. Phys. D Appl. Phys. 2016, 49, 363001. [Google Scholar] [CrossRef]
- Barman, A.; Gubbiotti, G.; Ladak, S.; Adeyeye, A.O.; Krawczyk, M.; Gräfe, J.; Adelmann, C.; Cotofana, S.; Naeemi, A.; Vasyuchka, V.I. The 2021 Magnonics Roadmap. J. Phys. Condens. Matter 2021, 33, 413001. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gómez, S.; Fernández-González, C.; Perez, L. Electrodeposition as a Tool for Nanostructuring Magnetic Materials. Micromachines 2022, 13, 1223. https://doi.org/10.3390/mi13081223
Ruiz-Gómez S, Fernández-González C, Perez L. Electrodeposition as a Tool for Nanostructuring Magnetic Materials. Micromachines. 2022; 13(8):1223. https://doi.org/10.3390/mi13081223
Chicago/Turabian StyleRuiz-Gómez, Sandra, Claudia Fernández-González, and Lucas Perez. 2022. "Electrodeposition as a Tool for Nanostructuring Magnetic Materials" Micromachines 13, no. 8: 1223. https://doi.org/10.3390/mi13081223
APA StyleRuiz-Gómez, S., Fernández-González, C., & Perez, L. (2022). Electrodeposition as a Tool for Nanostructuring Magnetic Materials. Micromachines, 13(8), 1223. https://doi.org/10.3390/mi13081223





