Optimizing Broadband Near-Infrared Emission in Bi/Sn-Doped Aluminosilicate Glass by Modulating Glass Composition
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murata, K.; Fujimoto, Y.; Kanabe, T. Bi-Doped SiO2 as a New Laser Material for an Intense Laser. Fusion Eng. Des. 1999, 44, 437–439. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Nakatsuka, M. Optical amplification in bismuth-doped silica glass. Appl. Phys. Lett. 2003, 82, 3325–3326. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Nakatsuka, M. Infrared luminescence from bismuth-doped silica glass. Jpn. J. Appl. Phys. 2001, 40, L279. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Matsubara, H.; Nakatsuka, M. New fluorescence from Bi-doped silica glass and its 1.3-/spl mu/m emission with 0.8-/spl mu/m excitation for fiber amplifier. In Proceedings of the Technical Digest. CLEO/Pacific Rim 2001. 4th Pacific Rim Conference on Lasers and Electro-Optics (Cat. No.01TH8557), Chiba, Japan, 15–19 July 2001; Volume 2, p. II. [Google Scholar]
- Dianov, E.M.; Dvoyrin, V.V.; Mashinsky, V.M. CW bismuth fibre laser. Quantum Electron. 2005, 35, 1083. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Yu, J.; Yang, W.; Lewis, E.; Wang, P. Bismuth-doped compound germanate glass microsphere lasing in the near-infrared region. Microw. Opt. Technol. Lett. 2020, 62, 67–71. [Google Scholar] [CrossRef]
- Zhang, N.; Qiu, J.; Dong, G.; Yang, Z.; Zhang, Q.; Peng, M. Broadband tunable near-infrared emission of Bi-doped composite germanosilicate glasses. J. Mater. Chem. 2012, 22, 3154–3159. [Google Scholar] [CrossRef]
- Dianov, E.M. Bi-doped optical fibers: A new active medium for NIR lasers and amplifiers. In Proceedings of the SPIE—The International Society for Optical Engineering, San Jose, CA, USA, 19 January 2008; p. 8601. [Google Scholar]
- Chen, D.; Wang, Y.; Bao, F.; Yu, Y. Broadband near-infrared emission from Tm3+∕Er3+ co-doped nanostructured glass ceramics. J. Appl. Phys. 2007, 101, 113511. [Google Scholar] [CrossRef]
- Afef, B.; Alqahtani, M.M.; Hegazy, H.H.; Yousef, E.; Damak, K.; Maâlej, R. Green and near infrared emission of Er3+ doped PZS and PZC glasses. J. Lumin. 2018, 194, 706–712. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Pan, H.; Zhang, X.; Fang, X.; Li, W.; Xiao, Z. Broadband near infrared emission of Er3+/Yb3+ co-doped fluorotellurite glass. J. Alloy. Compd. 2021, 866, 158568. [Google Scholar] [CrossRef]
- Alsingery, R.M.; Mudhafer, A. Development of Bismuth-Doped Fibers (BDFs) in Optical Communication Systems. In Bismuth-Fundamentals and Optoelectronic Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Fujimoto, Y.; Nakatsuka, M. 27Al NMR structural study on aluminum coordination state in bismuth doped silica glass. J. Non-Cryst. Solids 2006, 352, 2254–2258. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Li, X.; Jiao, Q.; Dai, S. Controllable ultra-broadband visible and near-infrared photoemissions in Bi-doped germanium-borate glasses. J. Am. Ceram. Soc. 2020, 103, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wondraczek, L.; Wang, Y.; Wang, L.; Li, J.; Xu, S.; Peng, M. Ultrabroadband near-infrared photoemission from bismuth-centers in nitridated oxide glasses and optical fiber. Acs Photonics 2018, 5, 4393–4401. [Google Scholar] [CrossRef]
- Chiodini, N.; Paleari, A.; Spinolo, G.; Chiasera, A.; Ferrari, M.; Brambilla, G.; Taylor, E.R. Photosensitive erbium doped tin-silicate glass. J. Non-Cryst. Solids 2002, 311, 217–222. [Google Scholar] [CrossRef]
- Peng, M.; Qiu, J.; Chen, D.; Meng, X.; Zhu, C. Superbroadband 1310 nm emission from bismuth and tantalum codoped germanium oxide glasses. Opt. Lett. 2005, 30, 2433–2435. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Peng, M.; Ren, J.; Meng, X.; Jiang, X.; Zhu, C. Novel Bi-doped glasses for broadband optical amplification. J. Non-Cryst. Solids 2008, 354, 1235–1239. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Huang, M.; Peng, M. Modulating broadband near infrared emission from Bi doped borate laser glass by codoping nonactive rare earth ions. J. Non-Cryst. Solids 2021, 553, 120477. [Google Scholar] [CrossRef]
- Meng, X.G.; Qiu, J.R.; Peng, M.Y.; Chen, D.P.; Zhao, Q.Z.; Jiang, X.W.; Zhu, C.S. Infrared broadband emission of bismuth-doped barium-aluminum-borate glasses. Opt. Express 2005, 13, 1635–1642. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, G.; Bao, J.; Yang, H.; Qiu, J. Broadband near-infrared emission from Bi-doped aluminosilicate glasses. J. Mater. Res. 2007, 22, 1435–1438. [Google Scholar] [CrossRef]
- Li, X.; Peng, M.; Cao, J.; Yang, Z.; Xu, S. Distribution and stabilization of bismuth NIR centers in Bi-doped aluminosilicate laser glasses by managing glass network structure. J. Mater. Chem. C 2018, 6, 7814–7821. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohishi, Y. Ultrabroadband near-infrared emission from Bi-doped Li2O-Al2O3-SiO2 glass. Appl. Phys. Lett. 2006, 88, 191912. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Wang, L.; Peng, M. Predictable tendency of Bi NIR emission in Bi-doped magnesium aluminosilicate laser glasses. J. Am. Ceram. Soc. 2018, 101, 1159–1168. [Google Scholar] [CrossRef]
- Dianov, E.M. Amplification in extended transmission bands using bismuth-doped optical fibers. J. Lightwave Technol. 2012, 31, 681–688. [Google Scholar] [CrossRef]
- Meng, X.G.; Qiu, J.R.; Peng, M.Y.; Chen, D.P.; Zhu, C.S. Near infrared broadband emission of bismuth-doped aluminophosphate glass. Opt. Express 2005, 13, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Tan, L.N.; Wang, L.P.; Peng, M.Y.; Xu, S.H. Superbroad visible to NIR photoluminescence from Bi+ evidenced in Ba2B5O9Cl: Bi crystal. Opt. Express 2016, 24, 2830–2835. [Google Scholar] [CrossRef]
- Romanov, A.N.; Veber, A.A.; Fattakhova, Z.T.; Vtyurina, D.N.; Kouznetsov, M.S.; Zaramenskikh, K.S. Spectral properties and NIR photoluminescence of Bi+ impurity in CsCdCl3 ternary chloride. Lumin 2014, 149, 292–296. [Google Scholar] [CrossRef]
- Doweidar, H. Density-structure correlations in Na2O-Al2O3-SiO2 glasses. J. Non-Cryst. Solids 1998, 240, 55–65. [Google Scholar] [CrossRef]
- Abd El-Moneim, A.; Youssof, I.M.; El-Latif, L.A. Structural role of RO and Al2O3 in borate glasses using an ultrasonic technique. Acta Mater. 2006, 54, 3811–3819. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Peng, M. The origin of the heterogeneous distribution of bismuth in aluminosilicate laser glasses. J. Am. Ceram. Soc. 2018, 101, 2921–2929. [Google Scholar] [CrossRef]
- Qian, M.; Cheng, J.; Hu, L. Dependence of spectroscopic properties on doping content and temperature of bismuth-doped lanthanum aluminosilicate glass. Chin. Opt. Lett. 2012, 10, 111602. [Google Scholar] [CrossRef] [Green Version]
- Cl, A.; Yz, B.; Jha, C.; Jian, R.; Chao, L.; Xza, C. Multi-band near-infrared emission in low concentration bismuth doped alkaline earth alumino-boro-germanate glass-sciencedirect. Ceram. Int. 2020, 46, 15544–15553. [Google Scholar]
- Zhang, Q.Y.; Zhang, W.J.; Wang, W.C.; Jiang, Z.H. Calculation of physical properties of glass via the phase diagram approach. Non-Cryst. Solids 2017, 457, 36–43. [Google Scholar] [CrossRef]
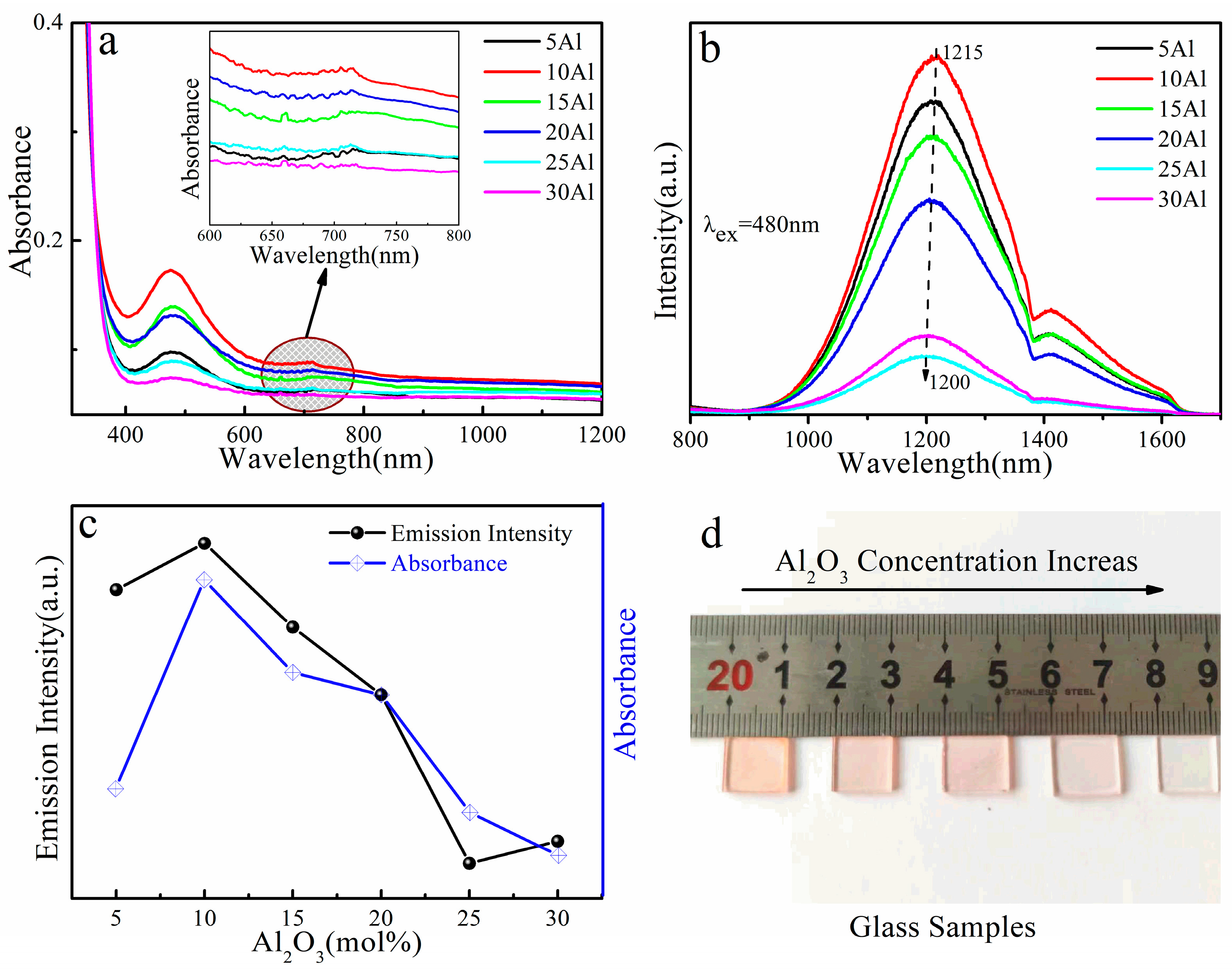
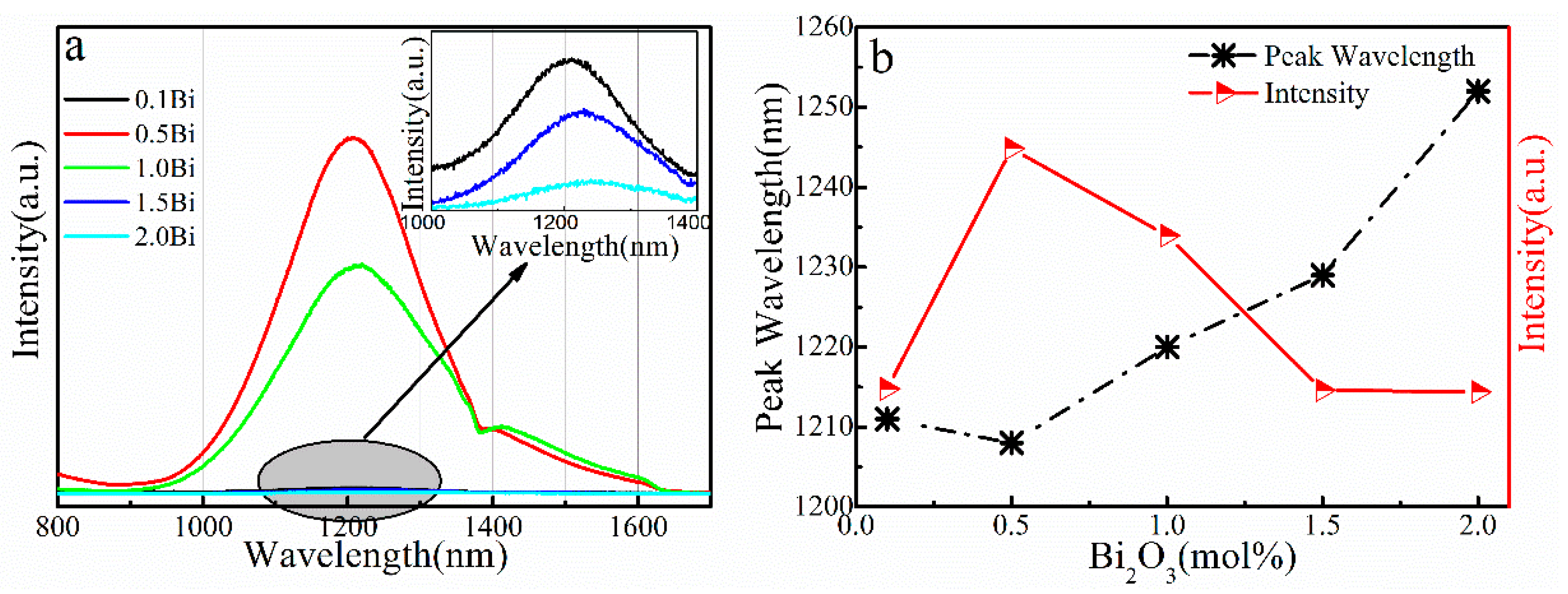
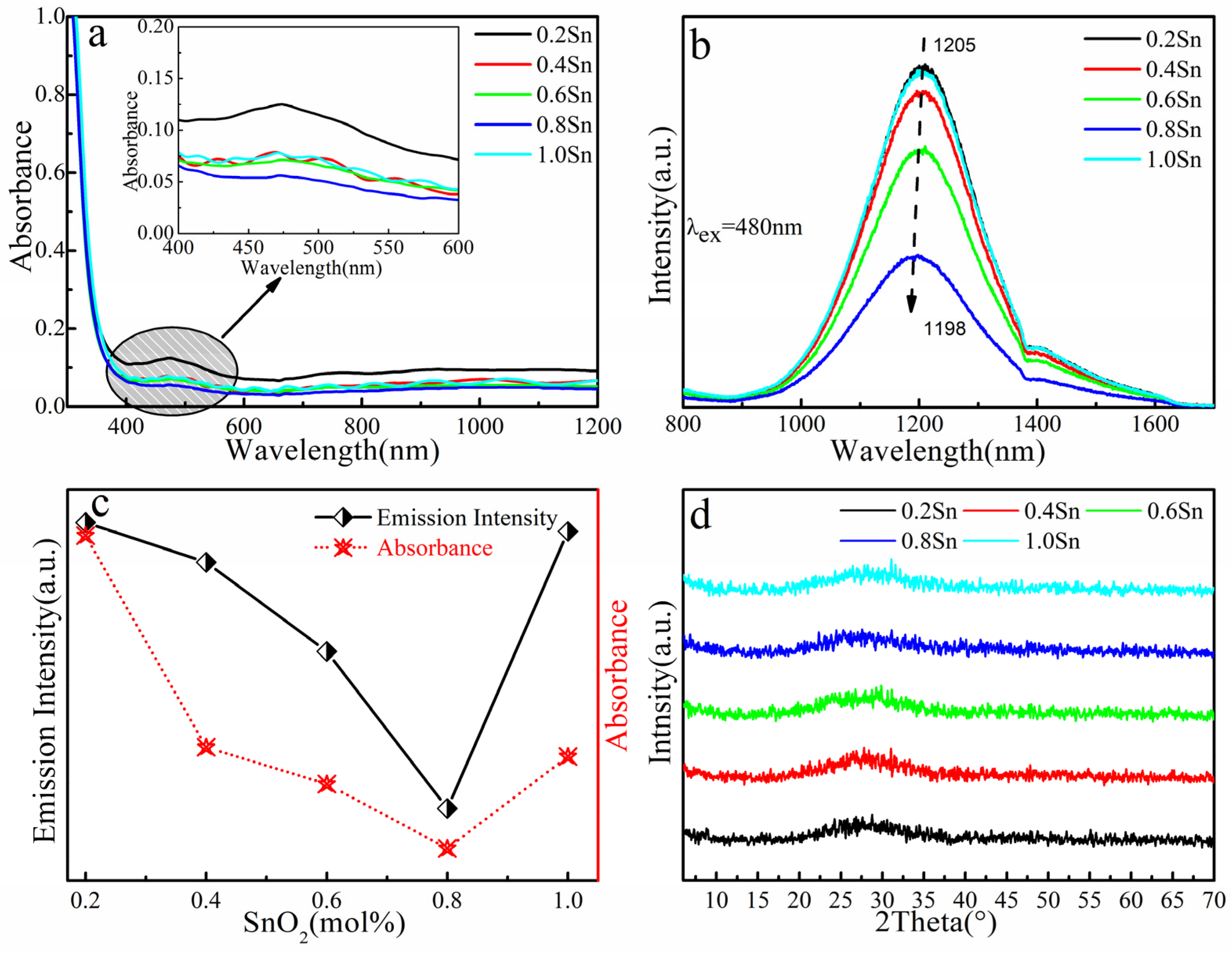
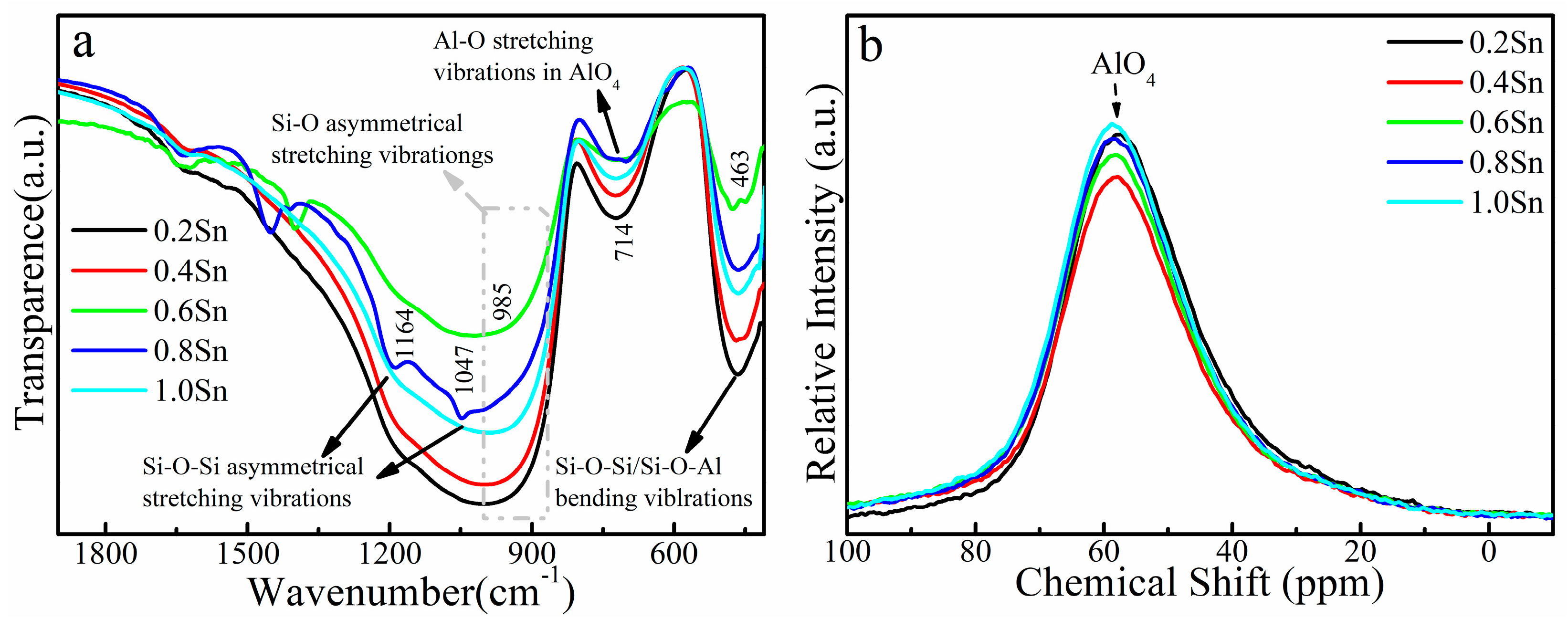
| xAl Sample | Eg ± 0.01 (eV) | xSn Sample | Eg ± 0.01 (eV) |
|---|---|---|---|
| 5Al | 3.83 | 0.2Sn | 3.76 |
| 10Al | 3.87 | 0.4Sn | 3.77 |
| 15Al | 3.85 | 0.6Sn | 3.79 |
| 20Al | 3.84 | 0.8Sn | 3.77 |
| 25Al | 3.82 | 1.0Sn | 3.72 |
| 30Al | 3.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, S.; Zhang, M.; Zeng, T.; Chen, J.; Zhang, F. Optimizing Broadband Near-Infrared Emission in Bi/Sn-Doped Aluminosilicate Glass by Modulating Glass Composition. Micromachines 2022, 13, 921. https://doi.org/10.3390/mi13060921
Xiang S, Zhang M, Zeng T, Chen J, Zhang F. Optimizing Broadband Near-Infrared Emission in Bi/Sn-Doped Aluminosilicate Glass by Modulating Glass Composition. Micromachines. 2022; 13(6):921. https://doi.org/10.3390/mi13060921
Chicago/Turabian StyleXiang, Song, Min Zhang, Tixian Zeng, Jiang Chen, and Feiquan Zhang. 2022. "Optimizing Broadband Near-Infrared Emission in Bi/Sn-Doped Aluminosilicate Glass by Modulating Glass Composition" Micromachines 13, no. 6: 921. https://doi.org/10.3390/mi13060921
APA StyleXiang, S., Zhang, M., Zeng, T., Chen, J., & Zhang, F. (2022). Optimizing Broadband Near-Infrared Emission in Bi/Sn-Doped Aluminosilicate Glass by Modulating Glass Composition. Micromachines, 13(6), 921. https://doi.org/10.3390/mi13060921





