Abstract
Natural polysaccharides are structures composed of highly diversified biological macromolecules whose properties have been exploited by a diversity of industries. Until 2018, the polysaccharides market raised more than US $ 12 billion worldwide, while an annual growth forecast of 4.8% is expected by 2026. The food industry is largely responsible for the consumption of this plant-source material, produced by microbiological fermentation. Among the used polysaccharides, gums are hydrocolloids obtained from a variety of sources and in different forms, being composed of salts of calcium, potassium, magnesium and sugar monomers. Their non-toxicity, hydrophilicity, viscosity, biodegradability, biocompatibility and sustainable production are among their main advantages. Although Brazil is amongst the largest producers of cashew gum, reaching 50 tons per year, the polysaccharide is not being used to its full potential, in particular, with regard to its uses in pharmaceuticals. Cashew gum (CG), obtained from Anacardium occidentale L., caught the attention of the industry only in 1970; in 1990, its production started to grow. Within the Brazilian academy, the groups from the Federal University of Ceará and Piauí are devoting the most efforts to the study of cashew gum, with a total of 31 articles already published. The number of patents in the country for innovations containing cashew tree gum has reached 14, including the technological process for the purification of cashew tree gum, comparison of physical and chemical methods for physicochemical characterizations, and optimum purification methodology. This scenario opens a range of opportunities for the use of cashew gum, mainly in the development of new pharmaceutical products, with a special interest in nanoparticles.
1. Introduction
Natural polysaccharides and their derivatives represent a group of polymers characterized by structurally diverse biological macromolecules, which are widely used by different industries due to their versatile physicochemical properties [1,2]. Among the polysaccharides of natural origin, cellulose is the most abundant polymer, representing about 1.5 × 1012 tons (metric ton) of annual biomass [3]. In addition to cellulose, other natural macromolecules (polysaccharides, proteins), such as chitosan, alginate, pectin, starch, collagen and gums, are also of great interest to the pharmaceutical and food industries [2,4,5].
Among natural polysaccharides, natural gums stand out as promising biodegradable polymeric materials, found most often in the woody elements of plants or inside the seed coatings [1,4]. These biomaterials can be used as gelling, thickening, emulsifying and stabilizing agents. Such properties are attributed to their ability to bind to water, to form films and gels, and to encapsulate different compounds, such as flavors, aromas and nutraceuticals [5]. They can also be chemically modified in order to obtain materials useful for the production of drug delivery systems. Other factors, such as low toxicity risk, wide availability in nature and low cost compared to synthetic materials, make natural gum products of great industrial interest [6].
Plant-derived gums are available worldwide [6]. Within this market, the Northeast region of Brazil stands out for its high production rates of cashew gum (95% of national production). The states of the Brazilian Northeast, known as Piauí, Ceará and Rio Grande do Norte, are the holders of the largest Anacardium occidentale L. crops, popularly known as cashew tree. This region already has a well-consolidated market for cashew fruit and pseudofruit, with a potential production of around 50,000 tons/year [7].
Cashew gum is, however, a hydrophilic polysaccharide; thus, to be used in pharmaceutical and food products, it needs to be chemically modified or mixed with other polymers. In this review, we discuss the main uses of polysaccharides for pharmaceutical applications, with a special focus on cashew gum, and present the state of the art of patents filled in Brazil that describe the use of cashew gum in pharmaceutical and food formulations.
2. Natural Polymers
Polymer is by definition a compound of high molecular weight, resulting from the covalent bond between several hundreds of monomers (monosaccharides) [2]. These monosaccharides are linked by glycosidic bonds and are one of the key biomacromolecules involved in the growth of living organisms, cell–cell communication, cell adhesion and molecular recognition in the immune system [8]. They can also be called glycans, and they differ in the types of constituent monomers, the length of the chains, the type of bonds between the units and the degree of branching [9,10].
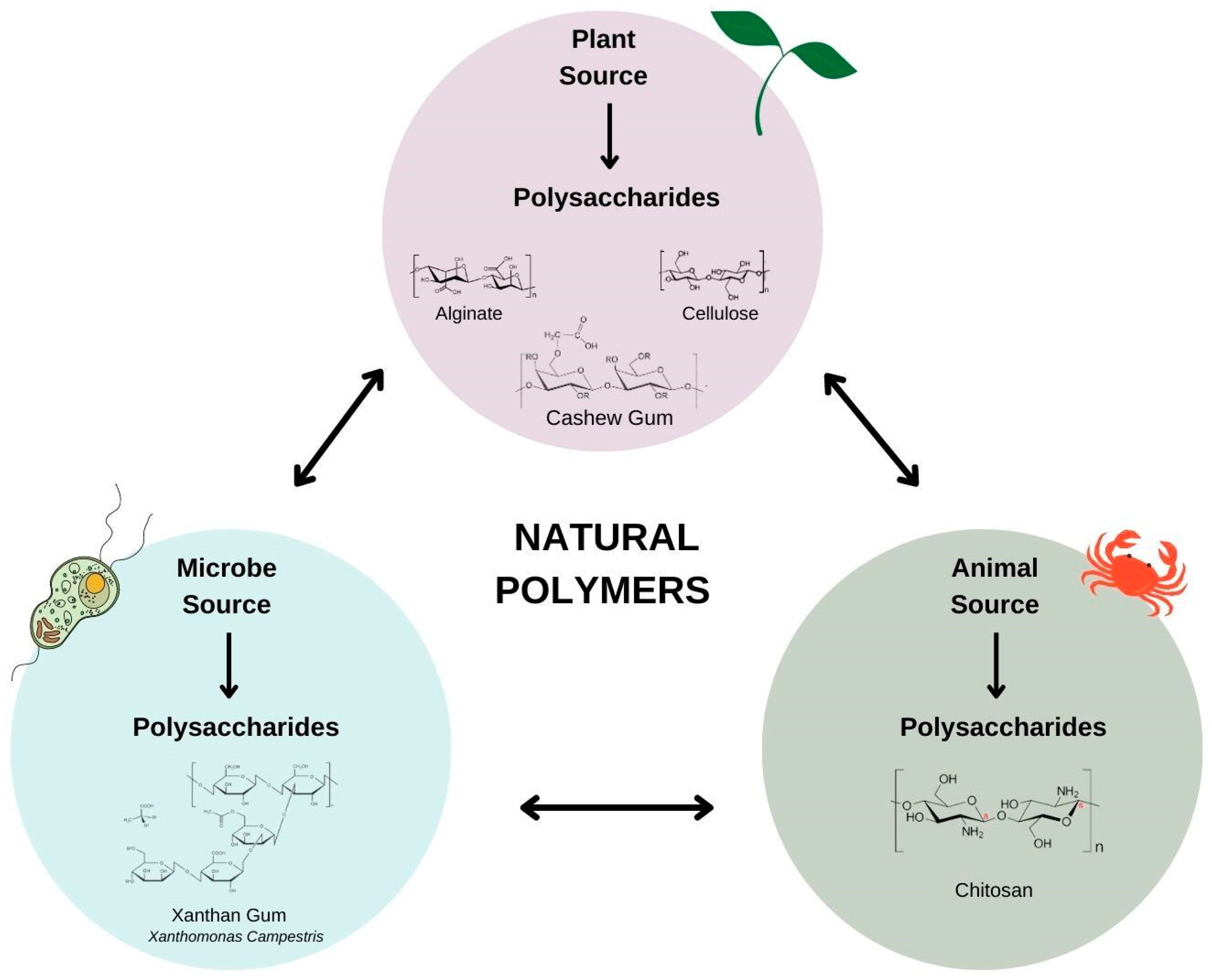
Natural polysaccharides (Figure 1) are produced naturally without human intervention and are found in plants (algae, plant exudate, seeds, fruits, tubers and cereals), animals (bovine, swine and crustaceans), fungi (P. ostreatus and Agaricus blazei) and bacteria (Xanthomonas ssp., Leuconostoc spp and Sphingomonas elodea) [7].
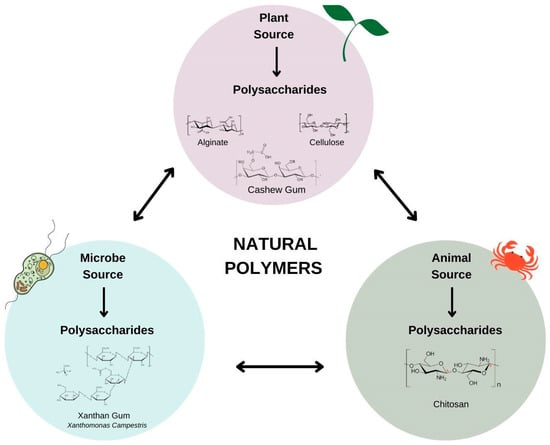
Figure 1.
Natural polysaccharides based on sources.
Due to their versatility, simple extraction process, biocompatibility and easy biodegradation, natural polysaccharides are of scientific and industrial interest as pharmaceutical excipients for the formulation of cosmetics, conventional-release drugs, modified-release drugs and a diversity of foodstuffs. In addition, they are referred to as biological macromolecules, described in the literature as immunological [11], antioxidant [12], anti-tumor [13], antidiabetic [14], anti-inflammatory [15], antiviral [16], antifungal [17] and anticoagulant [18] agentes, and also show cardiovascular [19] and hepatoprotective [20] effects.
Currently available sources report that these polysaccharides and oligosaccharides will have an annual growth rate of over 5% between 2020 and 2030, led primarily by the food and beverage industries [21]. In 2018. this market reached US $5.7 billion in Asia, followed by Europe, North America and Latin America markets. However, comparing both products, polysaccharides will dominate the market, with an estimated growth of 4.6% per year. In 2018, they reached a total of US $6.4 billion and have bacteria as their main source of production to supply the world market, followed by those obtained from plants, making up the second largest revenue [22].
There are several types of polysaccharides (Table 1) with potential pharmaceutical uses, such as Alginate, Agaranas, Carrageenans, Tragacante, Guar, Carob, Tamarind, Pectins, Starch, Inulin, Hyaluronic acid, Heparin, Chitin, Chitosan, Glucans, Xanthan, Dextran, Gelana and Gum. Among these, the most frequently used in the industry are mucilages, chitosan with different degrees of acetylation, alginate, pectin, starch, and different types of gums [2,23,24].

Table 1.
Pharmaceutical and food applications of the main gums on the market.
2.1. Gums
Gums are natural polysaccharides that are classified according to their charge (ionic or non-ionic), shape (branch or short branch), origin (seeds, plant exudate, microbial or algae), gelling behavior (cold, heating or reversible) and chemical structure (galactomannan, glucomannan, uronic acid, monoglycans or heteroglycans) [22].
Also known as gum hydrocolloids, they are a product developed by the plant as a defense mechanism in response lesions occurring mainly on the stem or due to unfavorable conditions such as the drying of the cell walls, as a form of pathological gummosis [4]. This biomaterial is composed, in general, of calcium, potassium and magnesium salts, and sugar monomers such as galactose and arabinose, from hemicellulose [24,49]. Xanthan, agar, Arabic, carob, ghatti, tragacanth, karaya, carrageenan and guar gums are amongst the most commonly occurring gums on the market [30].
Several natural gums are used by the food and pharmaceutical industries, e.g., as emulsifiers, disintegrants, emollients and additives. Because of their biodegradability, biocompatibility, non-toxicity, hydrophilicity and high viscosity, they are present in many pharmaceutical products as a component for suppositories (agar), in cosmetic products (gum Arabic, xanthan gum), as a stabilizer in toothpaste (xanthan gum), and in modified drug delivery systems (guar gum, locust bean gum, xanthan gum) [49]. Table 1 summarizes examples of natural gums and their applications in pharmaceutical and food products. Among all the studied and applied gums, some are still little explored in both areas, such as cashew gum.
2.1.1. Cashew Gum
Cashew gum is a complex heteropolysaccharide obtained from Anacardium occidentale L., a plant native to Venezuela and Brazil (North and Northeast). It was introduced in Africa and India by the Portuguese, and today it is spread over several Asian regions (Vietnam, United Republic of Tanzania and Indonesia) [50].
The cashew tree is a large tree that can reach up to 20 m in height. Its parts have several therapeutic uses; however, the fruits or cotyledons, known as cashews and cashew nuts, are the main economically valuable product; they are edible and are used as a snack or in the manufacture of sweets, having a higher competitiveness over the production of nuts, peanuts, hazelnuts and pistachios [51]. After the nut, the second main product of the cashew tree is the cashew nut shell liquid (CNSL), followed by the juice from the pulp of the pseudofruit or pendulum, called the cashew [50,51].
While these products are of unquestionable economic value, the cashew bark also has high commercial potential from the extraction of its exudate by incision, producing a yellow or reddish-brown gum resin. Exuded gums are polysaccharides produced by plant epithelial cells when the cortex is physically injured or suffers any microbial attack. The cashew gum is thus produced by the epithelial cells of the cashew tree in response to mechanical stimuli or pathogens [52].
Some studies report the possibility of using cashew gum in several sectors; however, its applications in an industrial environment are not well established. Although cashew gum has been explored since 1970, it is only since the 1990s that it has received increased interest from scientific community, mainly due to structural and chemical similarity with gum Arabic. The gum Arabic, also known as gum Acacia, is considered the oldest and the best known among natural gums; however, it has high cost, and occasionally its import is compromised by the difficulty of supply due to climatic, economic and political problems in the African producing region [53,54].
The cashew gum, in addition to the structural (branching) and chemical (component sugars) similarities with gum Arabic, has an important differential: it has high availability in the Northeast region of the Brazilian territory, which can generate profits in the period between the cashew season [55,56].
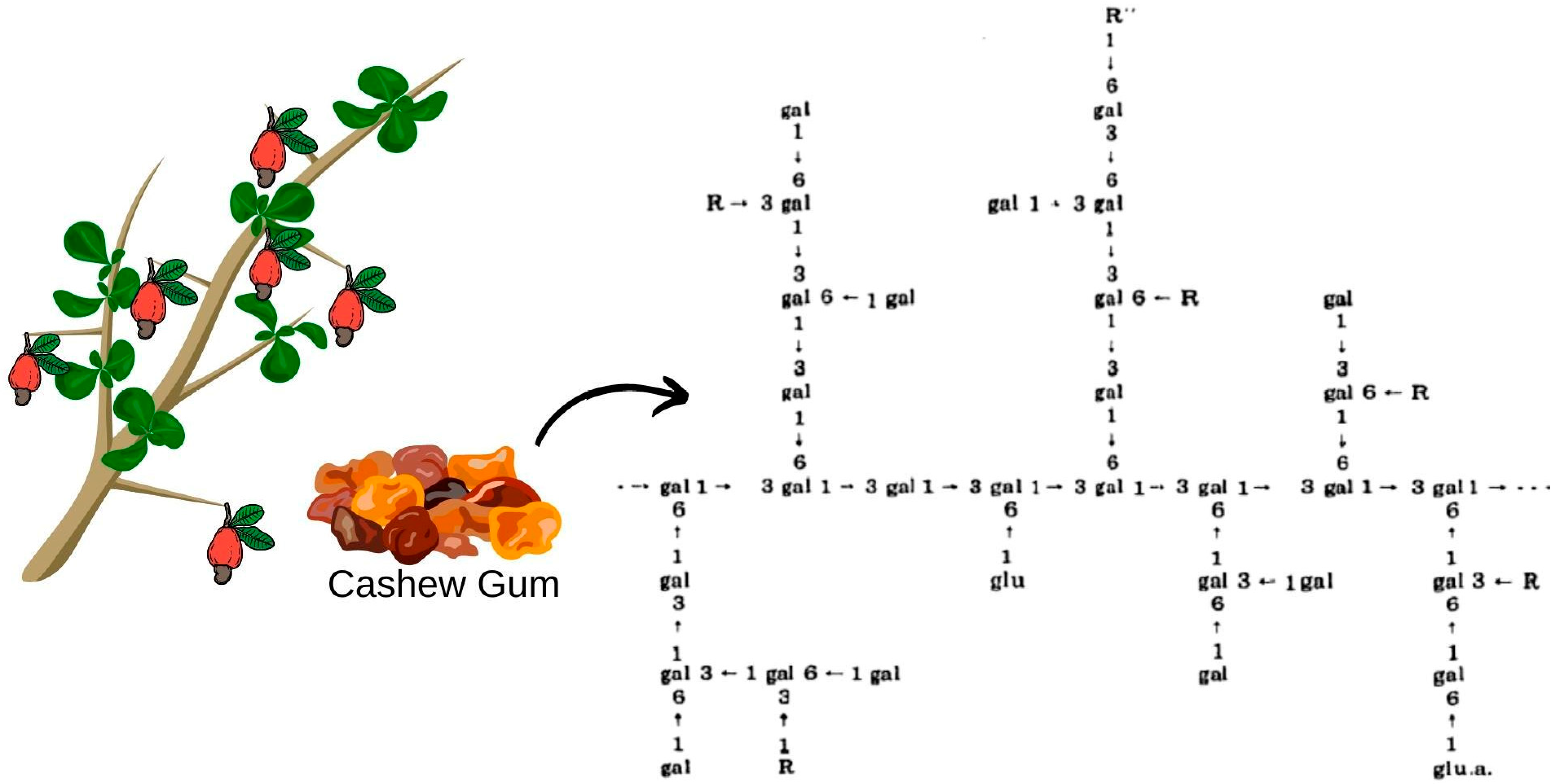
Cashew gum extracted from trees in the northeast region was characterized as a branched heteropolysaccharide containing in its structure (Figure 2): β-D-galactose (72–73%), α-D-glucose (11–14%), arabinose (4–6.5%), rhamnose (3.2–4%) and glucuronic acid (4.7–6.3%) by mass percentage [57]. In addition, it presents advantages in relation to gum Arabic, such as: lower ash content, indicating a lower degree of impurities even in the non-purified sample; higher protein content, which can provide better emulsifying properties, desired in the encapsulation processes of oils and aromas; high fiber content, being higher than gum Arabic and other traditional materials; and lower viscosity after extrusion, which can contribute to better dispersion and solubility in solutions [58,59]. Therefore, cashew gum could be indicated as a substitute for gum Arabic, and its commercial standardization would raise Brazil to a significant exporter in the international market, thus reducing the import dependency and creating a highly competitive product in its export share [58].
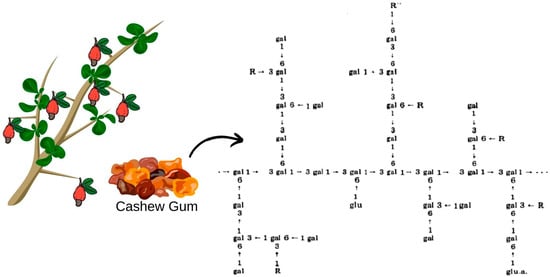
Figure 2.
Possible structure of Anacardium occidentale gum, L. R represents D-mannose, D-xylose, L-rhamnose, L-arabinose or 1–2 linkage with the arabinose chain. R” represents D-glucose or D-glucuronic acid.
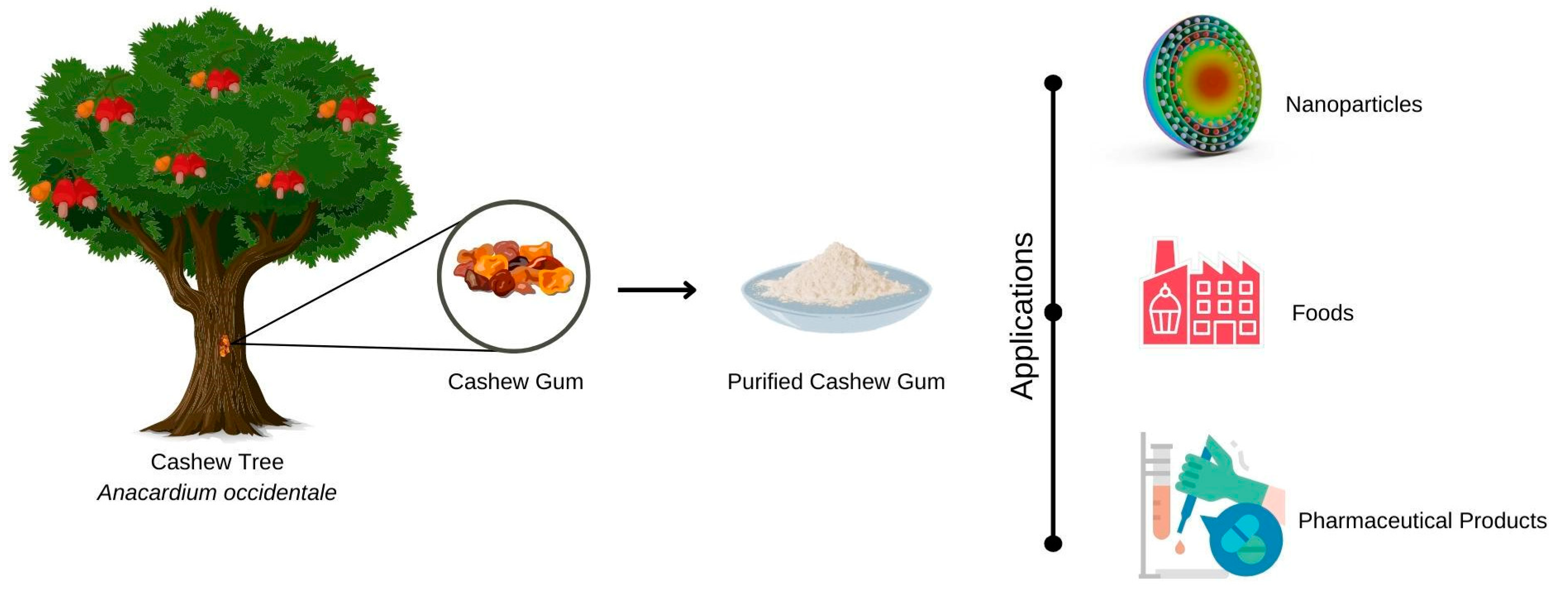
Cashew gum can be used, for example, in the manufacture of (Figure 3) pharmaceutical products, foodstuffs, cosmetics, adhesives and, more recently, in nanotechnology [57].
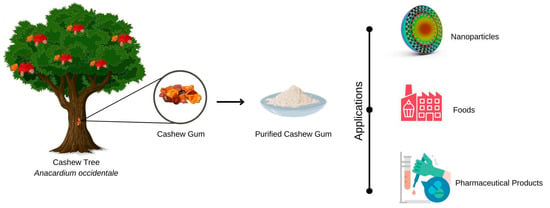
Figure 3.
Representation of potential applications of cashew tree gum in different fields, such as food, pharmaceutical excipients, and micro- and nanostructured systems.
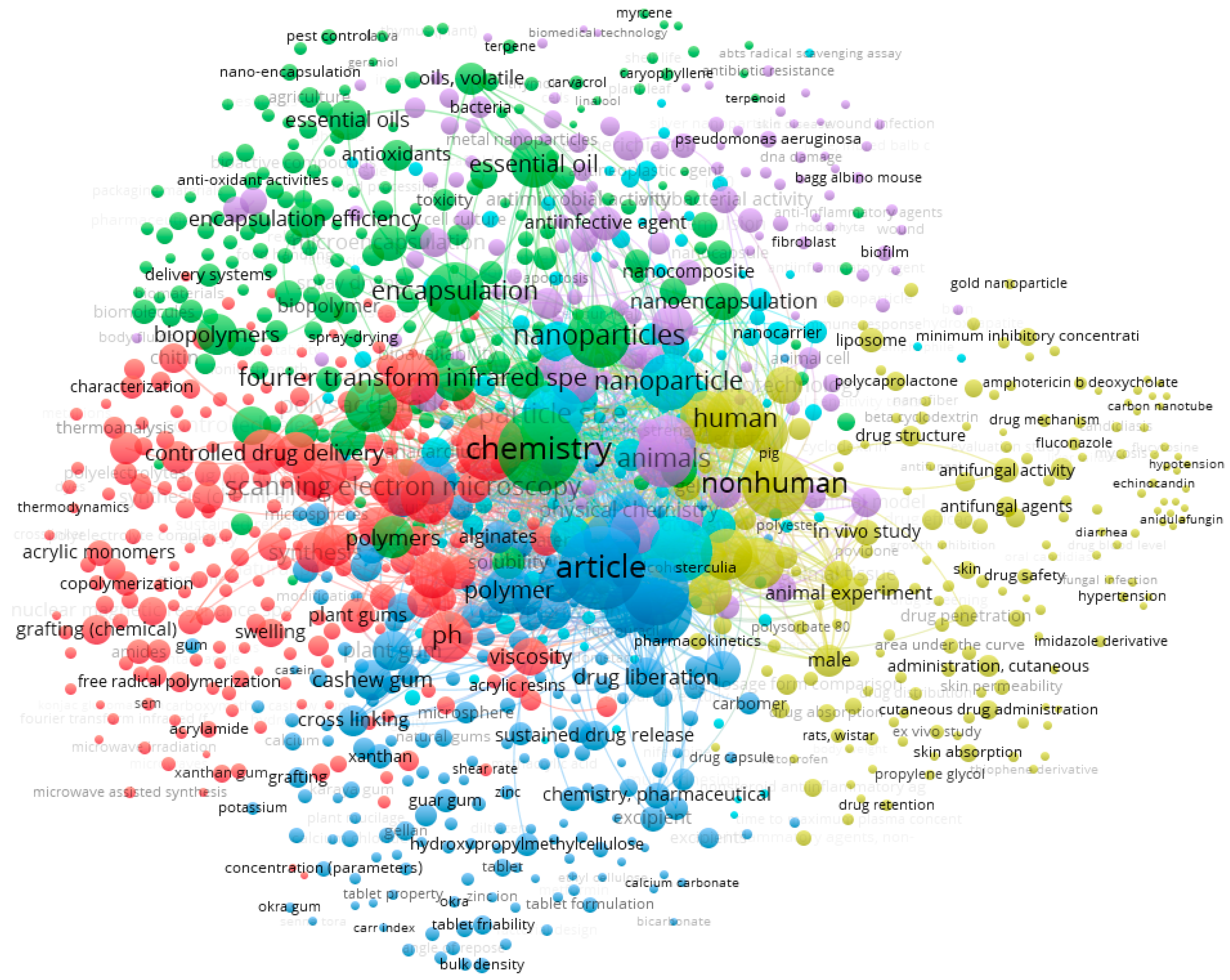
A survey in Scopus database generated a total of 824 published papers that combine both “cashew gum” AND “pharmaceutics”. VOSviewer was used to obtain the bibliometric map shown in Figure 4 [60,61]. Six clusters of terms were generated, demonstrating the wide range of uses of this polysaccharide in biomedical applications (e.g., development of nanocomposites, biodegradable films responsive to stimulus materials, gel dressings and controlled release tablets, and chemical engineering of new polymers, oxidation, sulfation, carboxymethylation, acetylation or copolymerization).
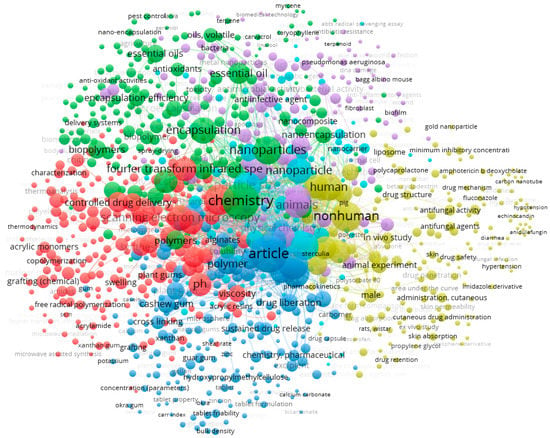
Figure 4.
Bibliometric map obtained by VOSviewer software version 1.6.16 (https://www.vosviewer.com), using “cashew gum” AND “pharmaceutics” as keywords, recorded from Scopus database.
2.1.2. Applications of Cashew Gum in the Development of Micro- and Nanoparticles
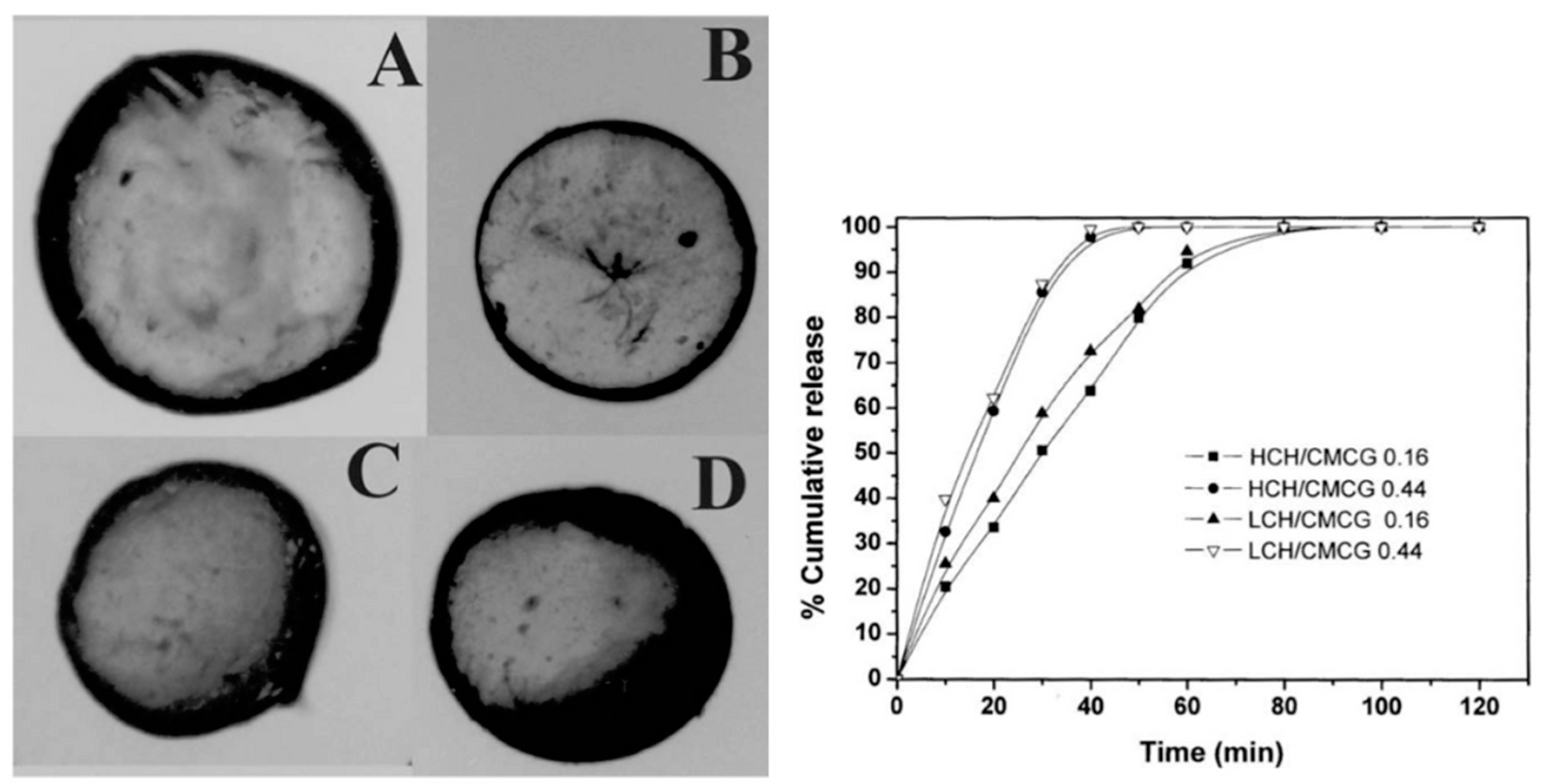
Cashew gum (CG) has been recently suggested as a biopolymer for the production of nanoparticles (NPs) and nanogels through various methods, with nanoprecipitation the most frequently used. In addition to several advantages, such as low cost, biocompatibility and self-assembly, several studies have highlighted the gastroprotective, anti-inflammatory and healing properties of cashew gum (Table 2) [62]. Magalhães et al. (2009) [63] developed chitosan/carboxymethyl cashew gum (CH/CMCG) microspheres (Figure 5) with different degrees of substitution and evaluated the release rate of the drug incorporated into the CG/chitosan matrix, which revealed high release rates of microparticles prepared using high density carboxymethyl CG, while those prepared with a low density (DS = 0.16) promoted a prolonged release of the drug.
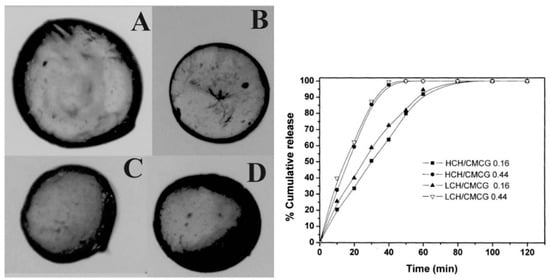
Figure 5.
Optical microscopy of different chitosan/carboxymethyl cashew gum (CH/CMCG) microspheres. (A) HCH/CMCG 0.16; (B) HCH/CMCG 0.44; (C) LCH/CMCG 0.16; and (D) HCH/CMCG 0.44 and effect of chitosan (CH) molar mass and degrees of substitution (DS) of chitosan/carboxymethyl cashew gum (CMCG) on in vitro release profile of BSA (Adapted from Magalhães et al. (2009) [63], Copyright© 2009, Elsevier Ltd).
De Oliveira et al. (2020) [64] combined alginate and cashew gum for the production of nanoparticles by spray-drying for the loading of Lippia sidoides essential oil. The obtained particles were in the range of 223–399 nm, and the encapsulation efficiency reached 55%, achieving 95% of the oil released within 30–50 h in vitro.
In recent years, the use of coacervation has become one of the most effective methods for micro/nanoencapsulation in pharmaceutical and food industries. This process is formed by a three-phase system in relation to the solvent, the active and the coating material, which involves four steps: (i) preparation of aqueous solution containing two or more polymers, (ii) mixing the hydrophobic phase with the aqueous solution of the polymer, (iii) changing the pH and temperature to induction of coacervation and phase separation and (iv) resistance of polymer matrices (temperature, crosslinking or dissolving agent) [65].
Gomez et al. (2016) [66] used the coacervation method for the development of CG and gelatin microcapsules for the encapsulation of shrimp lipid extract (astaxanthin). The study described the production of microparticles of approximately 32.7 ± 9.7 µm and an astaxanthin encapsulation efficiency of 59.9 ± 0.01%. The results showed that the presence of cashew tree gum in the microparticles resulted in the delay of the lipid extract degradation compared to non-encapsulated ones, thus demonstrating a high potential for the use of cashew gum to replace gum Arabic in the development of new technological products as a food ingredient.
Araruna et al. (2020) [67] developed silver nanoparticles combined with cashew gum (CG) and carboxymethylated cashew gum (CCG), exploring the role of microwave heating in the process of reducing silver ions at different pH (10 and 11) and the activity of the gum in stabilizing the nanoparticles. The cashew gum carboxylation process was carried out by back titration to GC mixed in 10 M NaOH, followed by the addition of monochloroacetic acid and by neutralization with 1M HCl. The synthesis of the nanoparticles was performed by mixing GC and GCC with an AgNO3 solution (1 mM) in a domestic microwave oven with exposure to microwaves at 2450 MHz for 3 min, which led to the reduction of Ag+ ions and the formation of nanoparticles: CGAgNP and CCGAgNP.

Table 2.
Applications of cashew gum (CG) for the loading of bioactives in the production of micro/nanoparticles.
Table 2.
Applications of cashew gum (CG) for the loading of bioactives in the production of micro/nanoparticles.
| Variation of CG | Polymers | Encapsulated Bioactive | Method | Size | Objective | References |
|---|---|---|---|---|---|---|
| CG carboxymethylated | Chitosan | Bovine serum albumin | Nanoprecipitation | 500–580 μm | Albumin release due to swelling behavior. | [63] |
| CG copolymerized | Acrylic acid | - | Self-embedding copolymerization | 71–603 nm | Prepare CG particles and acrylic acid and evaluate the responsive pH behavior. | [68] |
| CG | Chitosan | Lippia menosides essential oil | Emulsion | 1.50–1.56 mm | Larvicidal activity. | [69] |
| CG | Chitosan | Lippia menosides essential oil | Emulsion | 219–674 nm | Effects of spray-drying and the concentration of polymers in the preparation of particles. | [70] |
| CG | Alginate | Lippia menosides essential oil | Emulsion | 223–399 nm | Effects of spray-drying and the concentration of polymers in the preparation of the particles. | [64] |
| CG acetylated | - | Lippia menosides indomethacin | Self-assembly | 140–179 nm | Evaluation of the release profile of the produced particle confirming its application in drug release. | [71] |
| CG | Inulin | Ginger essential oil | Emulsion | 13.43–18.52 μm | To evaluate the influence of CG and inulin, in powder particles, in order to obtain functional products with ginger essential oil. | [72] |
| CG copolymerized | N-isopropylacrylamide (97%) | - | Radical polymerization | 11–23 nm | Copolymerize the cashew gum in order to make it sensitive to stimuli for the purpose of drug administration. | [73] |
| CG | Type B gelatin | Carotenoid | Emulsion | 113 μm 23–42.4 μm | Encapsulate astaxanthin in the polymer particle without the use of solvents. | [66] |
| CG acetylated | - | Diclofenac diethylamine | Nanoprecipitation/Dialysis | 79.32 nm/ 302 nm | Encapsulate the drug using different methodologies and compare them, in order to develop a transdermal delivery device. | [74] |
| CG | - | Omega 3 | Emulsion | 29.9 μm | Substitute potential for CG in the encapsulation of Omega 3. | [55] |
| CG | Maltodextrin | Green tea leaf extracts | Emulsion | 2.50–3.64 μm | Develop alternative microcapsules of green tea extract for the food industry for health benefits. | [75] |
| CG | - | D-limonene | Emulsion | 17–26.01 μm | Evaluate the effects of high dynamic pressure (APD) on emulsifying and encapsulating characteristics of CG. | [76] |
| CG acetylated | Monobasic sodium phosphate, bibasic sodium phosphate and sodium lauryl sulfate | Amphotericin B | Self-assembly | 50–900 nm | To investigate the influence of temperature, time and proportion of the acetylating agent on the acetylation of cashew gum as well as the influence of the degree of substitution of derivatives on their properties. | [77] |
| CG | Poly (L-lactide) | Amphotericin B | Nanoprecipitation and Pickering Emulsion | 100–3500 nm | Combine different particle production methodologies to encapsulate amphotericin B and improve its oral absorption, enhancing the treatment of leishmaniasis. | [78] |
| CG acetylated | - | Epi-isopiloturine | Dialysis | 107–156 nm | Increase the solubility of the alkaloid and enable its controlled release. | [79] |
| CG acetylated | - | Indomethacin | Pickering emulsion | 263.7–325 nm | Evaluate the points that make it possible to develop CG particles acetylated by Pickering Emulsion without surfactant, with and without Indomethacin. | [80] |
| CG | Gelatin | Green coffee oil | Complex coacervation | 13.9–25.7 μm | Produce green coffee oil microcapsules by complex coacervation for addition to juices. | [81] |
| CG copolymerized | L-Lactide | Amphotericin B | Dialysis | 223–233 nm | Produce copolymerized CG particles by dialysis to encapsulate amphotericin B and compare with previous study. | [82] |
| CG | Potassium hexacyanoferrate (II) trihydrate and iron (III) chloride | - | Nanoprecipitation | 63.5–85.0 nm | Develop a hybrid nanomaterial (Prussian Blue + CG (used to stabilize the matrix)) to act as an electrochemical sensor for the oxidation of some drugs. | [83] |
| CG Carboxymethylated | Cashew gum and carboxymethylated | cashew gum | Green synthesis | 100.9–144.7 nm | Antibacterial activity of silver nanoparticles based on cashew gum and carboxymethylated cashew gum. | [67] |
Natural polymers have also been intensively evaluated in the development of micro- and nanoparticles for pharmaceutical purposes [84,85,86,87]. Nanoparticles are colloidal systems with diameters of 1–1000 nm that show important advantages in the improvement of the bioavailability of loaded drugs while also reducing the risk of toxicological events, provided that a lower drug dose will be required to reach the site of action given that nanoparticles have site-specific delivery features [88]. Nanoparticles obtained from naturally occurring polymers are also reported to be stable and useful in the protection of bioactives against degradation [86]. Polysaccharides are among the most used materials in the development of nanoparticles due to their biocompatibility, biodegradability and structural flexibility [89]. A study performed by Richter et al. (2020) [82] used different ratios of copolymers of cashew tree gum and L-lactide to carry amphotericin B (AmB) by the self-assembled method. The nanoparticles showed mean size values between 230 and 250 nm, with the capacity to inhibit all tested strains of C. albicans. Pickering emulsion was used to produce cashew gum-poly-l-lactide (CGPLAP) nanoparticles copolymer by Richter et al. (2018) [78] for the loading of amphotericin B (AmB) and was compared to a commercial AmB formulation. The CGPLAP nanoparticles resulted in an encapsulation efficiency of 21% and 47% using the initial AmB concentrations of 5 and 10 mg (respectively). These nanoparticles were stable for up to 28 days under refrigeration.
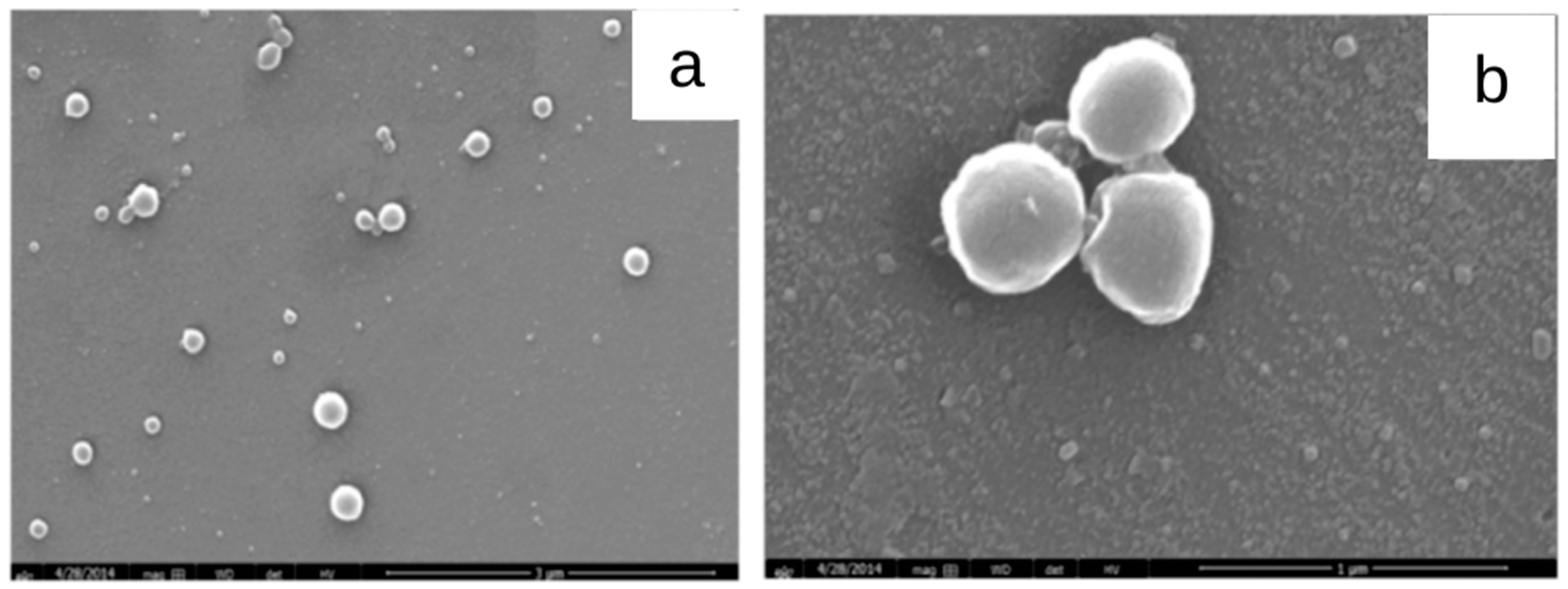
Dias et al. (2016) [74] developed cashew gum nanoparticles (Figure 6) using the nanoprecipitation method followed by dialysis, with the aim to encapsulate diclofenac diethylamine. An encapsulation efficiency greater than 60% was obtained. The loaded nanoparticles of particle sizes between 23 nm and 1.5 mm showed biocompatibility when evaluated in OSCC cell lines, demonstrating that cashew gum may be a promising polysaccharide to develop delivery systems for anti-inflammatory drugs.
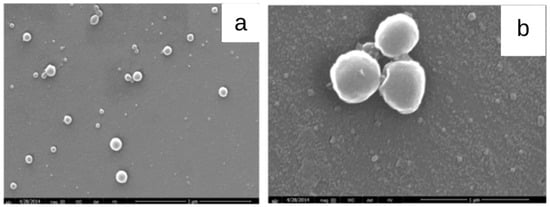
Figure 6.
SEM images of acetylated cashew gum (ACG) nanoparticles (a) without and (b) with diclofenac diethylamine (DDA) (adapted from Dias et al. (2016) [74], Copyright© 2009, Elsevier Ltd).
Pickering emulsions were also used by Lima Cardial et al. (2019) [80] in the development of nanoparticles containing acetylated derivatives of cashew gum, with the aim of loading indomethacin. The CG nanoparticles carrying the drug showed particle size and zeta potential influenced by the different degrees of acetylation of the polysaccharide, presenting an encapsulation efficiency of about 50% for indomethacin.
From these studies, there is still a range of opportunities to be explored using CG in nano- and microemulsion systems. Cashew gum as a potential substitute for other gums, added to the large growing market in production, mainly in the Brazilian northeast, make the development of new products a promising field of pharmaceutical research using this polysaccharide.
3. Cashew Gum Patent Perspectives in Brazil
Cashew gum (CG) has been tested for various applications in a range of industries (plastics/polymerics, agri-food and pharmaceuticals). The use of this biopolymer in scientific research has become an object of interest mainly in the last decades due to its promising results as a food additive and in the development of nanoparticles for drug encapsulation. Different techniques to obtain, modify and use cashew gum have been developed and patented. The patents related to the use of cashew gum and its applications are presented in Table 3.

Table 3.
Patents filed in Brazil that contain ‘gum AND cashew’ in the title and summary.
The study published on the website of the National Institute of Industrial Property (INPI) showed a total of 14 patents between 1990 and 2018 that relate isolation method, technological development and applications of cashew gum. The first patent related to cashew tree gum was patented by Paula and Rodrigues in 1990 [52]. The inventors disclosed the method of isolating cashew gum from the raw gum exuded from the tree. By isolating the polysaccharide composing the gum, it was separated from impurities by applying steps of dialysis, lyophilization and pH adjustment. Mothé (2000) [102] disclosed the invention of the process of obtaining and the composition of the purified cashew tree gum. The purification process results from solubilization, centrifugation and addition of ethyl alcohol to precipitate the polysaccharide and, finally, vacuum drying to obtain the purified gum, which appears as a white powder that is odorless and has a mild taste. An invention related to a superabsorbent hydrogel was disclosed by Rubira et al. (2004) [100]; the patent describes the chemical modification of cashew gum with glycidyl methacrylate, leading to the insertion of carbon–carbon unsaturation in the cashew gum structure. The development of a hydrogel matrix associating cashew gum copolymerized with acrylamide showed a superabsorbent material and delayed water loss. Soares et al. (2014) [104] disclosed the invention of a cashew gum and chitosan hydrogel without modification in the chemical structures of the polysaccharides. The cashew gum and chitosan hydrogels showed a water absorption capacity of about 270% (1:4) and 320% (2:3) of the initial weight under immersion in water for 24 h, showing the practical use of this material in the pharmaceutical industry for the development of new materials for application in topical dressings.
Sobrinho et al. (2015) [97] patented an invention of a new excipient containing a processed blend of cashew tree gum and chitosan for the development of topically applied products. The mucoadhesive polymer blend described by the inventor was obtained by solubilizing cashew tree gum in distilled water and chitosan in acetic acid (2%) followed by rotational evaporation and lyophilization steps. Subsequently, the mucoadhesive and release properties were evaluated by incorporating the excipient into a pharmaceutical form for the development of a mucoadhesive product containing pilocarpine; the results showed that the mucoadhesive formulation containing GC/chitosan and pilocarpine had a mucoadhesion time of 510 min. According to the invention, the polymeric blend (cashew gum and chitosan) had high performance, high safety and low cost, granting it mucoadhesive and modified release properties.
The use of cashew gum in the encapsulation of bioactives has been patented in the last decades. Torres et al. (2016) [96] invented cashew gum microcapsules encapsulating green tea extract (Camelliasinesis), with the aim of protecting the phytochemicals of the tea extract. In addition to the preservation of bioactive compounds, the encapsulation process opens new markets for the use of cashew gum, which is of low cost and easily accessible.
In addition to micro- and nanoparticles, some therapeutic/nutraceutical properties of cashew tree gum have been demonstrated in studies. A patent disclosed by Mothé et al. (2016) [57] used cashew gum as a food additive in chocolate production. This invention used cashew gum as a partial substitute for cocoa butter normally used for the production of chocolates, creating a chocolate with fewer calories and with potential nutraceutical effects on human health. The use of cashew gum has also been reported in the development of biomaterials with potential use in surgical applications and as a support (scaffold) for cell growth in the process of tissue regeneration. The scaffold was developed using a cashew gum solution modified with phthalic anhydride (2.5–4.0%) and mixed with a chitosan solution dissolved in acetic acid (2.5%). The in vitro cell culture showed that the scaffold with cashew gum is an effective support for the growth of mesenchymal stem cells (MSC). Positive results in the cytotoxicity test indicated biocompatibility and non-toxicity, making cashew gum a possible component of synthetic matrices for therapeutic purposes and tissue repair.
Silvia et al. (2018) [90] published an invention from purified cashew gum for the preparation of nanotechnology-based products. The patent disclosed the extraction of cashew tree gum followed by the process of purification and chemical modification through the chemical insertion of acetyl groups transforming the hydrophobic cashew gum.
4. Conclusions
A review of recent works, patents and the main techniques for the development of micro- and nanoparticles using cashew gum was presented, highlighting the potential of this biopolymer in the creation of new products applied to human health. This review emphasizes that exploitation of cashew gum has the potential to widen the food and pharmaceutical markets, especially the domestic Brazilian markets. A number of patents covering isolation methods, composition, technological developments and applications of cashew gum have been filled in the last decades, with the potential to create a portfolio of products of high market value.
Author Contributions
Conceptualization, methodology, validation, formal analysis, writing—original draft preparation: R.G.A., L.R.M.d.A. and L.N.A.; investigation, resources, data curation, writing—review and editing, supervision, project administration, funding acquisition, K.C.L., E.B.S. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPQ, Bolsa de Produtividade em Pesquisa #301964/2019-0, CNPQ.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhardwaj, T.R.; Kanwar, M.; Lal, R.; Gupta, A. Natural gums and modified natural gums as sustained-release carriers. Drug Dev. Ind. Pharm. 2000, 26, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Halake, K.; Kim, H.J.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.; Kim, Y.J.; Kim, S.; Ahn, S.; An, S.Y. Recently developed applications for natural hydrophilic polymers. J. Ind. Eng. Chem. 2016, 40, 16–22. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently investigated natural gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Goswami, S.; Naik, S. Natural gums and its pharmaceutical application. J. Sci. Innov. Res. 2014, 3, 112–121. [Google Scholar] [CrossRef]
- Cunha, P.L.R.d.; Paula, R.C.M.d.; Feitosa, J. Polissacarídeos da biodiversidade brasileira: Uma oportunidade de transformar conhecimento em valor econômico. Química Nova 2009, 32, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Wang, H.-X.; Huang, F.; Wang, L.-M. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural polysaccharides with immunomodulatory activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Mei, X.; Hu, J. The antioxidant activities of natural polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, Q.; Yin, J.-J.; Yao, Y.; Zhang, J.-L. Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int. J. Biol. Macromol. 2015, 72, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Guo, Q.; Wang, M.; Li, Y.; Meng, Y.; Huang, L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Iriqui, A.C.; García-Romo, J.S.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Burboa-Zazueta, M.G.; Luque-Alcaraz, A.G.; Calderón-Santoyo, M.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Phytotoxicity, cytotoxicity, and in vivo antifungal efficacy of chitosan nanobiocomposites on prokaryotic and eukaryotic cells. Environ. Sci. Pollut. Res. 2021, 28, 3051–3065. [Google Scholar] [CrossRef]
- Cai, W.; Xu, H.; Xie, L.; Sun, J.; Sun, T.; Wu, X.; Fu, Q. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016, 140, 308–313. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015, 125, 32–41. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, J.; Di, L.; Li, J.; Hu, L.; Qiao, H.; Wang, L.; Feng, Y. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohydr. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef]
- Polysaccharides and Oligosaccharides Market. 2022. Available online: https://www.factmr.com/report/427/polysaccharides-oligosaccharides-market (accessed on 15 June 2022).
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Lyons, J.G.; Devine, D.M.; Kennedy, J.E.; Geever, L.M.; O’Sullivan, P.; Higginbotham, C.L. The use of Agar as a novel filler for monolithic matrices produced using hot melt extrusion. Eur. J. Pharm. Biopharm. 2006, 64, 75–81. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lu, E.-X.; Jiang, Z.-Q.; Zhang, Q.-Z.; Jiang, X.-G. A water-insoluble drug monolithic osmotic tablet system utilizing gum arabic as an osmotic, suspending and expanding agent. J. Control. Release 2003, 92, 375–382. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 627–652. [Google Scholar]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar]
- Bonferoni, M.; Rossi, S.; Tamayo, M.; Pedraz, J.; Dominguez-Gil, A.; Caramella, C. On the employment of λ-carrageenan in a matrix system. II. λ-Carrageenan and hydroxypropylmethylcellulose mixtures. J. Control. Release 1994, 30, 175–182. [Google Scholar] [CrossRef]
- Prajapat, P.; Talesara, G.L. Synthesis and Anti-inflammatory Screening of Some Mono and Bis-Alkoxyphthalimide Linked Benzimidazole and their Quinazoline and Pyrimidine Derivatives. J. Heterocycl. Chem. 2016, 53, 1603–1610. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Setty, C.M.; Badiger, A.M.; Muralikrishna, K. Gum ghatti: A promising polysaccharide for pharmaceutical applications. Carbohydr. Polym. 2012, 87, 980–986. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Nie, K.; Gao, Z.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F. Effect of gum arabic, gum ghatti and sugar beet pectin as interfacial layer on lipid digestibility in oil-in-water emulsions. Food Biophys. 2016, 11, 292–301. [Google Scholar] [CrossRef]
- Madni, A.; Khalid, A.; Wahid, F.; Ayub, H.; Khan, R.; Kousar, R. Preparation and Applications of Guar Gum Composites in Biomedical, Pharmaceutical, Food, and Cosmetics Industries. Curr. Nanosci. 2021, 17, 365–379. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Krishnaiah, Y.; Satyanarayana, S. In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J. Control. Release 1998, 51, 281–287. [Google Scholar] [CrossRef]
- Krishnaiah, Y.; Satyanarayana, V.; Kumar, B.D.; Karthikeyan, R. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef]
- Krishnaiah, Y.; Satyanarayana, S.; Prasad, Y.R.; Rao, S.N. Evaluation of guar gum as a compression coat for drug targeting to colon. Int. J. Pharm. 1998, 171, 137–146. [Google Scholar] [CrossRef]
- Sreenivasa, B.; Prasanna, R.; Mary, S. Design and studies of gum karaya matrix tablet. Int. J. Pharm. Excip. 2000, 2, 239–242. [Google Scholar]
- Munday, D.L.; Cox, P.J. Compressed xanthan and karaya gum matrices: Hydration, erosion and drug release mechanisms. Int. J. Pharm. 2000, 203, 179–192. [Google Scholar] [CrossRef]
- Park, C.R.; Munday, D.L. Evaluation of selected polysaccharide excipients in buccoadhesive tablets for sustained release of nicotine. Drug Dev. Ind. Pharm. 2004, 30, 609–617. [Google Scholar] [CrossRef]
- Soumya, R.; Raghu, K.; Abraham, A. Locust bean gum-based micro-and nanomaterials for biomedical applications. In Micro-and Nanoengineered Gum-Based Biomaterials for Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 241–253. [Google Scholar]
- Tian, H.; Xiang, D.; Li, C. Tea polyphenols encapsulated in W/O/W emulsions with xanthan gum–locust bean gum mixture: Evaluation of their stability and protection. Int. J. Biol. Macromol. 2021, 175, 40–48. [Google Scholar] [CrossRef]
- Nagaraja, K.; Rao, K.M.; Reddy, G.V.; Rao, K. Tragacanth gum-based multifunctional hydrogels and green synthesis of their silver nanocomposites for drug delivery and inactivation of multidrug resistant bacteria. Int. J. Biol. Macromol. 2021, 174, 502–511. [Google Scholar] [CrossRef]
- Komeilyfard, A.; Fazel, M.; Akhavan, H.; Mousakhani Ganjeh, A. Effect of Angum gum in combination with tragacanth gum on rheological and sensory properties of ketchup. J. Texture Stud. 2017, 48, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; He, W.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Effect of pH and xanthan gum on emulsifying property of ovalbumin stabilized oil-in water emulsions. LWT 2021, 147, 111621. [Google Scholar] [CrossRef]
- Dhopeshwarkar, V.; Zatz, J.L. Evaluation of xanthan gum in the preparation of sustained release matrix tablets. Drug Dev. Ind. Pharm. 1993, 19, 999–1017. [Google Scholar] [CrossRef]
- Pan, X.; Veroniaina, H.; Su, N.; Sha, K.; Jiang, F.; Wu, Z.; Qi, X. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J. Pharm. Sci. 2021, 16, 687–703. [Google Scholar] [CrossRef]
- Ye, F.; Astete, C.E.; Sabliov, C.M. Entrapment and delivery of α-tocopherol by a self-assembled, alginate-conjugated prodrug nanostructure. Food Hydrocoll. 2017, 72, 62–72. [Google Scholar] [CrossRef]
- Mendes, C.; Costa, J.; Vicente, A.A.; Oliveira, M.B.P.; Mafra, I. Cashew nut allergy: Clinical relevance and allergen characterisation. Clin. Rev. Allergy Immunol. 2019, 57, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, N.N.; Mothé, C.G.; Mothé, M.G.; de Oliveira, L.G. Cashew nut and cashew apple: A scientific and technological monitoring worldwide review. J. Food Sci. Technol. 2020, 57, 12–21. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.; Rodrigues, J. Composition and rheological properties of cashew tree gum, the exudate polysaccharide from Anacardium occidentale L. Carbohydr. Polym. 1995, 26, 177–181. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Phillips, G.O. Acacia gum (Gum Arabic): A nutritional fibre; metabolism and calorific value. Food Addit. Contam. 1998, 15, 251–264. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; de Barros Fernandes, R.V.; Antoniassi, R.; de Faria-Machado, A.F.; de Andrade Feitosa, J.P.; de Paula, R.C.M. Application of cashew tree gum on the production and stability of spray-dried fish oil. Food Chem. 2017, 221, 1522–1529. [Google Scholar] [CrossRef]
- Loureiro, K.C.; Lima-Verde, I.B.; Johannisson, A.; Ntallaris, T.; Jager, A.; Štěpánek, P.; da Costa Mendonça, M.; Severino, P.; Morrell, J.M. Effects of cashew gum and nanoparticles on cooled stallion semen. Acta Vet. Scand. 2020, 62, 31. [Google Scholar] [CrossRef] [PubMed]
- Mothé, C.G.; Oliveira, N.N.; de Freitas, J.S.; Mothé, M.G. Cashew tree gum: A scientific and technological review. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238716. [Google Scholar] [CrossRef]
- Andrade, K.; de Carvalho, C.W.; Takeiti, C.Y. Goma de cajueiro (Anacardium occidentale): Avaliação das modificações químicas e físicas por extrusão termoplástica. Polímeros 2013, 23, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Olorunsola, E.O.; Bhatia, P.G.; Tytler, B.A.; Adedokun, M.O.; Adikwu, M.U. Dissolution and permeation characteristics of artemether tablets formulated with two gums of different surface activity. Trop. J. Pharm. Res. 2017, 16, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. VosViewer—Visualizing Scientific Landscapes. Available online: https://www.vosviewer.com (accessed on 26 June 2022).
- Amorim, A.d.G.N.; Sánchez-Paniagua, M.; de Oliveira, T.M.; Mafud, A.C.; da Silva, D.A.; de Almeida, J.R.d.S.; López-Ruiz, B. Synthesis, characterization and use of enzyme cashew gum nanoparticles for biosensing applications. J. Mater. Chem. B 2021, 9, 6825–6835. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, G.A., Jr.; Santos, C.M.; Silva, D.A.; Maciel, J.S.; Feitosa, J.P.; Paula, H.C.; de Paula, R.C. Microspheres of chitosan/carboxymethyl cashew gum (CH/CMCG): Effect of chitosan molar mass and CMCG degree of substitution on the swelling and BSA release. Carbohydr. Polym. 2009, 77, 217–222. [Google Scholar]
- De Oliveira, E.F.; Paula, H.C.; de Paula, R.C. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Fávaro-Trindade, C.S. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocoll. 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Araruna, F.B.; de Oliveira, T.M.; Quelemes, P.V.; de Araújo Nobre, A.R.; Plácido, A.; Vasconcelos, A.G.; de Paula, R.C.M.; Mafud, A.C.; de Almeida, M.P.; Delerue-Matos, C.; et al. Antibacterial application of natural and carboxymethylated cashew gum-based silver nanoparticles produced by microwave-assisted synthesis. Carbohydr. Polym. 2020, 241, 115260. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.A.; Feitosa, J.P.; Paula, H.C.; de Paula, R.C. Synthesis and characterization of cashew gum/acrylic acid nanoparticles. Mater. Sci. Eng C 2009, 29, 437–441. [Google Scholar] [CrossRef]
- Paula, H.C.; Sombra, F.M.; de Freitas Cavalcante, R.; Abreu, F.O.; de Paula, R.C. Preparation and characterization of chitosan/cashew gum beads loaded with Lippia sidoides essential oil. Mater. Sci. Eng. C 2011, 31, 173–178. [Google Scholar] [CrossRef]
- Abreu, F.O.; Oliveira, E.F.; Paula, H.C.; de Paula, R.C. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Pitombeira, N.A.; Neto, J.G.V.; Silva, D.A.; Feitosa, J.P.; Paula, H.C.; de Paula, R.C. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef]
- De Barros Fernandes, R.V.; Botrel, D.A.; Silva, E.K.; Borges, S.V.; de Oliveira, C.R.; Yoshida, M.I.; de Andrade Feitosa, J.P.; de Paula, R.C.M. Cashew gum and inulin: New alternative for ginger essential oil microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef]
- Abreu, C.M.; Paula, H.C.; Seabra, V.; Feitosa, J.P.; Sarmento, B.; de Paula, R.C. Synthesis and characterization of non-toxic and thermo-sensitive poly (N-isopropylacrylamide)-grafted cashew gum nanoparticles as a potential epirubicin delivery matrix. Carbohydr. Polym. 2016, 154, 77–85. [Google Scholar] [CrossRef]
- Dias, S.F.L.; Nogueira, S.S.; de França Dourado, F.; Guimarães, M.A.; de Oliveira Pitombeira, N.A.; Gobbo, G.G.; Primo, F.L.; de Paula, R.C.M.; Feitosa, J.P.A.; Tedesco, A.C. Acetylated cashew gum-based nanoparticles for transdermal delivery of diclofenac diethyl amine. Carbohydr. Polym. 2016, 143, 254–261. [Google Scholar] [CrossRef]
- Silva, F.; Torres, L.; Silva, L.; Figueiredo, R.; Garruti, D.; Araújo, T.; Duarte, A.; Brito, D.; Ricardo, N. Cashew gum and maltrodextrin particles for green tea (Camellia sinensis var Assamica) extract encapsulation. Food Chem. 2018, 261, 169–175. [Google Scholar] [CrossRef]
- Porto, B.C.; Cristianini, M. Effect of dynamic high pressure on emulsifying and encapsulant properties of cashew tree gum. Carbohydr. Polym. 2018, 186, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.R.; Paula, H.C.; Abreu, F.O.; da Silva, R.B.; Sombra, F.M.; de Paula, R.C. Hydrophobization of cashew gum by acetylation mechanism and amphotericin B encapsulation. Int. J. Biol. Macromol. 2018, 108, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Feitosa, J.; Paula, H.; Goycoolea, F.; de Paula, R. Pickering emulsion stabilized by cashew gum-poly-l-lactide copolymer nanoparticles: Synthesis, characterization and amphotericin B encapsulation. Colloids Surf. B Biointerfaces 2018, 164, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral Rodrigues, J.; de Araújo, A.R.; Pitombeira, N.A.; Plácido, A.; de Almeida, M.P.; Veras, L.M.C.; Delerue-Matos, C.; Lima, F.C.D.A.; Neto, A.B.; de Paula, R.C.M. Acetylated cashew gum-based nanoparticles for the incorporation of alkaloid epiisopiloturine. Int. J. Biol. Macromol. 2019, 128, 965–972. [Google Scholar] [CrossRef]
- Lima Cardial, M.R.; Paula, H.C.B.; da Silva, R.B.C.; da Silva Barros, J.F.; Richter, A.R.; Sombra, F.M.; de Paula, R.C.M. Pickering emulsions stabilized with cashew gum nanoparticles as indomethacin carrier. Int. J. Biol. Macromol. 2019, 132, 534–540. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, W.Q.; Wurlitzer, N.J.; de Oliveira Araújo, A.W.; Comunian, T.A.; Bastos, M.d.S.R.; de Oliveira, A.L.; Magalhães, H.C.R.; Ribeiro, H.L.; de Figueiredo, R.W.; de Sousa, P.H.M. Complex coacervates of cashew gum and gelatin as carriers of green coffee oil: The effect of microcapsule application on the rheological and sensorial quality of a fruit juice. Food Res. Int. 2020, 131, 109047. [Google Scholar] [CrossRef]
- Richter, A.R.; Carneiro, M.J.; de Sousa, N.A.; Pinto, V.P.; Freire, R.S.; de Sousa, J.S.; Mendes, J.F.; Fontenelle, R.O.; Feitosa, J.P.; Paula, H.C. Self-assembling cashew gum-graft-polylactide copolymer nanoparticles as a potential amphotericin B delivery matrix. Int. J. Biol. Macromol. 2020, 152, 492–502. [Google Scholar] [CrossRef]
- Moraes, R.R.; de Oliveira Farias, E.A.; Carvalho, C.L.; Cantanhêde, W.; Eiras, C. Development of cashew gum-based bionanocomposite as a platform for electrochemical trials. Int. J. Biol. Macromol. 2020, 153, 118–127. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef]
- Ataide, J.A.; Gerios, E.F.; Cefali, L.C.; Fernandes, A.R.; Teixeira, M.D.C.; Ferreira, N.R.; Tambourgi, E.B.; Jozala, A.F.; Chaud, M.V.; Oliveira-Nascimento, L.; et al. Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain-Chitosan Nanoparticles. Polymers 2019, 11, 1681. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Chaud, M.V.; Shimojo, A.; Antonini, D.; Lancelloti, M.; Santana, M.H.; Souto, E.B. Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: Antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids Surf. B Biointerfaces 2015, 129, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Dmour, I.; Taha, M.O. Natural and semisynthetic polymers in pharmaceutical nanotechnology. Org. Mater. Smart Nanocarriers Drug Deliv. 2018, 35–100. [Google Scholar] [CrossRef]
- Silvia, D.R.e.a. Micro e Nanopartículas do Biopolímero da Goma do Cajueiro Acetilada Para Veiculação de Fármacos. Patent No. BR 10 2018 014996 2 A2, 23 July 2018. [Google Scholar]
- Silvia, K.F.F. Plástico Biodegradável à Base de Goma de Cajueiro Para Aplicação Como Embalagem de Produtos Comerciais Desidratados. Patent No. BR 10 2017 020813 3 A2, 28 September 2017. [Google Scholar]
- De Carvalho, M.D. Matriz Porosa Desenvolvida à Base de Quitosana e Polissacarídeo Exsudato da Anacardium Occidentale L. Modificado com Anidrido Ftálico Para Cultivo de Células-Tronco Mesenquimais. Patent No. BR 10 2017 012139 9 A2, 8 June 2017. [Google Scholar]
- Silvia, K.F.F. Espuma Sólida Nanoporosa Hidrossolúvel Para Liberação Controlada de Drogas em mucosas. Patent No. BR 10 2017 007322 0 A2, 10 April 2017. [Google Scholar]
- Mothé, C.G.; Lannes, S.C.S.; Mothé, M.G. Composições Alimentícias de Chocolate Contendo Goma de Cajueiro, em Barra, Bombom e Chocolate em pó, Úteis Como Alimento Funcional e Nutracêutico. Patent No. BR 10 2016 027801 5 A2, 25 November 2016. [Google Scholar]
- Brasil, I.M.; Figueiredo, R.W.; Figueiredo, E.A.T.; Pontes, D.F.; Oliveira, L.S.; Zambell, R.A. Nanoencapsulados de Resíduos da Industria de Processamento de Frutas em Matriz Polieletrolitica de Goma de Cajueiro e Quitosana Para Uso Como Revestimento em Frutas Minimamente Processadas. Patent No. BR 10 2016 018308 1 A2, 9 August 2016. [Google Scholar]
- Torres, L.B.V.; Silva, F.M.R.; Zocolo, G.J.; Ricardo, N.M.P.S.; Garruti, D.S.; Figueiredo, R.W. Encapsulamento de Chá Verde (Camelliasinensis) por “Spray Drier” Com Goma de Cajueiro/Maltodextrina. Patent No. BR 10 2016 002436 6 A2, 3 February 2016. [Google Scholar]
- Sobrinho, J.L.S.S.; Cordeiro, M.S.F.; De Sá, L.L.F.; Da Silva, C.M.B.; De Souza, F.R.L.; Nunes, L.C.C.; Filho, E.C.S.N.; Neto, P.J.R. Blenda Polimérica Mucoadesiva Para Liberação Prolongada de Fármacos. Patent No. BR 10 2015 027337 1 A2, 28 October 2015. [Google Scholar]
- Klein, J.M.; Forte, M.M.C. Process of Obtaining a Biodegradable Flocculant from Cashew gum and Its Use for Water and Effluent Treatment. Patent No. BR 102015005684A2, 13 March 2015. [Google Scholar]
- Cunha, C.M.D.G.; Soares, P.A.G.; Neto, A.C.A.; Pessoa, A.J. Hidrogel a Base de Polissacarídeos Naturais, Processos e Usos. Patent No. BR 10 2014 014009 3 A2, 10 June 2014. [Google Scholar]
- Rubira, A.F.; Muniz, E.C.; Feitosa, J.P.d.A.; Guilherme, M.R. Hidrogéis Superabsorventes Constituídos da Goma do Cajueiro Modificada e Acrilamida. Patent No. PI 0404265-4 A2, 29 September 2004. [Google Scholar]
- Correia, J.C.G.; Ribeiro, R.C.d.C.; Monte, M.B.d.M.; Seidl, P.R. Processo Para a Utilização da Goma de Cajueiro como depressor na flotação de minerais calcários. Patent No. PI 0304986-8 A2, 15 September 2003. [Google Scholar]
- Mothé, C.G. Processo de Obtenção de Goma de Cajueiro Purificada e Composição de Goma de Cajueiro Purificada. Patent No. PI 0004114-9 B1, 12 September 2000. [Google Scholar]
- Paula, R.C.M.d.; Rodrigues, J.F. Método de isolamento da goma do cajueiro (Anacardium occidentale L.). Patent No. PI 9005645-0 A2, 31 October 1990. [Google Scholar]
- Soares, P.A.; Bourbon, A.I.; Vicente, A.A.; Andrade, C.A.; Barros, W., Jr.; Correia, M.T.; Pessoa, A., Jr.; Carneiro-da-Cunha, M.G. Development and characterization of hydrogels based on natural polysaccharides: Policaju and chitosan. Mater. Sci. Eng. C 2014, 42, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).