Understanding the Mechanisms of SiC–Water Reaction during Nanoscale Scratching without Chemical Reagents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Method
2.2. RMD Model
3. Results
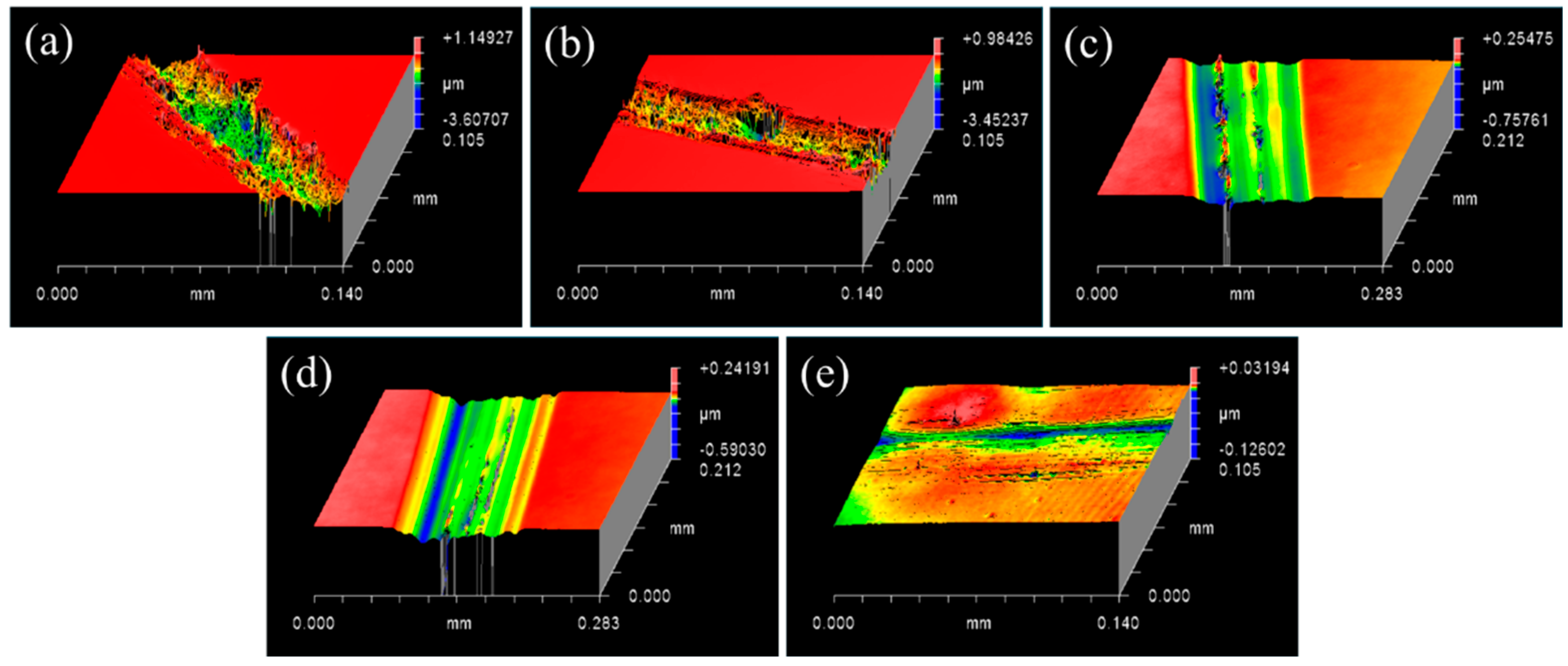
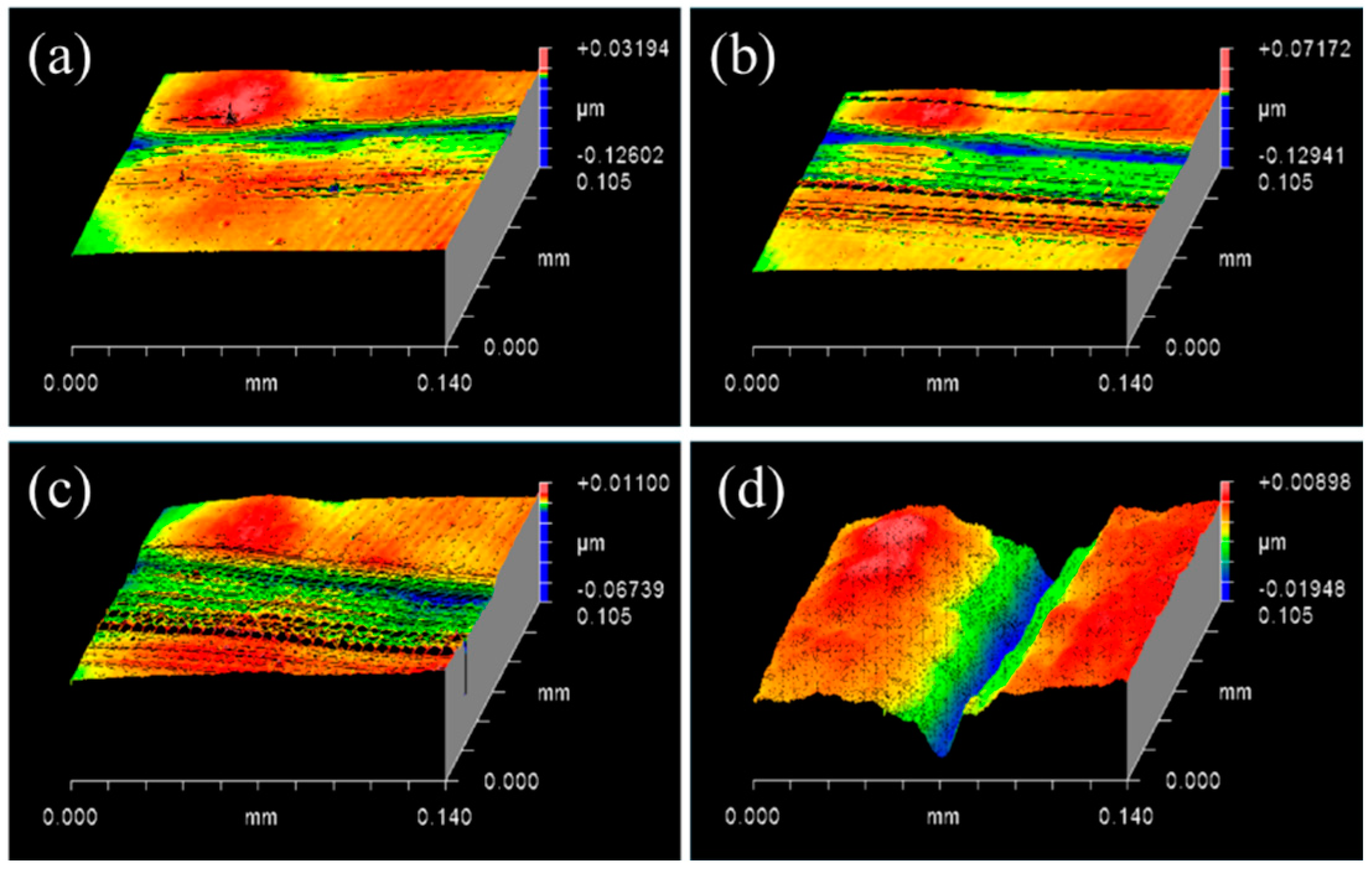
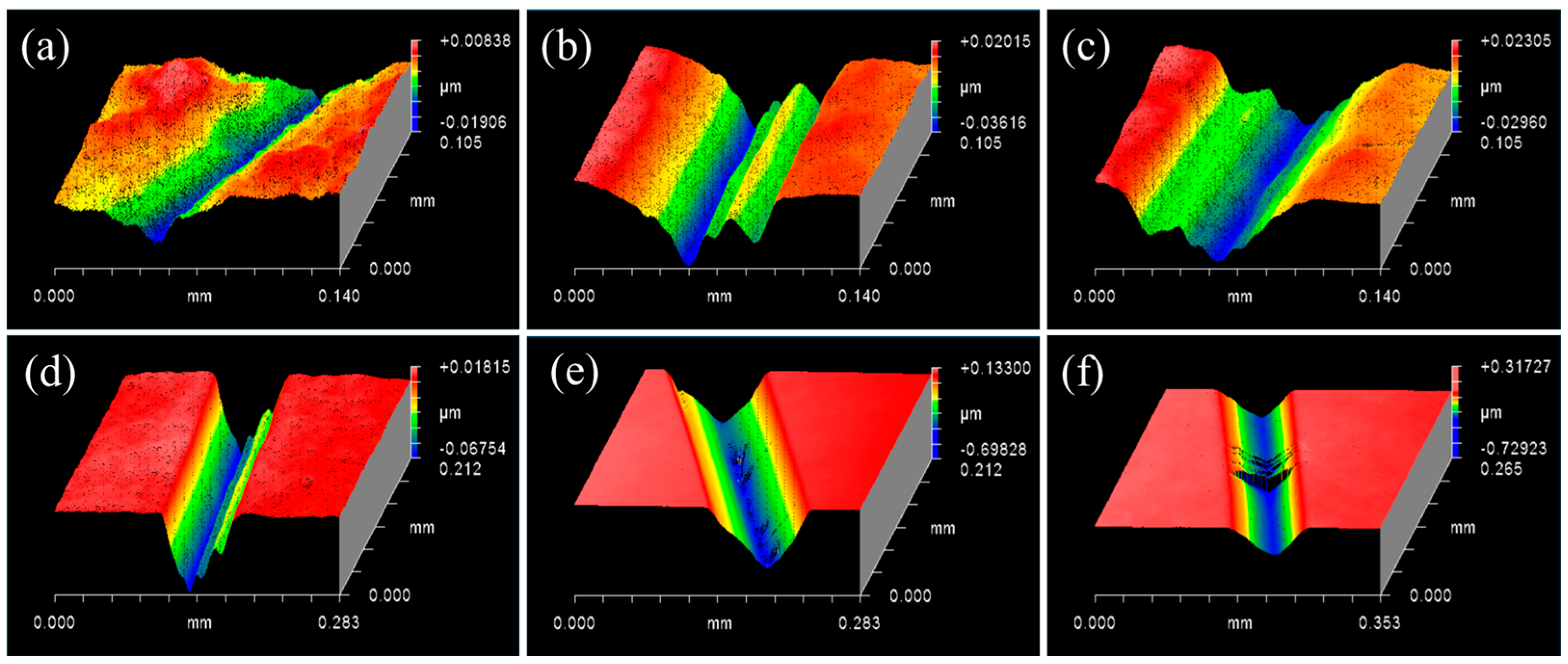
3.1. Controlling the Material Removal Behaviour of SiC Wafer
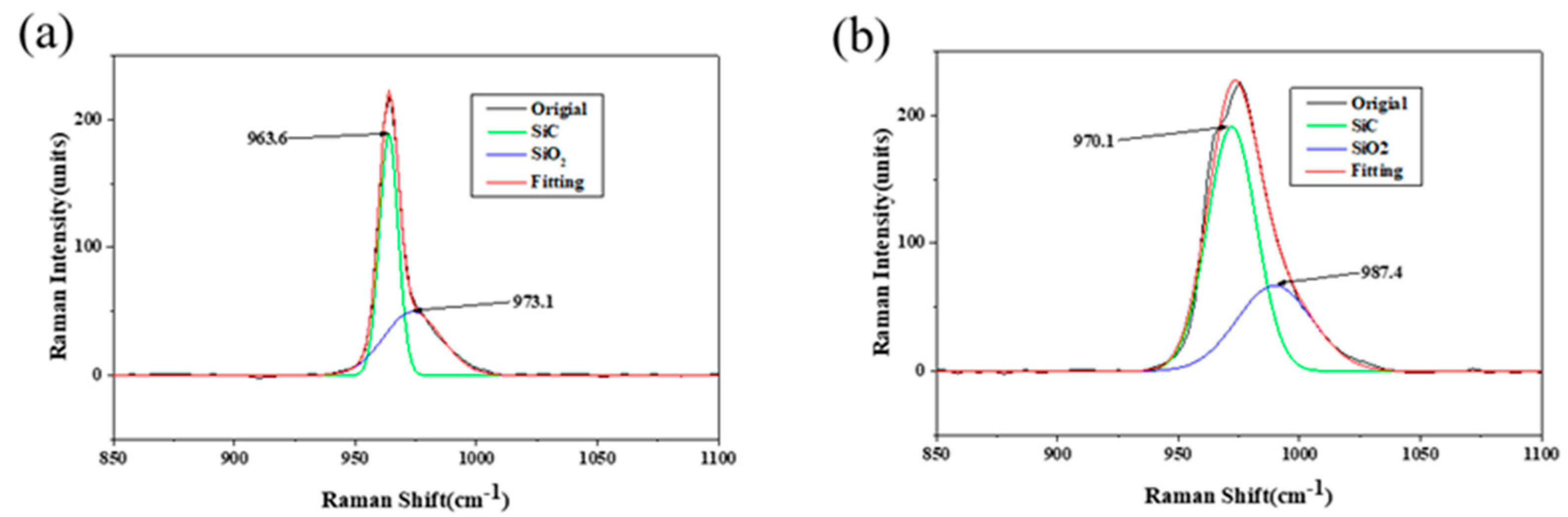
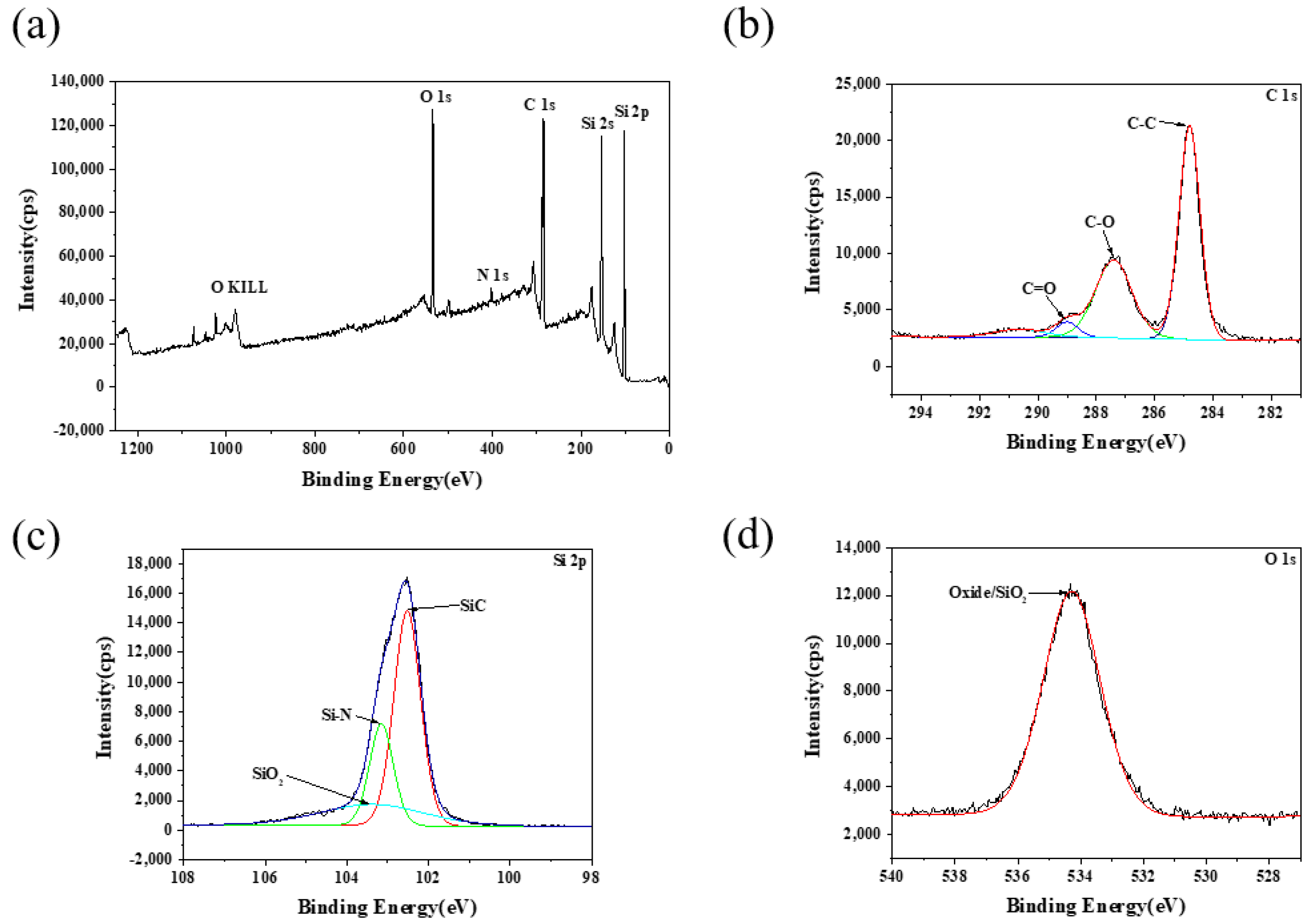
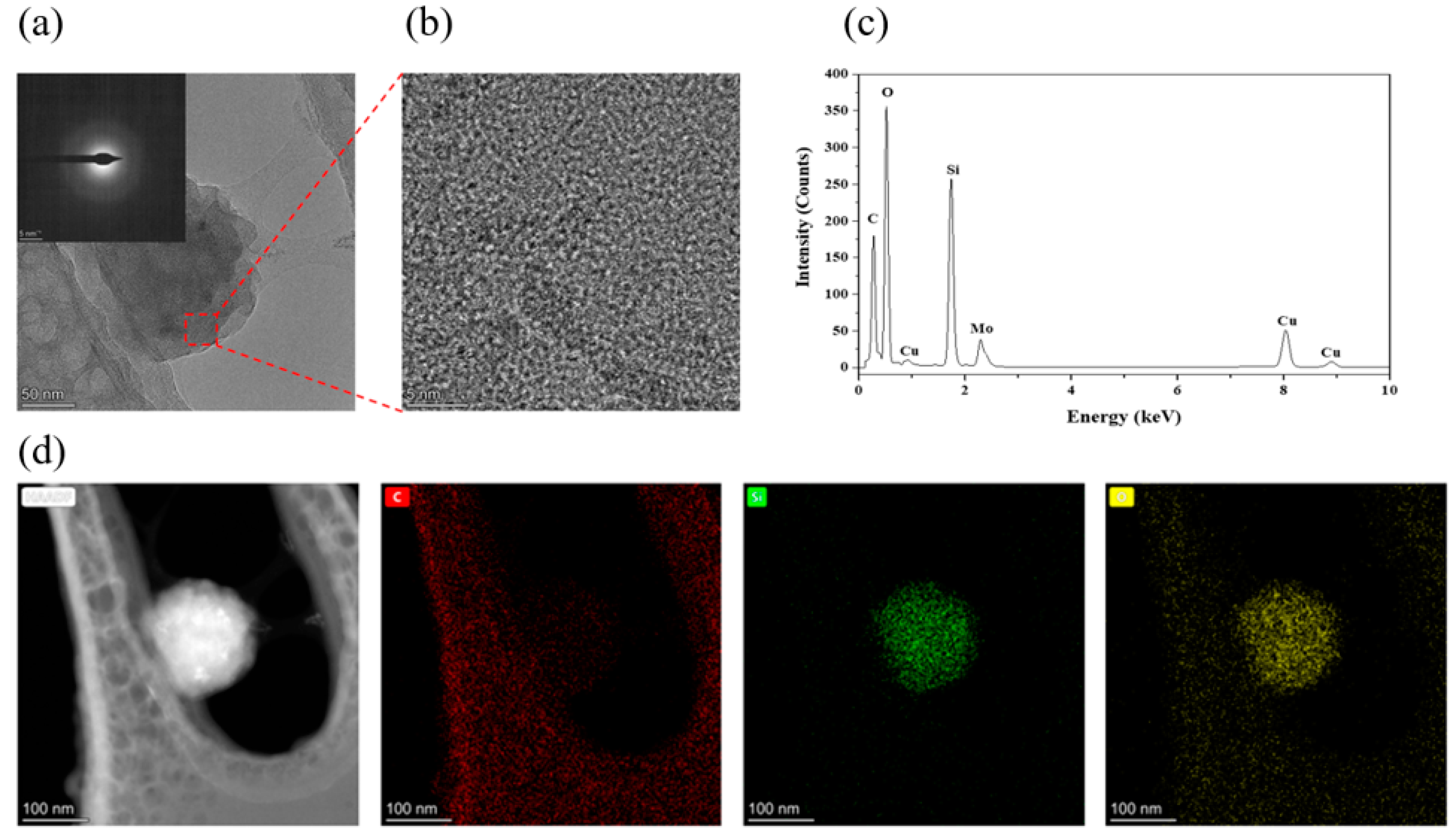
3.2. Characterisations of SiC–Water Reaction on C-Face (000)
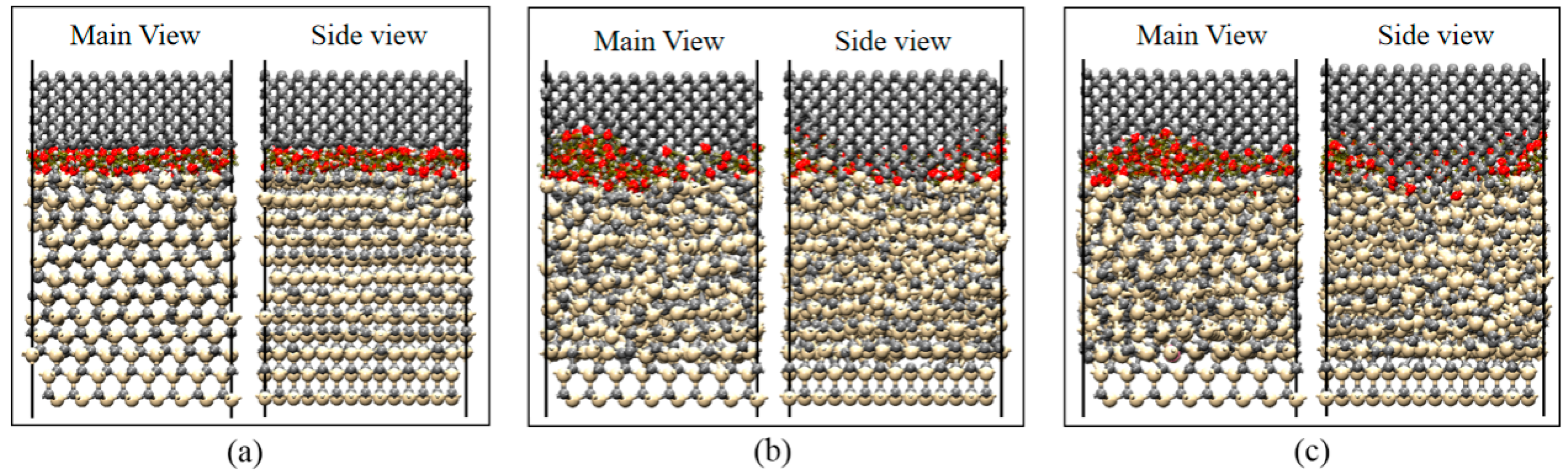
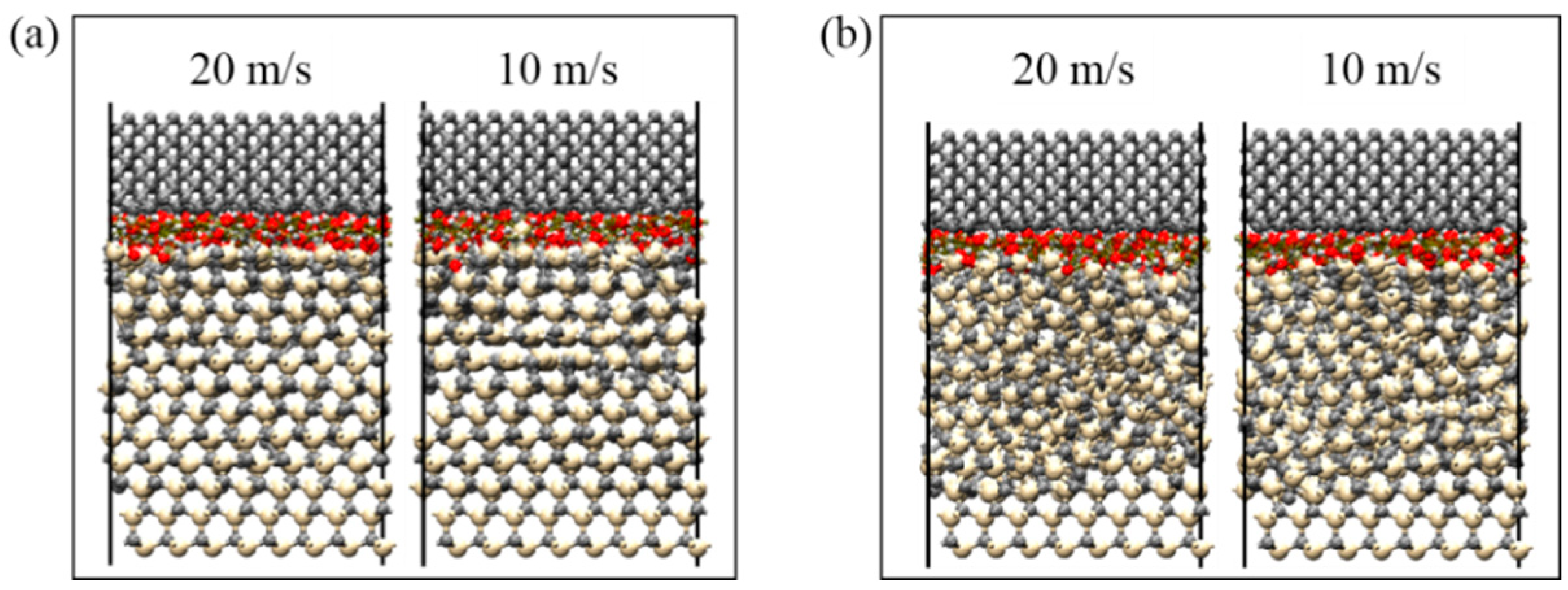
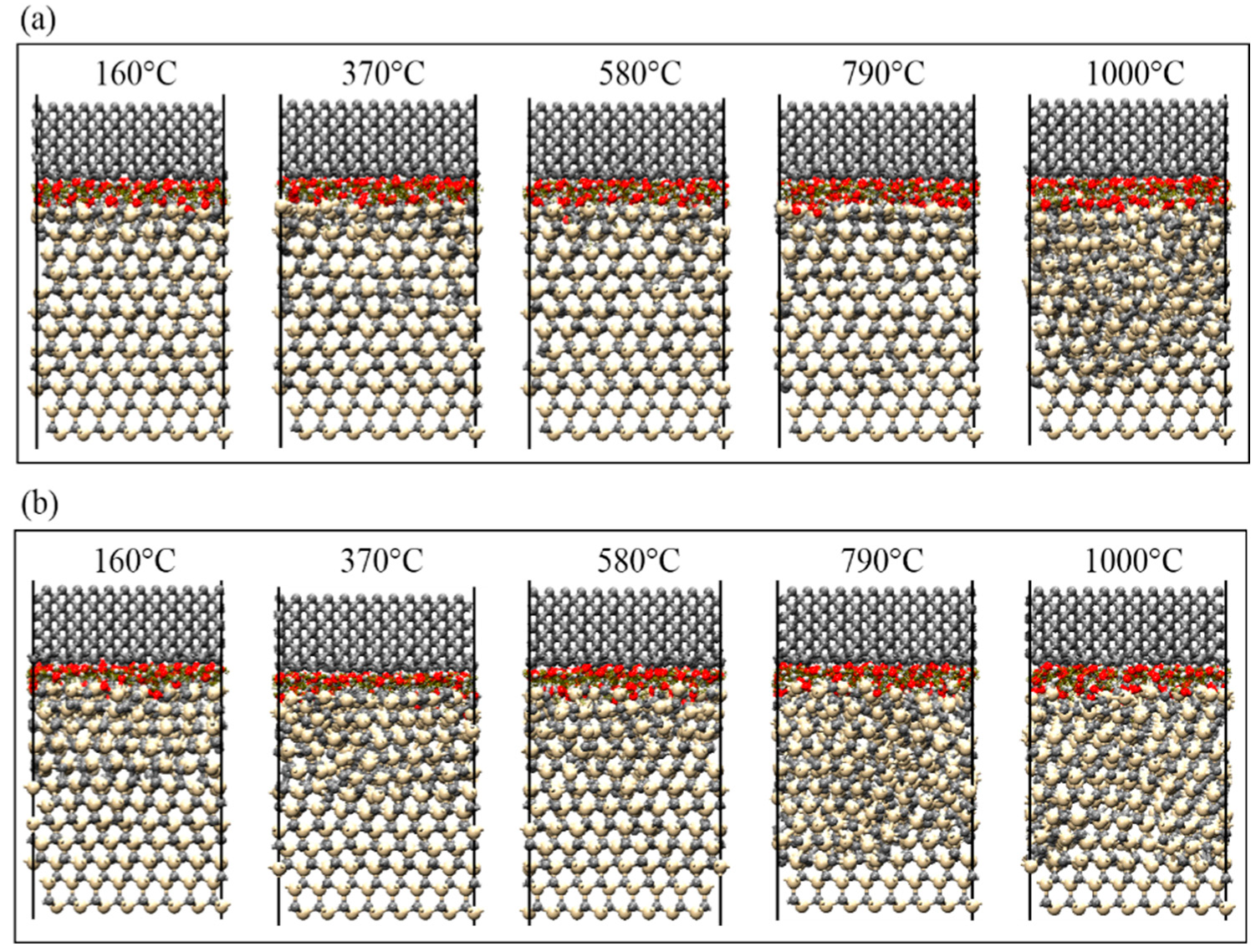
3.3. Mechanism of SiC–Water Reaction in RMD Simulation
4. Discussion
5. Conclusions
- (1)
- A regular plastic scratch with nanometer depth and almost no evident cracks was obtained using diamond abrasives to scratch 4H-SiC C face (000) without any chemical agent, and the formation of amorphous SiO2 was clearly observed, which verified that the SiC–water reaction occurred in the SiC plastic removal stage.
- (2)
- When the contact radius was gradually increased to 2 mm, the scratches that developed on the surface were converted from brittle to plastic removal mode, which were further concluded from the RMD simulations. The SiC crystal structure was damaged to a lesser extent owing to the larger contact radius and smaller contact stress. The plastic removal of SiC materials was not significantly affected by the increase in speed; only the removal rate of SiC materials was increased.
- (3)
- With the increase in load, the scratch depth could be in the range of tens to hundreds of nanometers during scratching. Furthermore, the removal mode of SiC transitioned from plastic to brittle and thereby achieved a controllable behavior in the plastic domain of SiC under controlled loading, which indirectly controlled the SiC–water reaction. Conversely, the controllable behavior of the SiC–water reaction at the nanoscale during RMD simulations was primarily achieved by adjusting the friction depth. Therefore, both experiments and simulations regulated the load, thereby regulating the friction depth to control the occurrence of the SiC–water reaction.
- (4)
- The main factors controlling the SiC–water reaction were the load and contact states, and the secondary factors were the speed and temperature. The SiC–water reaction could occur smoothly at low temperatures as long as a suitable load and contact state were maintained. Therefore, microcracks could be reasonably avoided by the effective application of the SiC–water reaction in the subsequent SiC thinning process, thus improving the surface quality of SiC wafers.
Author Contributions
Funding
Conflicts of Interest
References
- Yu, K.; Zhao, W.; Wu, X.; Zhuang, J.; Hu, X.; Zhang, Q.; Sun, L. In situ atomic-scale observation of monolayer graphene growth from SiC. Nano Res. 2018, 11, 2809–2820. [Google Scholar] [CrossRef]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.X.; Huang, P.J. Study on material removal mechanism of 6H-SiC single crystal wafer based on different nano-scratch order. Diam. Abras. Eng. 2022, 41, 92–97. [Google Scholar]
- Deng, J.; Yan, Q.; Lu, J.; Xiong, Q.; Pan, J. Optimisation of lapping process parameters for single-crystal 4H–SiC using orthogonal experiments and grey relational analysis. Micromachines 2021, 12, 910. [Google Scholar] [CrossRef]
- Neslen, C.L.; Mitchel, W.C.; Hengehold, R.L. Effects of process parameter variations on the removal rate in chemical mechanical polishing of 4H-SiC. J. Electron. Mater. 2001, 30, 1271–1275. [Google Scholar] [CrossRef]
- Evans, C.J.; Paul, E.; Dornfeld, D.A.; Lucca, D.A.; Byrne, G.; Tricard, M.; Klocke, F.; Dambon, O.; Mullany, B.A. Material removal mechanisms in lapping and polishing. CIRP Ann.- Manuf. Techn. 2003, 52, 611–633. [Google Scholar] [CrossRef]
- Endoh, N.; Akiyama, S.; Tashima, K.; Suwa, K.; Kamogawa, T.; Kohama, R.; Fukidome, H. High-quality few-layer graphene on single-crystalline SiC thin film grown on affordable wafer for device applications. Nanomaterials 2021, 11, 392. [Google Scholar] [CrossRef]
- Royet, A.S.; Ouisse, T.; Cabon, B.; Noblanc, O.; Arnodo, C.; Brylinski, C. Self-heating effects in silicon carbide mesfets. IEEE Trans. Electron. Devices 2000, 47, 2221–2227. [Google Scholar] [CrossRef]
- Anzalone, R.; Piluso, N.; Litrico, G.; Lorenti, S.; Arena, G.; Coffa, S.; La Via, F. Stress relaxation mechanism after thinning process on 4H-SiC substrate. Mater. Sci. Forum 2018, 924, 535–538. [Google Scholar] [CrossRef]
- Zhao, M.; Hayakawa, S.; Nishida, Y.; Jourdain, A.; Tabuchi, T.; Leunissen, L.H. Wafer backside thinning process integrated with post-thinning clean and TSV exposure recess etch. ECS Trans. 2012, 44, 865. [Google Scholar] [CrossRef]
- Huo, F.W.; Guo, D.M.; Kang, R.K. Nanogrinding of SiC wafers with high flatness and low subsurface damage. Trans. Nonferrous Met. Soc. China 2012, 22, 3027–3033. [Google Scholar] [CrossRef]
- Presser, V.; Krummhauer, O.; Kailer, A.; Nickel, K.G. In situ monitoring and depth-resolved characterization of wet wear of silicon carbide. Wear 2011, 271, 2665–2672. [Google Scholar] [CrossRef]
- Rupp, R.; Gerlach, R.; Kirchner, U.; Schlogl, A.; Kern, R. Performance of a 650 V SiC diode with reduced chip thickness. Mater. Sci. Forum 2012, 717–720 Pt 2, 921–924. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, F.; Luo, X. Subsurface damages beneath fracture pits of reaction-bonded silicon carbide after ultra-precision grinding. Appl. Surf. Sci. 2018, 448, 341–350. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Touge, M.; Nakano, T.; Watanabe, J. Study on lapping and constant-pressure grinding of single-crystal SiC. Adv. Mater. Res. 2009, 76–78, 282–287. [Google Scholar] [CrossRef]
- Tsukimoto, S.; Ise, T.; Maruyama, G.; Hashimoto, S.; Sakurada, T.; Senzaki, J. Correlation between local strain distribution and microstructure of grinding-induced damage layers in 4H-SiC (000). Mater. Sci. Forum 2017, 897, 177–180. [Google Scholar] [CrossRef]
- Feng, K.; Zhao, T.; Lyu, B.; Zhou, Z. Ultra-precision grinding of 4H-SiC wafer by pav/pf composite sol-gel diamond wheel. Adv. Mech. Eng. 2021, 13, 16878140211044929. [Google Scholar] [CrossRef]
- Sano, Y.; Tajiri, K.; Inoue, Y.; Mukai, R.; Nakanishi, Y.; Matsuyama, S.; Yamauchi, K. High-speed etching of silicon carbide wafer using high-pressure sf6 plasma. ECS J. Solid State Sci. Technol. 2021, 10, 014005. [Google Scholar] [CrossRef]
- Guan, J.; Zhao, Y. Non-contact grinding/thinning of silicon carbide wafer by pure EDM using a rotary cup wheel electrode. Precis. Eng. 2022, 74, 209–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Shi, X.; Gong, H.; Luo, G.; Gu, Z. Chemical mechanical planarization (CMP) of on-axis Si-face SiC wafer using catalyst nanoparticles in slurry. Surf. Coat. Technol. 2014, 251, 48–55. [Google Scholar] [CrossRef]
- Deng, J.Y.; Pan, J.S.; Zhang, Q.X.; Guo, X.H.; Yan, Q.S. Research progress in chemical mechanical polishing of single crystal SiC substrates. Diam. Abras. Eng. 2020, 40, 13. [Google Scholar]
- Qi, W.; Cao, X.; Xiao, W.; Wang, Z.; Su, J. Study on the mechanism of solid-phase oxidant action in tribochemical mechanical polishing of SiC single crystal substrate. Micromachines 2021, 12, 1547. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Xu, X. The effects of abrasive yielding on the polishing of SiC wafers using a semi-fixed flexible pad. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 170–177. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X. A comparative study on the material removal mechanisms of 6H-SiC polished by semi-fixed and fixed diamond abrasive tools. Wear 2016, 350, 99–106. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Xu, X.; Jiang, F. Removal mechanism of sapphire substrates (0001, 110 and 100) in mechanical planarization machining. Ceram. Int. 2017, 43, 16178–16184. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Xu, X.; Chen, C.; Lin, Y. Study on high efficient sapphire wafer processing by coupling SG-mechanical polishing and GLA-CMP. Int. J. Mach. Tool. Manu. 2018, 130, 12–19. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Tian, Z.; Jiang, F. Controllable material removal behavior of 6H-SiC wafer in nanoscale polishing. Appl. Surf. Sci. 2021, 562, 150219. [Google Scholar] [CrossRef]
- Chrobak, D.; Tymiak, N.; Beaber, A.; Ugurlu, O.; Gerberich, W.W.; Nowak, R. Deconfinement leads to changes in the nanoscale plasticity of silicon. Nat. Nanotechnol. 2011, 6, 480–484. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Zhang, L. Revealing the deformation mechanisms of 6H-silicon carbide under nano-cutting. Comput. Mater. Sci. 2017, 137, 282–288. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Van Duin, A.C. The ReaxFF reactive force-field: Development, applications and future directions. NPJ Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; Psofogiannakis, G.; van Duin, A.C.; Chen, L.; Lu, X. Atomic insight into tribochemical wear mechanism of silicon at the Si/SiO2 interface in aqueous environment: Molecular dynamics simulations using ReaxFF reactive force field. Appl. Surf. Sci. 2016, 390, 216–223. [Google Scholar] [CrossRef]
- Fogarty, J.C.; Aktulga, H.M.; Grama, A.Y.; Van Duin, A.C.; Pandit, S.A. A reactive molecular dynamics simulation of the silica-water interface. J. Chem. Phys. 2010, 132, 174704. [Google Scholar] [CrossRef]
- Roehrl, J.; Hundhausen, M.; Emtsev, K.V.; Seyller, T.; Graupner, R.; Ley, L. Raman spectra of epitaxial graphene on SiC (0001). Appl. Phys. Lett. 2008, 92, 201918. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, W.; Fan, X. Stress/strain characterization in electronic packaging by micro-Raman spectroscopy: A review. Microelectron. Reliab. 2021, 118, 114045. [Google Scholar] [CrossRef]
- Malinovsky, V.K.; Novikov, V.N.; Surovtsev, N.V.; Shebanin, A.P. Investigation of amorphous states of SiO2 by Raman scattering spectroscopy. Phys. Solid State 2000, 42, 65–71. [Google Scholar] [CrossRef]
- Kusunoki, I.; Igari, Y. XPS study of a SiC film produced on Si (100) by reaction with a C2H2 beam. Appl. Surf. Sci. 1992, 59, 95–104. [Google Scholar] [CrossRef]
- Ferrah, D.; Penuelas, J.; Bottela, C.; Grenet, G.; Ouerghi, A. X-ray photoelectron spectroscopy (XPS) and diffraction (XPD) study of a few layers of graphene on 6H-SiC (0001). Surf. Sci. 2013, 615, 47–56. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Luo, Q.; Xu, X. Photocatalysis assisting the mechanical polishing of a single-crystal SiC wafer utilizing an anatase TiO2-coated diamond abrasive. Precis. Eng. 2017, 49, 235–242. [Google Scholar] [CrossRef]
- Borysiuk, J.; Bozek, R.; Grodecki, K.; Wysmołek, A.; Strupinski, W.; Stępniewski, R.; Baranowski, J.M. Transmission electron microscopy investigations of epitaxial graphene on C-terminated 4H-SiC. J. Appl. Phys. 2010, 108, 013518. [Google Scholar] [CrossRef]
- Tian, L.; Fu, Y.; Xu, J.; Li, H.; Ding, W. The influence of speed on material removal mechanism in high speed grinding with single grit. Int. J. Mach. Tool. Manu 2015, 89, 192–201. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Luo, Q.; Lu, J.; Tian, Z. Understanding the Mechanisms of SiC–Water Reaction during Nanoscale Scratching without Chemical Reagents. Micromachines 2022, 13, 930. https://doi.org/10.3390/mi13060930
Cheng Z, Luo Q, Lu J, Tian Z. Understanding the Mechanisms of SiC–Water Reaction during Nanoscale Scratching without Chemical Reagents. Micromachines. 2022; 13(6):930. https://doi.org/10.3390/mi13060930
Chicago/Turabian StyleCheng, Zhihao, Qiufa Luo, Jing Lu, and Zige Tian. 2022. "Understanding the Mechanisms of SiC–Water Reaction during Nanoscale Scratching without Chemical Reagents" Micromachines 13, no. 6: 930. https://doi.org/10.3390/mi13060930
APA StyleCheng, Z., Luo, Q., Lu, J., & Tian, Z. (2022). Understanding the Mechanisms of SiC–Water Reaction during Nanoscale Scratching without Chemical Reagents. Micromachines, 13(6), 930. https://doi.org/10.3390/mi13060930





