Agarose Gel-Templating Synthesis of a 3D Wrinkled Graphene Architecture for Enhanced Supercapacitor Performance
Abstract
1. Introduction
2. Materials and Methods
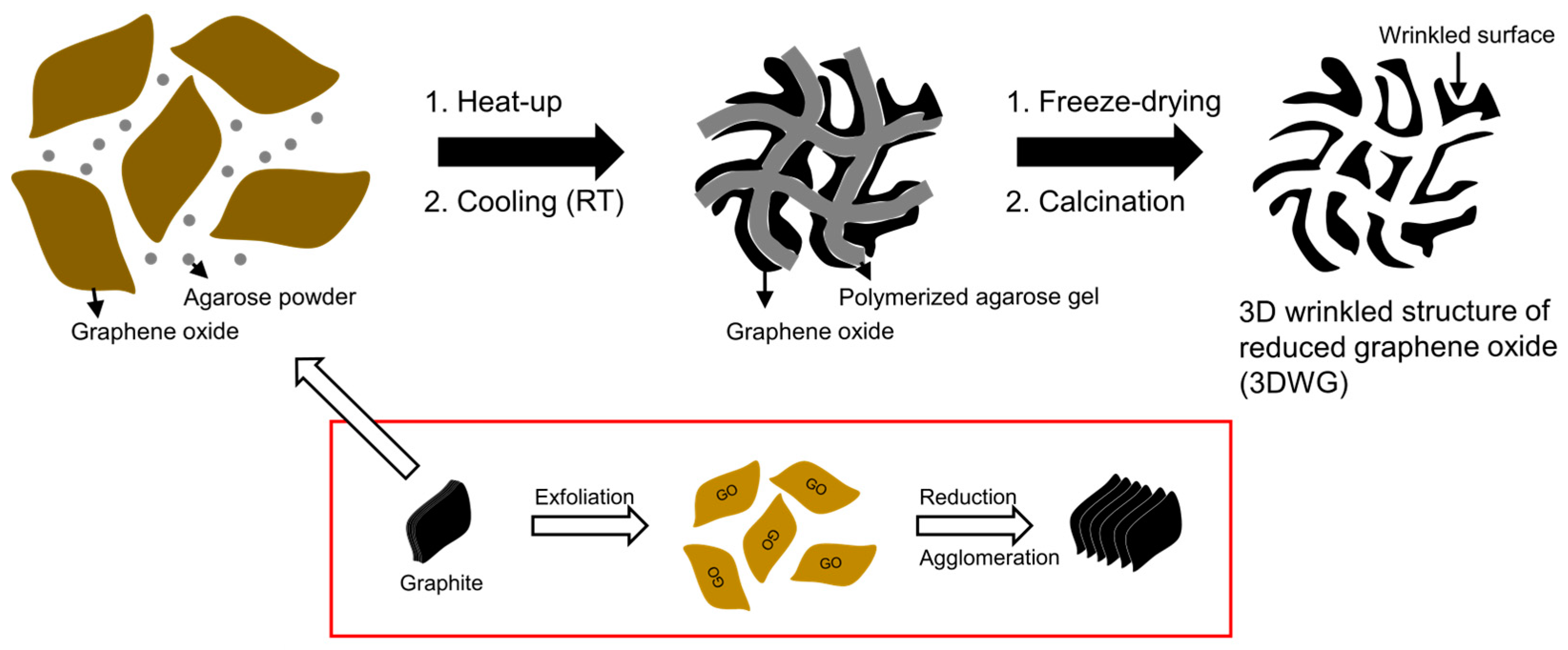
2.1. Synthesis of the 3DWG
2.2. Characterization
2.3. Calculations
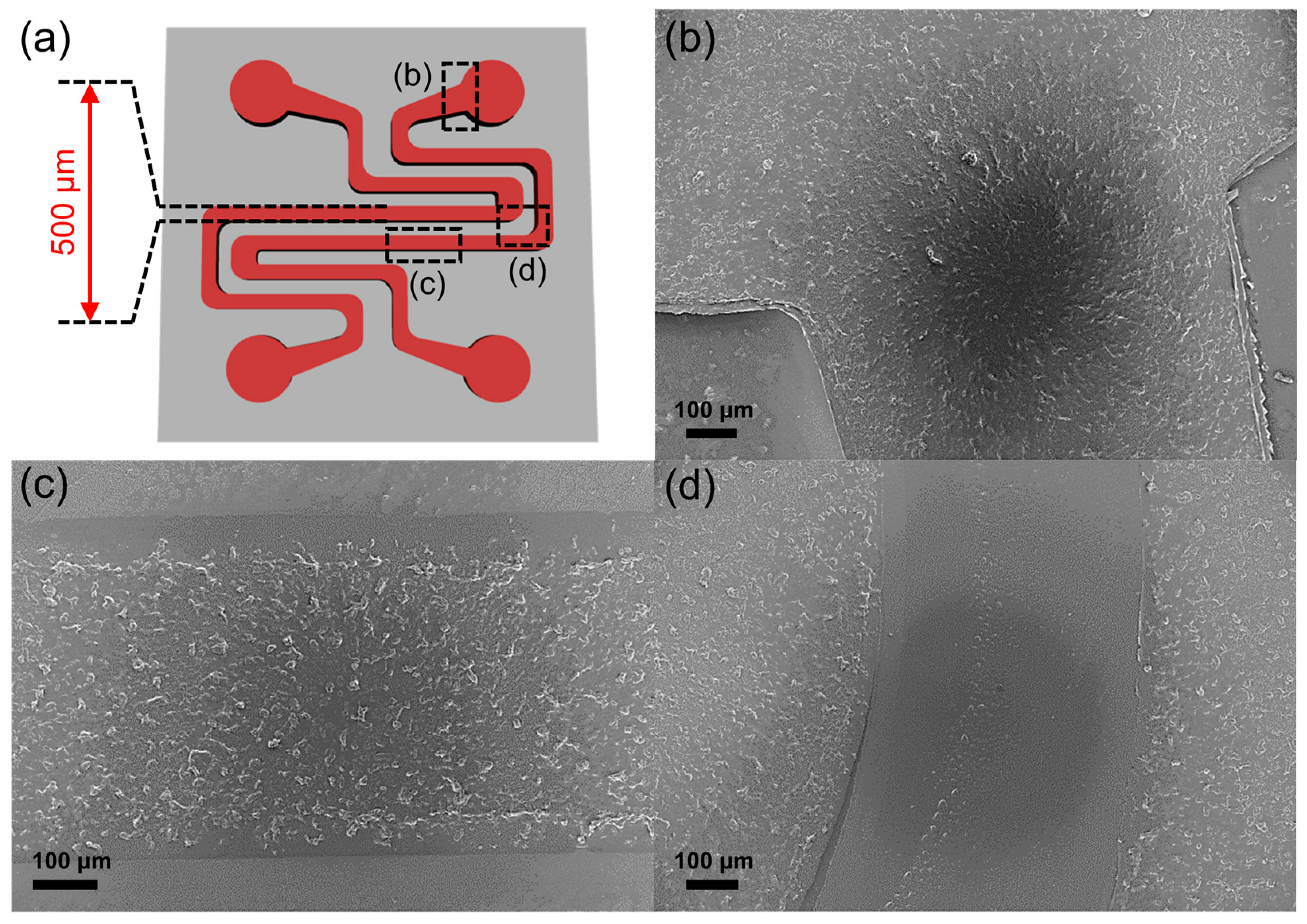
2.4. Fabrication of Thin-Lined Patterns of the 3DWG
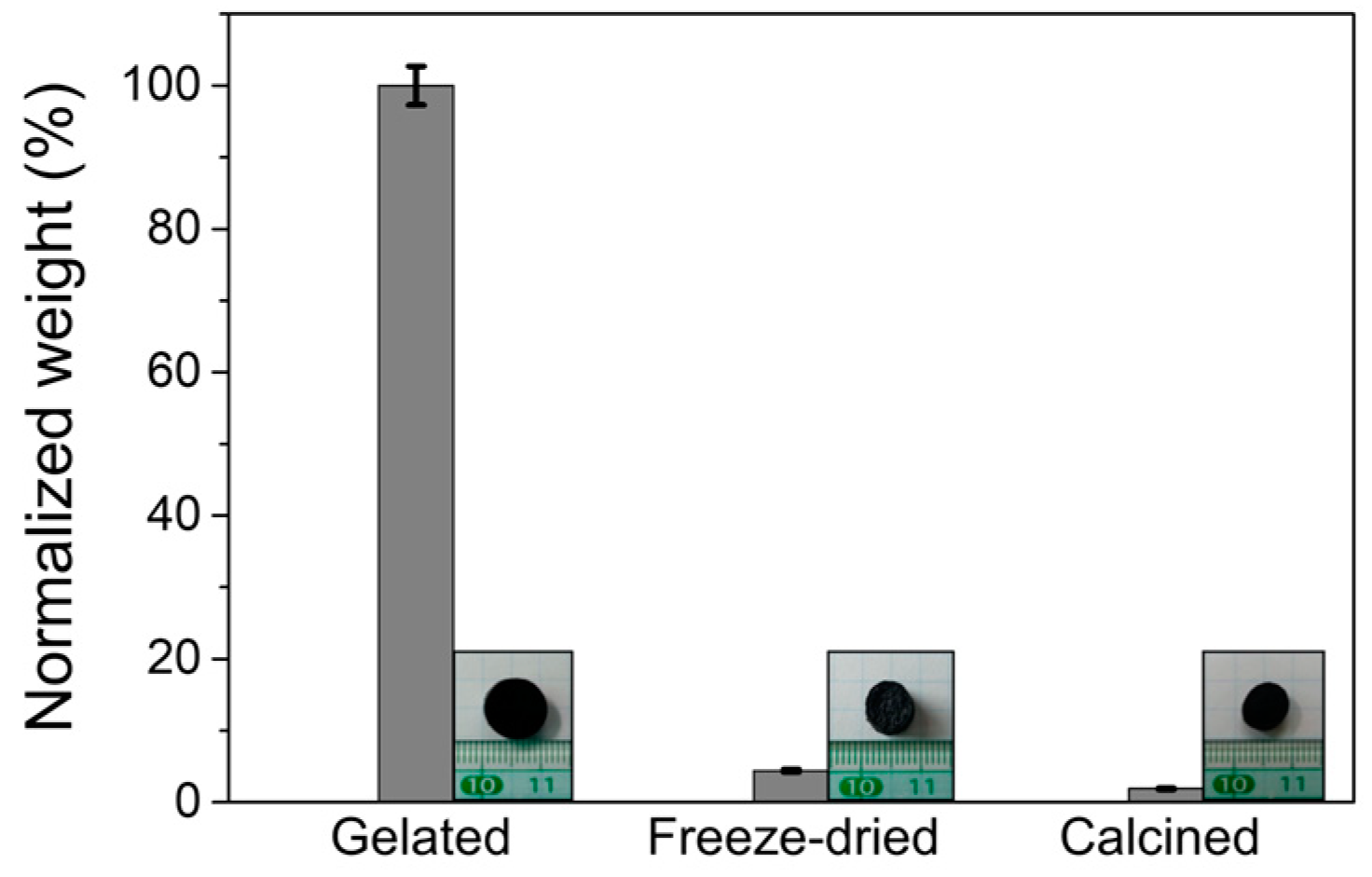
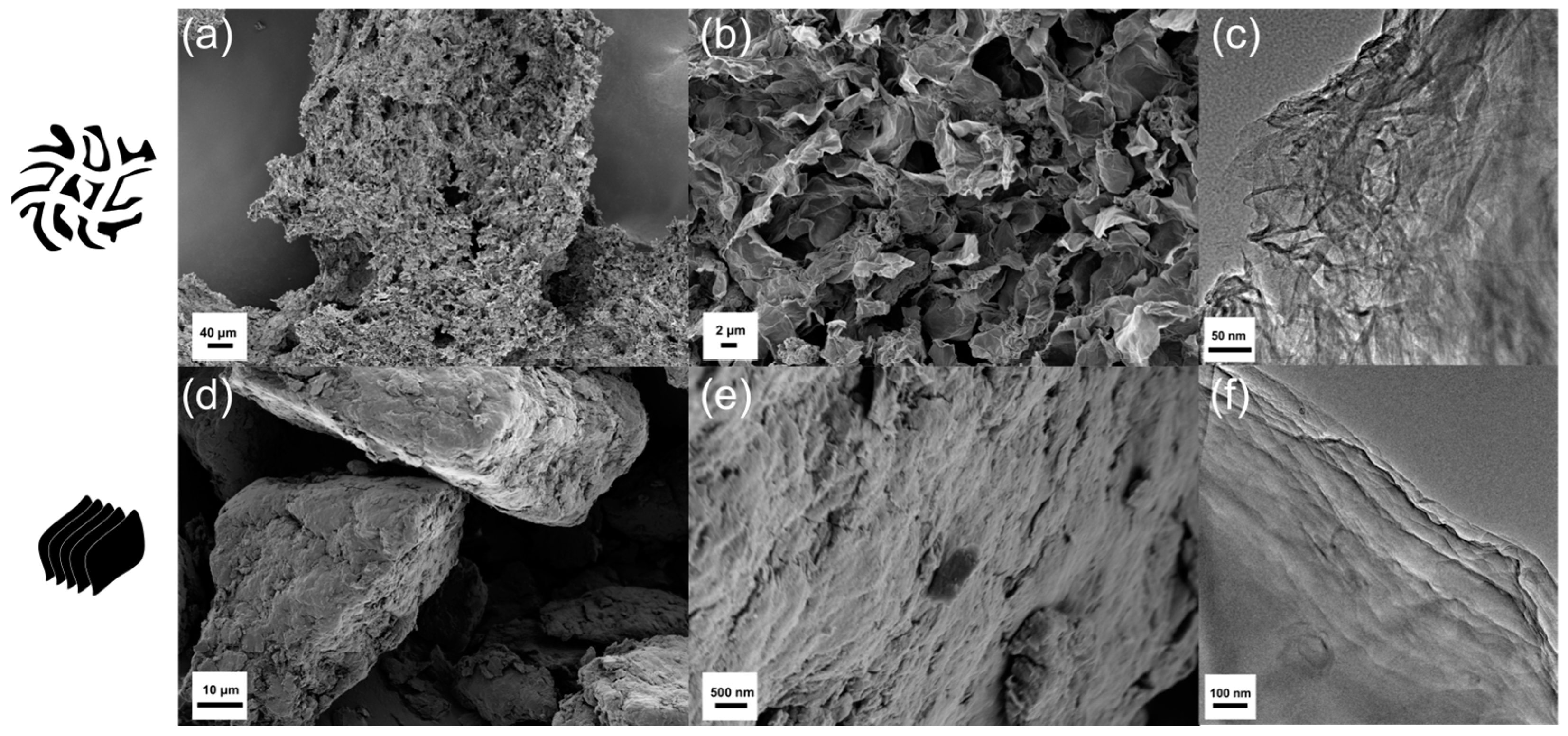
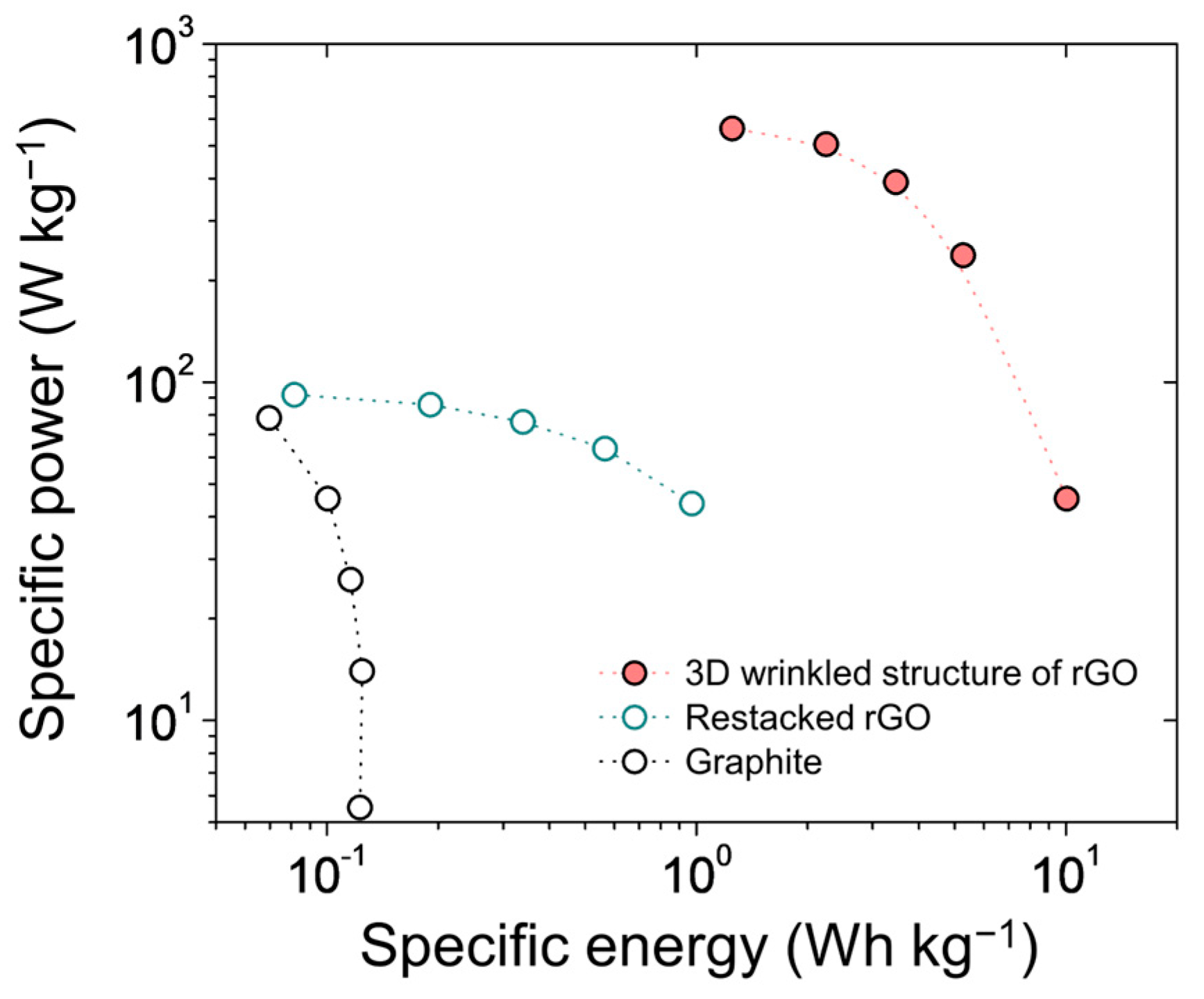
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Navarro, G.; Torres, J.; Blanco, M.; Nájera, J.; Santos-Herran, M.; Lafoz, M. Present and Future of Supercapacitor Technology Applied to Powertrains, Renewable Generation and Grid Connection Applications. Energies 2021, 14, 3060. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.-P.; Umh, H.N.; Nam, I.; Park, S.; Kim, Y.; Yi, J. Co3O4 Nanoparticles Embedded in Ordered Mesoporous Carbon with Enhanced Performance as an Anode Material for Li-Ion Batteries. J. Nanopart. Res. 2013, 15, 1943. [Google Scholar] [CrossRef]
- Kwak, S.; Eom, H.; Kang, J.; Jang, S.; Choi, S.; Kwon, O.; Kim, T.Y.; Nam, I. Mesoporous Carbon Hollow Sphere with Dandelion-Like Radial-Hierarchy for High-Performance Supercapacitors. Int. J. Energy Res. 2022, 46, 4935–4946. [Google Scholar] [CrossRef]
- Young, C.; Chen, H.-T.; Guo, S.-Z. Highly Porous Holey Carbon for High Areal Energy Density Solid-State Supercapacitor Application. Micromachines 2022, 13, 916. [Google Scholar] [CrossRef]
- Park, S.; Nam, I.; Kim, G.-P.; Park, J.; Kim, N.D.; Kim, Y.; Yi, J. A Brain-Coral-Inspired Metal-Carbon Hybrid Synthesized Using Agarose Gel for Ultra-Fast Charge and Discharge Supercapacitor Electrodes. Chem. Commun. 2013, 49, 1554–1556. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, K.; Kwon, S.; Seo, S.; Yoo, H.; Kim, S.; Shin, Y.; Park, Y.; Kim, D.; Choi, J.-Y.; et al. Vertical Alignments of Graphene Sheets Spatially and Densely Piled for Fast Ion Diffusion in Compact Supercapacitors. ACS Nano 2014, 8, 4580–4590. [Google Scholar] [CrossRef]
- Hu, J.; Kang, Z.; Huang, X. Graphene with Three-Dimensional Architecture for High Performance Supercapacitor. Carbon 2014, 67, 221–229. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired Effective Prevention of Restacking in Multilayered Graphene Films: Towards the Next Generation of High-Performance Supercapacitors. Adv. Mater. 2011, 23, 2833–2838. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, A.; Zhang, J.; Kumar, M. Recent advances and prospects in reduced graphene oxide-based photodetectors. J. Mater. Chem. C 2021, 9, 8129–8157. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, B.; Halsted, J.K.; Subashchandrabose, R.; Stickle, W.F.; Ji, X. Direct fabrication of nanoporous graphene from graphene oxide by adding a gasification agent to a magnesiothermic reaction. Chem. Commun. 2015, 51, 1969–1971. [Google Scholar] [CrossRef]
- Xing, Z.; Lu, J.; Ji, X. A Brief Review of Metallothermic Reduction Reactions for Materials Preparation. Small Methods 2018, 2, 1800062. [Google Scholar] [CrossRef]
- Araki, C. Structure of the Agarose Constituent of Agar-Agar. Bull. Chem. Soc. Jpn. 1956, 29, 543–544. [Google Scholar] [CrossRef]
- Kwak, S.; Jang, S.; Park, S.; Kang, J.; Kim, T.Y.; Nam, I. Synthesis of Au Sponges Based on Agarose Template. Scr. Mater. 2021, 196, 113769. [Google Scholar] [CrossRef]
- Park, S.; Nam, I.; Kim, G.-P.; Han, J.W.; Yi, J. Hybrid MnO2 Film with Agarose Gel for Enhancing the Structural Integrity of Thin Film Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2013, 5, 9908–9912. [Google Scholar] [CrossRef]
- Kim, G.-P.; Park, S.; Nam, I.; Park, J.; Yi, J. Synthesis of Porous NiO Materials with Preferentially Oriented Crystalline Structures with Enhanced Stability as Lithium Ion Battery Anodes. J. Power Sources 2013, 237, 172–177. [Google Scholar] [CrossRef]
- Kim, G.-P.; Park, S.; Nam, I.; Park, J.; Yi, J. Preferential Growth of Co3O4 Anode Materials with Improved Cyclic Stability for Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 3872–3876. [Google Scholar] [CrossRef]
- Park, S.; Yoo, Y.G.; Nam, I.; Bae, S.; Yi, J. All-Solid-State, Washable, Wearable Supercapacitors Fabricated by using a Fibrous Graphite Network and Self-Adhering Architecture. Energy Technol. 2014, 2, 677–684. [Google Scholar] [CrossRef]
- Wang, K.; Zou, W.; Quan, B.; Yu, A.; Wu, H.; Jiang, P.; Wei, Z. An All-Solid-State Flexible Micro-Supercapacitor on a Chip. Adv. Energy Mater. 2011, 1, 1068–1072. [Google Scholar] [CrossRef]
- Nam, I.; Park, S.; Kim, G.-P.; Park, J.; Yi, J. Transparent and Ultra-Bendable All-Solid-State Supercapacitors without Percolation Problems. Chem. Sci. 2013, 4, 1663–1667. [Google Scholar] [CrossRef]
- Du, K.-F.; Yang, D.; Sun, Y. Controlled Fabrication of Porous Titania Beads by a Sol−Gel Templating Method. Ind. Eng. Chem. Res. 2009, 48, 755–762. [Google Scholar] [CrossRef]
- Ma, X.; Klosterman, L.; Hu, Y.-Y.; Liu, X.; Schmidt-Rohr, K.; Mallapragada, S.; Akinc, M. Aqueous Route Synthesis of Mesoporous ZrO2 by Agarose Templation. J. Am. Ceram. Soc. 2012, 95, 3455–3462. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Su, Y.; Ruan, H.; Hu, R.; Zhang, L. MnO2 nanorods/3D-rGO composite as high performance anode materials for Li-ion batteries. App. Surf. Sci. 2017, 392, 777–784. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the Quantum Capacitance of Graphene. Nat. Nanotechnol. 2009, 4, 505–509. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Li, J. Preparation and Electrochemical Performance for Methanol Oxidation of Pt/Graphene Nanocomposites. Electrochem. Commun. 2009, 11, 846–849. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Folch, A.; Ayon, A.; Hurtado, O.; Schmidt, M.A.; Toner, M. Molding of Deep Polydimethylsiloxane Microstructures for Microfluidics and Biological Applications. J. Biomech. Eng. 1999, 121, 28–34. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Jo, S.; Kim, Y.; Zaman, S.; Kim, D. Electrospun Nanofiber Covered Polystyrene Micro-Nano Hybrid Structures for Triboelectric Nanogenerator and Supercapacitor. Micromachines 2022, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Thekkekara, L.; Jia, B.; Zhang, Y.; Qiu, L.; Li, D.; Gu, M. On-Chip Energy Storage Integrated with Solar Cells Using a Laser Scribed Graphene Oxide Film. Appl. Phys. Lett. 2015, 107, 031105. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, S.; Pan, B.; Dai, X.; Zhao, W.; Liu, Y.; Xie, W.; Kuang, Y.; Liu, X. Stable and Durable Laser-Induced Graphene Patterns Embedded in Polymer Substrates. Carbon 2020, 163, 85–94. [Google Scholar] [CrossRef]
- Yi, J.; Chen, J.; Yang, Z.; Dai, Y.; Li, W.; Cui, J.; Ciucci, F.; Lu, Z.; Yang, C. Facile Patterning of Laser-Induced Graphene with Tailored Li Nucleation Kinetics for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1901796. [Google Scholar] [CrossRef]
- Hofmann, M.; Hsieh, Y.-P.; Hsu, A.L.; Kong, J. Scalable, Flexible and High Resolution Patterning of CVD Graphene. Nanoscale 2014, 6, 289–292. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Park, J.-K.; Kim, G.W.; Nam, I.; Park, S. Agarose Gel-Templating Synthesis of a 3D Wrinkled Graphene Architecture for Enhanced Supercapacitor Performance. Micromachines 2022, 13, 1113. https://doi.org/10.3390/mi13071113
Shin J, Park J-K, Kim GW, Nam I, Park S. Agarose Gel-Templating Synthesis of a 3D Wrinkled Graphene Architecture for Enhanced Supercapacitor Performance. Micromachines. 2022; 13(7):1113. https://doi.org/10.3390/mi13071113
Chicago/Turabian StyleShin, Junhyeop, Jong-Kwon Park, Geon Woo Kim, Inho Nam, and Soomin Park. 2022. "Agarose Gel-Templating Synthesis of a 3D Wrinkled Graphene Architecture for Enhanced Supercapacitor Performance" Micromachines 13, no. 7: 1113. https://doi.org/10.3390/mi13071113
APA StyleShin, J., Park, J.-K., Kim, G. W., Nam, I., & Park, S. (2022). Agarose Gel-Templating Synthesis of a 3D Wrinkled Graphene Architecture for Enhanced Supercapacitor Performance. Micromachines, 13(7), 1113. https://doi.org/10.3390/mi13071113





