Bio-Inspired Hierarchical Micro-/Nanostructures for Anti-Icing Solely Fabricated by Metal-Assisted Chemical Etching
Abstract
:1. Introduction
2. Experiment
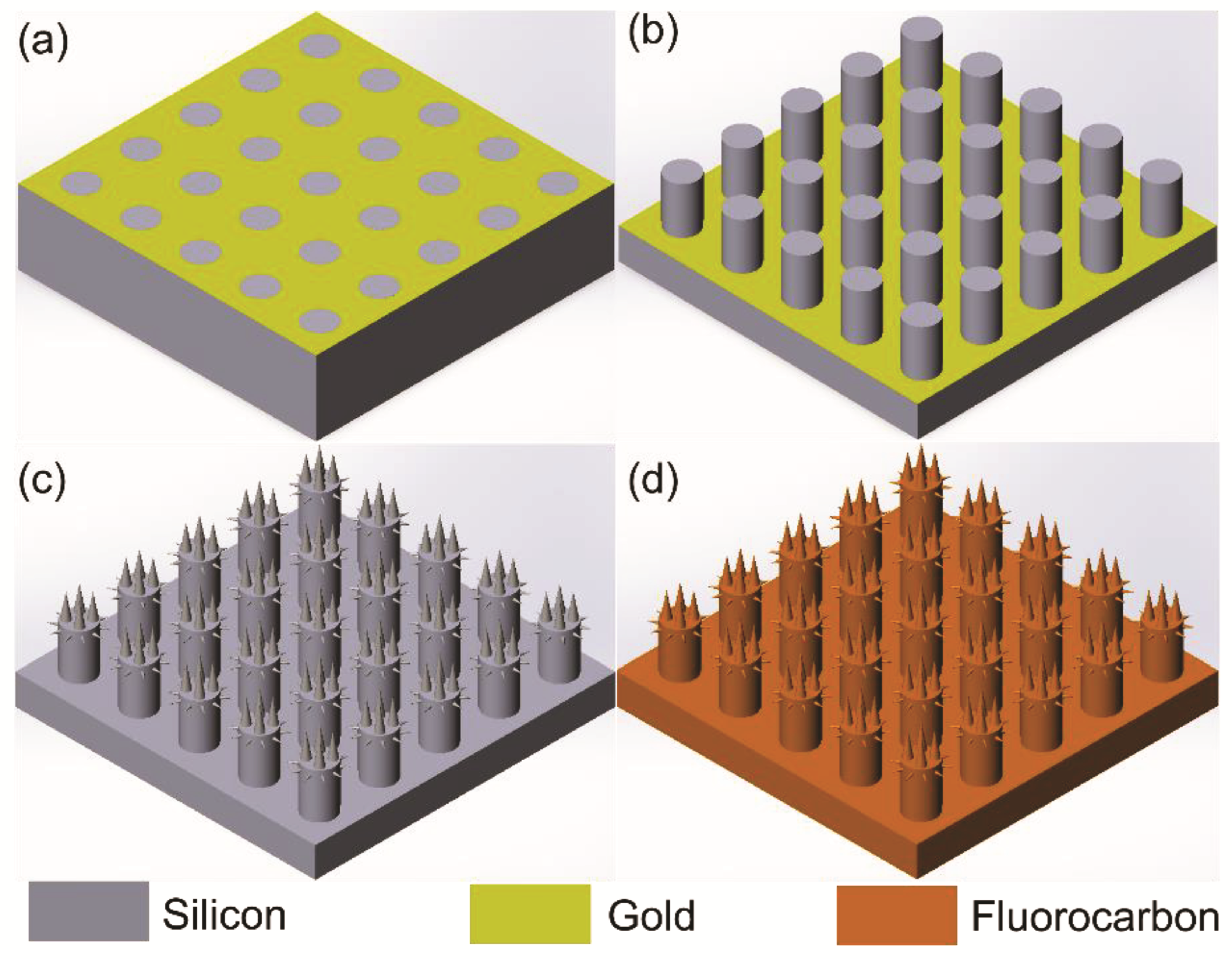
2.1. Fabrication Process
2.2. Metal-Assisted Chemical Etching Process for Hierarchical Micro-/Nanostructured Surface
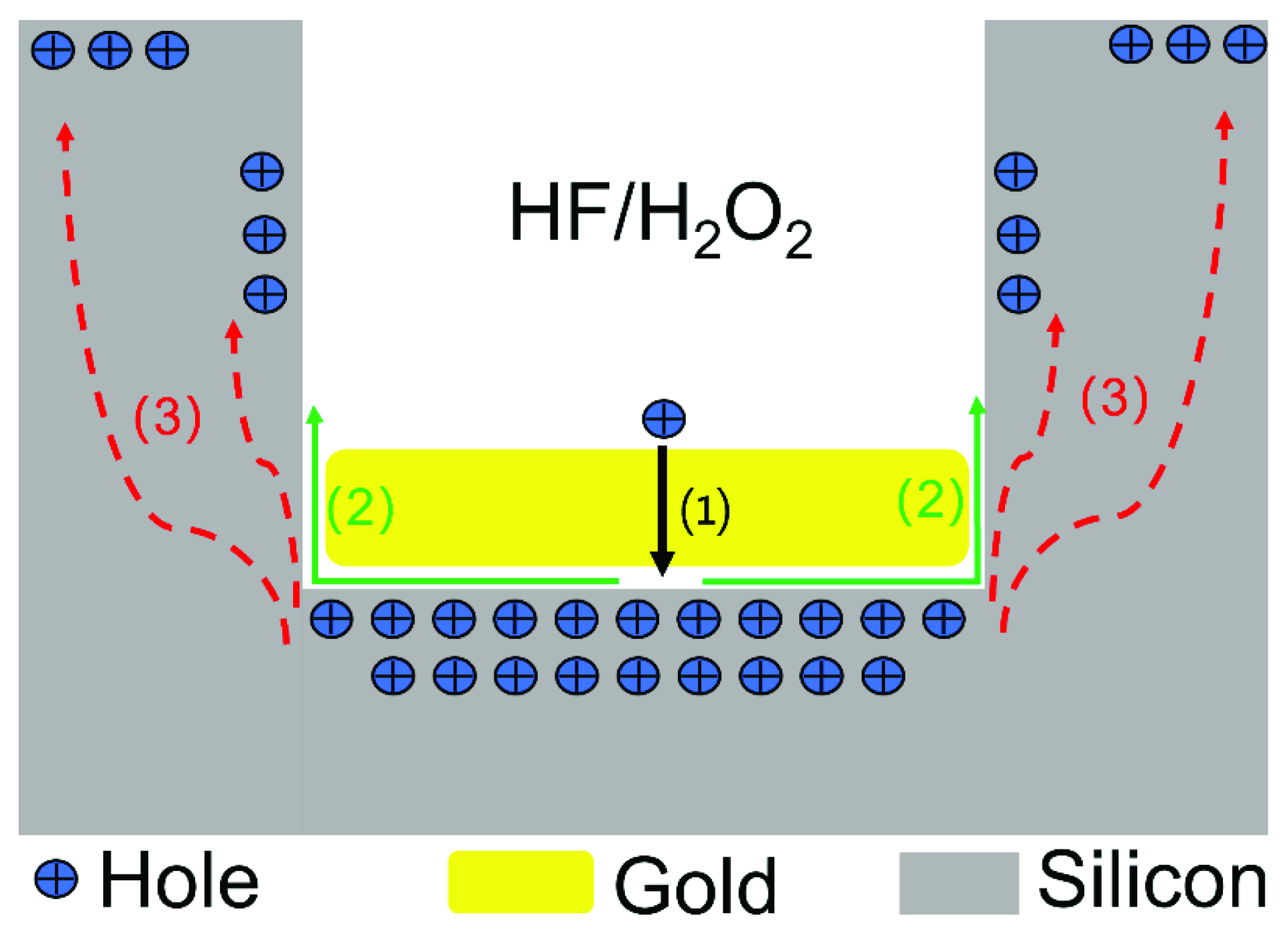
2.2.1. Metal-Assisted Chemical Etching Process with Gold
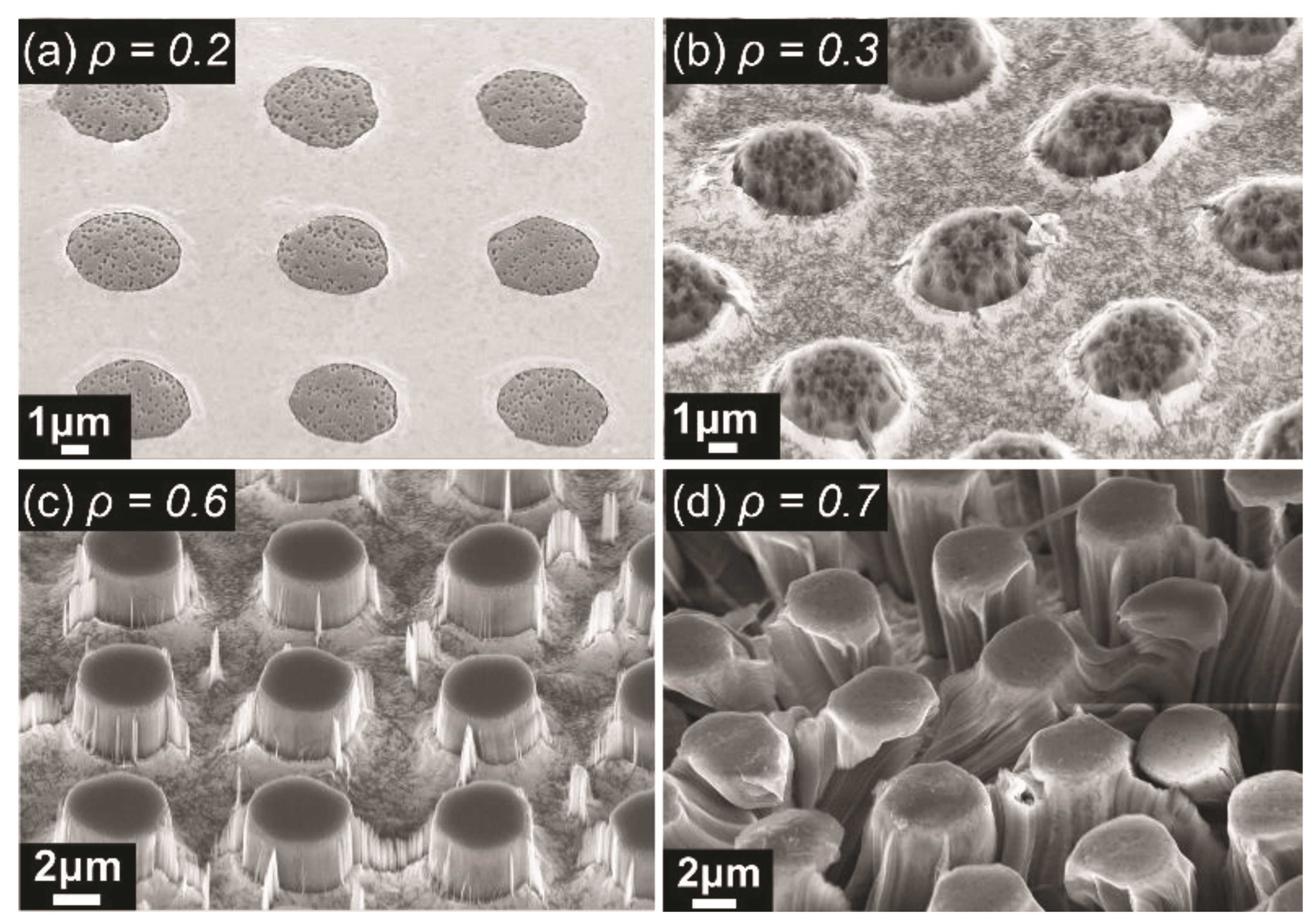
2.2.2. Metal-Assisted Chemical Etching for Micropillars
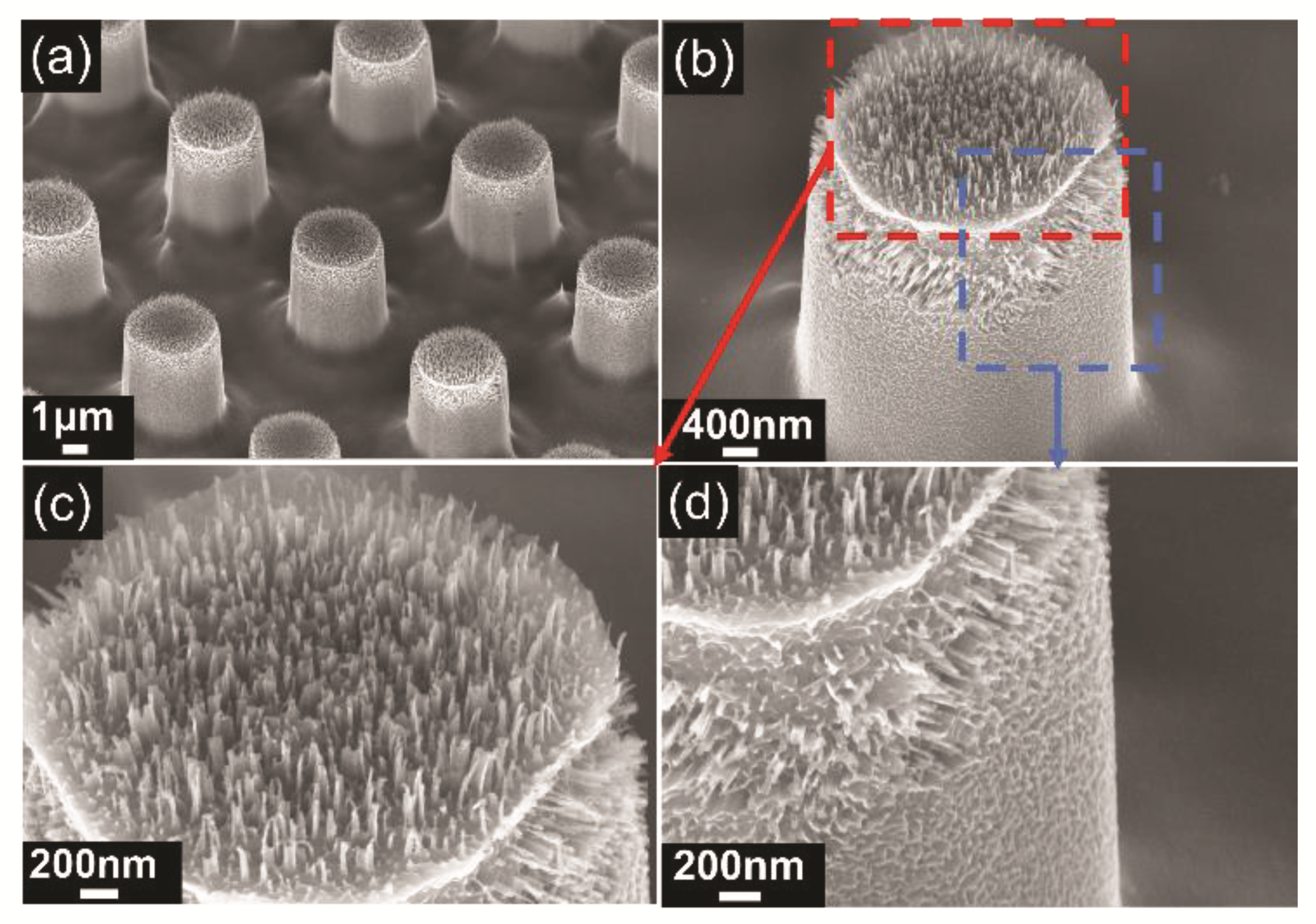
2.2.3. Maskless MacEtch for Producing Silicon Nanowires
2.3. Wettability Measurements
2.4. Anti-Icing Property Measurements
3. Results and Discussion
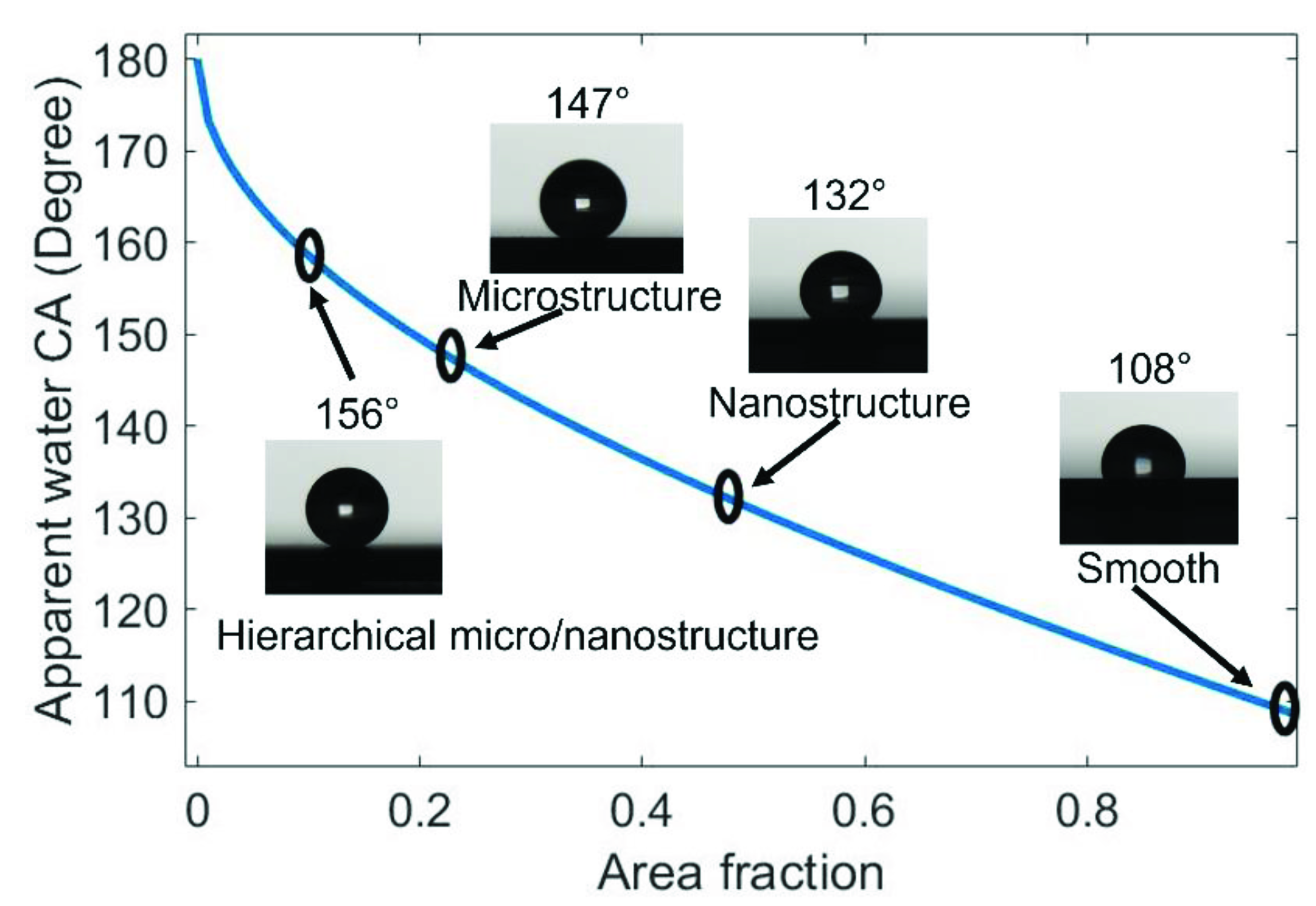
3.1. Wettability Analysis
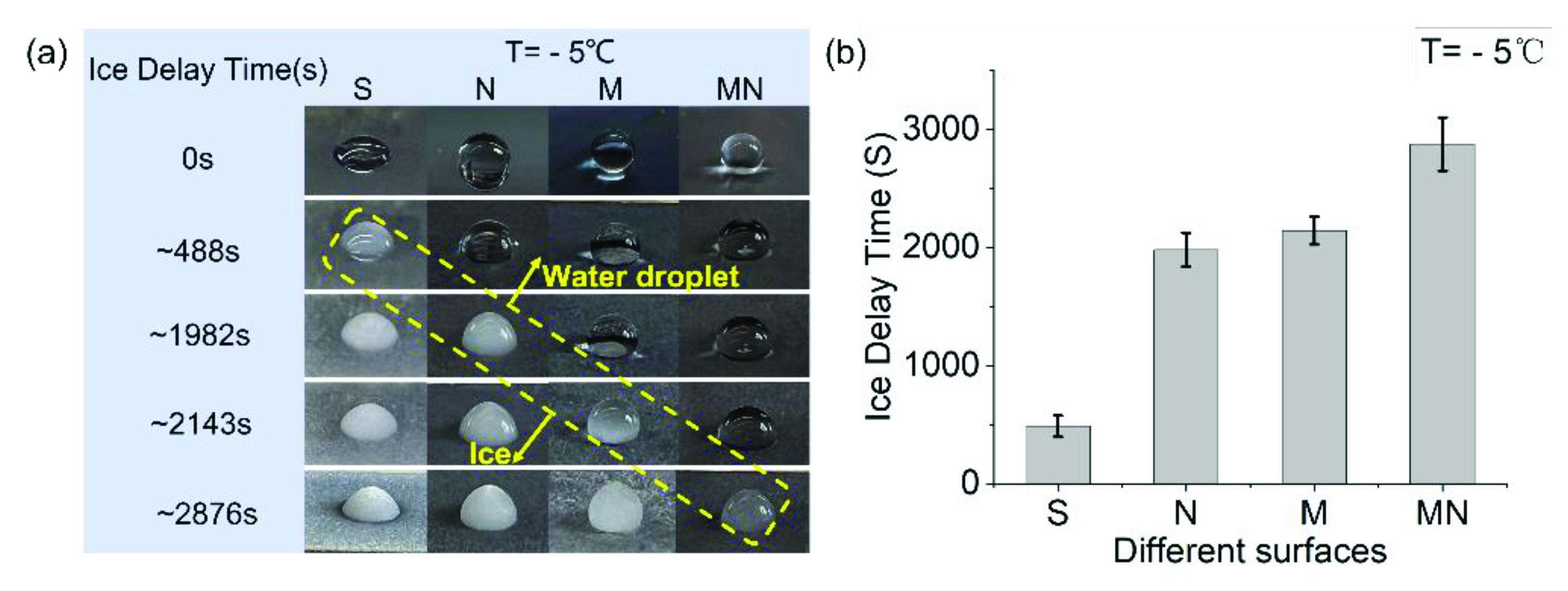
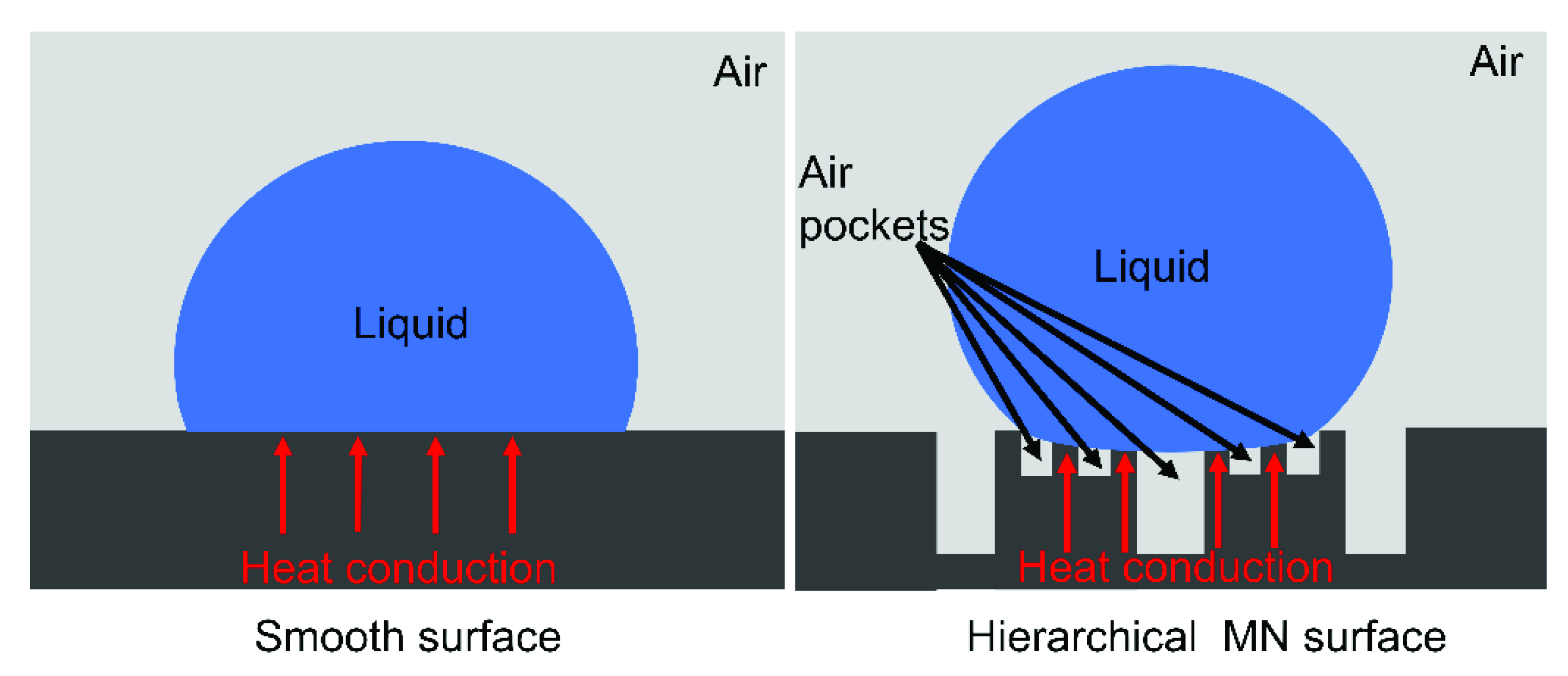
3.2. Anti-Icing Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethi, S.K.; Kadian, S.; Manik, G. A review of recent progress in molecular dynamics and coarse-grain simulations assisted understanding of wettability. Arch. Comput. Methods Eng. 2022, 1–27. [Google Scholar] [CrossRef]
- Sethi, S.K.; Soni, L.; Shankar, U.; Chauhan, R.P.; Manik, G. A molecular dynamics simulation study to investigate poly(vinyl acetate)-poly(dimethyl siloxane) based easy-clean coating: An insight into the surface behavior and substrate interaction. J. Mol. Struct. 2020, 1202, 127342. [Google Scholar] [CrossRef]
- Sethi, S.K.; Shankar, U.; Manik, G. Fabrication and characterization of non-fluoro based transparent easy-clean coating formulations optimized from molecular dynamics simulation. Prog. Org. Coat. 2019, 136, 105306. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto Joseph, G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Manoharan, K.; Bhattacharya, S. Superhydrophobic surfaces review: Functional application, fabrication techniques and limitations. J. Micromanufacturing 2019, 2, 59–78. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym.-Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Rus, A.Z.M.; Mohid, S.R.; Nurulsaidatulsyida, S.; Marsi, N. Biopolymer doped with titanium dioxide superhydrophobic photocatalysis as self-clean coating for lightweight composite. Adv. Mater. Sci. Eng. 2013, 2013, 486253. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Mortazavi, V.; Khonsari, M.M. On the degradation of superhydrophobic surfaces: A review. Wear 2017, 372, 145–157. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Nyankson, E.; Agbe, H.; Takyi, G.K.S.; Bensah, Y.D.; Sarkar, D.K. Recent advances in nanostructured superhydrophobic surfaces: Fabrication and long-term durability challenges. Curr. Opin. Chem. Eng. 2022, 36, 100790. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86, 144101. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Superhydrophobic surfaces: From the lotus leaf to the submarine. Comptes Rendus Mécanique 2012, 340, 18–34. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-cleaning coatings. J. Mater. Chem. 2005, 15, 1689–1695. [Google Scholar] [CrossRef]
- Karaman, M.; Çabuk, N.; Özyurt, D.; Köysüren, Ö. Self-supporting superhydrophobic thin polymer sheets that mimic the nature′s petal effect. Appl. Surf. Sci. 2012, 259, 542–546. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Shao, J.; Ding, Y.; Wang, W.; Mei, X.; Zhai, H.; Tian, H.; Li, X.; Liu, B. Generation of fully-covering hierarchical micro-/nano- structures by nanoimprinting and modified laser swelling. Small 2014, 10, 2595–2601. [Google Scholar] [CrossRef]
- Murphy, M.P.; Kim, S.; Sitti, M. Enhanced adhesion by gecko-inspired hierarchical fibrillar adhesives. ACS Appl. Mater. Interfaces 2009, 1, 849–855. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Z.; Cao, B.; Zhang, Z.; Zhu, K.; Wu, B.; Jiang, S.; Chai, G. Wetting and dewetting transitions on submerged superhydrophobic surfaces with hierarchical structures. Langmuir 2017, 33, 407–416. [Google Scholar] [CrossRef]
- Kwon, Y.; Patankar, N.; Choi, J.; Lee, J. Design of surface hierarchy for extreme hydrophobicity. Langmuir 2009, 25, 6129–6136. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wu, J.; Zhang, Y.; Zhang, N.; Chen, J.; Lei, Z.; Yi, Z.; Ye, X. Ultra-low-reflective, self-cleaning surface by fabrication dual-scale hierarchical optical structures on silicon. Coatings 2021, 11, 1541. [Google Scholar] [CrossRef]
- Du, X.; He, J. Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications. Nanoscale 2011, 3, 3984–4002. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaee, S.Y.; Bagheri, R.; Vossoughi, M.; Ahmadi Seyedkhani, S.; Samadikuchaksaraei, A. Bioinspired multifunctional TiO2 hierarchical micro/nanostructures with tunable improved bone cell growth and inhibited bacteria adhesion. Ceram. Int. 2020, 46, 9669–9679. [Google Scholar] [CrossRef]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Xiao, B.; Zhao, Q.; Zhang, M. Facile synthesis cedar-like SnO2 hierarchical micro-nanostructures with improved formaldehyde gas sensing characteristics. J. Alloys Compd. 2017, 724, 121–129. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Luo, T.; Jia, Y.; Zhang, Y.-X.; Liu, J.-H.; Huang, X.-J. Porous hierarchically micro-/nanostructured MgO: Morphology control and their excellent performance in As(III) and As(V) removal. J. Phys. Chem. C 2011, 115, 22242–22250. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. A Math Phys. Eng. Sci. 2009, 367, 1631–1672. [Google Scholar] [CrossRef]
- Jin, Z.; Mei, H.; Pan, L.; Liu, H.; Cheng, L. Superhydrophobic self-cleaning hierarchical micro-/nanocomposite coating with high corrosion resistance and durability. ACS Sustain. Chem. Eng. 2021, 9, 4111–4121. [Google Scholar] [CrossRef]
- Wang, X.; Handschuh-Wang, S.; Xu, Y.; Xiang, L.; Zhou, Z.; Wang, T.; Tang, Y. Hierarchical micro/nanostructured diamond gradient surface for controlled water transport and fog collection. Adv. Mater. Interfaces 2021, 8, 2100196. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.-H. A durable, fluorine-free, and repairable superhydrophobic aluminum surface with hierarchical micro/nanostructures and its application for continuous oil-water separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Song, D.; Swarctz, C.; Lee, J.; Choi, C.H. Anti-Icing or deicing: Icephobicities of superhydrophobic surfaces with hierarchical structures. Langmuir 2018, 34, 13821–13827. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Xing, L.; Liao, Y.; Qiu, Y.; Yang, S.; Li, W. Morphology-conserved transformations of metal-based precursors to hierarchically porous micro-/nanostructures for electrochemical energy conversion and storage. Adv. Mater. 2017, 29, 1607015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, J.; Parida, K.; Guo, M.; Wang, C.; Wang, C.; Li, X.; Shao, J.; Lee, P.S. Transparent and stretchable bimodal triboelectric nanogenerators with hierarchical micro-nanostructures for mechanical and water energy harvesting. Nano Energy 2019, 64, 103904. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Hierarchically micro/nanostructured photoanode materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 15475–15489. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Huang, X.; Hong, J.; Cao, B.; Hao, J.; Fan, Q.; Zhou, T.; Guo, Z. Structural engineering of hierarchical micro-nanostructured Ge–C framework by controlling the nucleation for ultralong-life li storage. Adv. Energy Mater. 2019, 9, 1900081. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Peng, Z.; Wu, W.; Shi, T.; Liao, G. Gas sensing using hierarchical micro/nanostructures of Morpho butterfly scales. Sens. Actuators A Phys. 2014, 213, 63–69. [Google Scholar] [CrossRef]
- Wang, C.; Huang, H.; Qian, Y.; Zhang, Z.; Yan, J. One-step fabrication of regular hierarchical micro/nano-structures on glassy carbon by nanosecond pulsed laser irradiation. J. Manuf. Processes 2021, 62, 108–118. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Singh, S.C.; Zhan, Z.; Yu, Z.; Xin, W.; Huang, T.; Guo, C. Hierarchical micro/nanostructured TiO2/Ag substrates based on femtosecond laser structuring: A facile route for enhanced SERS performance and location predictability. Appl. Surf. Sci. 2019, 478, 737–743. [Google Scholar] [CrossRef]
- Wang, C.; Shao, J.; Lai, D.; Tian, H.; Li, X. Suspended-Template Electric-Assisted Nanoimprinting for Hierarchical Micro-Nanostructures on a Fragile Substrate. ACS Nano 2019, 13, 10333–10342. [Google Scholar] [CrossRef]
- Lim, C.H.; Han, S.Y.; Eo, J.D.; Kim, K.; Kim, W.B. Superhydrophobic hierarchical structures produced through novel low-cost stamp fabrication and hot embossing of thermoplastic film. J. Mech. Sci. Technol. 2015, 29, 5107–5111. [Google Scholar] [CrossRef]
- Choi, I.; Kim, Y.; Yi, J. Fabrication of hierarchical micro/nanostructures via scanning probe lithography and wet chemical etching. Ultramicroscopy 2008, 108, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Uzoma, P.C.; Xiaoyang, C.; Penkov, O.V.; Hu, H. Bio-inspired hierarchical micro/nanostructured surfaces for superhydrophobic and anti-ice applications. Front Bioeng. Biotechnol. 2022, 10, 872268. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wu, H.; Higaki, Y.; Otsuka, H.; Takahara, A. A “non-sticky” superhydrophobic surface prepared by self-assembly of fluoroalkyl phosphonic acid on a hierarchically micro/nanostructured alumina gel film. Chem. Commun. 2012, 48, 6824–6826. [Google Scholar] [CrossRef] [PubMed]
- Schauer, S.; Worgull, M.; Holscher, H. Bio-inspired hierarchical micro- and nano-wrinkles obtained via mechanically directed self-assembly on shape-memory polymers. Soft Matter 2017, 13, 4328–4334. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Fang, M.; Cheung, H.-Y.; Xiu, F.; Yip, S.; Wong, C.-Y.; Ho, J.C. Hierarchical silicon nanostructured arrays via metal-assisted chemical etching. RSC Adv. 2014, 4, 50081–50085. [Google Scholar] [CrossRef]
- Feng, J.S.; Tuominen, M.T.; Rothstein, J.P. Hierarchical superhydrophobic surfaces fabricated by dual-scale electron-beam-lithography with well-ordered secondary nanostructures. Adv. Funct. Mater. 2011, 21, 3715–3722. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gosele, U. Metal-assisted chemical etching of silicon: A review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Kim, S.M.; Khang, D.Y. Bulk micromachining of Si by metal-assisted chemical etching. Small 2014, 10, 3761–3766. [Google Scholar] [CrossRef]
- Hu, H.; Swaminathan, V.V.; Farahani, M.R.Z.; Mensing, G.; Yeom, J.; Shannon, M.A.; Zhu, L. Hierarchically structured re-entrant microstructures for superhydrophobic surfaces with extremely low hysteresis. J. Micromech. Microeng. 2014, 24, 95023. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wu, C.-S.; Chou, C.-J.; Yen, T.-J. Morphological control of single-crystalline silicon nanowire arrays near room temperature. Adv. Mater. 2008, 20, 3811–3815. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, W.H.; Zhou, Y.; Chen, X.H.; Xiao, Y.Y.; Ma, M.Y.; Zhu, W.J.; Wei, F. Fabrication of porous silicon nanowires by MACE method in HF/H2O2/AgNO3 system at room temperature. Nanoscale Res. Lett. 2014, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, N.; Bhushan, B. Hierarchical roughness makes superhydrophobic states stable. Microelectron. Eng. 2007, 84, 382–386. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Peng, K.-Q.; Fan, X.; Jie, J.-S.; Zhang, R.-Q.; Lee, S.-T.; Wong, N.-B. Preparation of large-area uniform silicon nanowires arrays through metal-assisted chemical etching. J. Phys. Chem. C 2008, 112, 4444–4450. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Hsu, M.; Bhate, N.; Yang, W.; Deng, T. Spatial control in the heterogeneous nucleation of water. Appl. Phys. Lett. 2009, 95, 3200951. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 3524513. [Google Scholar] [CrossRef]
- Shah, A.V.; Schade, H.; Vanecek, M.; Meier, J.; Vallat-Sauvain, E.; Wyrsch, N.; Kroll, U.; Droz, C.; Bailat, J. Thin-film silicon solar cell technology. Prog. Photovolt. Res. Appl. 2004, 12, 113–142. [Google Scholar] [CrossRef]
- Jelle, B.P.; Gao, T.; Mofid, S.A.; Kolås, T.; Stenstad, P.M.; Ng, S. Avoiding snow and ice formation on exterior solar cell surfaces—A review of research pathways and opportunities. Procedia Eng. 2016, 145, 699–706. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chu, X.; Tian, F.; Xu, Y.; Hu, H. Bio-Inspired Hierarchical Micro-/Nanostructures for Anti-Icing Solely Fabricated by Metal-Assisted Chemical Etching. Micromachines 2022, 13, 1077. https://doi.org/10.3390/mi13071077
Zhang L, Chu X, Tian F, Xu Y, Hu H. Bio-Inspired Hierarchical Micro-/Nanostructures for Anti-Icing Solely Fabricated by Metal-Assisted Chemical Etching. Micromachines. 2022; 13(7):1077. https://doi.org/10.3390/mi13071077
Chicago/Turabian StyleZhang, Lansheng, Xiaoyang Chu, Feng Tian, Yang Xu, and Huan Hu. 2022. "Bio-Inspired Hierarchical Micro-/Nanostructures for Anti-Icing Solely Fabricated by Metal-Assisted Chemical Etching" Micromachines 13, no. 7: 1077. https://doi.org/10.3390/mi13071077
APA StyleZhang, L., Chu, X., Tian, F., Xu, Y., & Hu, H. (2022). Bio-Inspired Hierarchical Micro-/Nanostructures for Anti-Icing Solely Fabricated by Metal-Assisted Chemical Etching. Micromachines, 13(7), 1077. https://doi.org/10.3390/mi13071077







