Characterization of Fluidic-Barrier-Based Particle Generation in Centrifugal Microfluidics
Abstract
:1. Introduction
2. Theory and Governing Equations
3. Materials and Method
3.1. Material Preparation
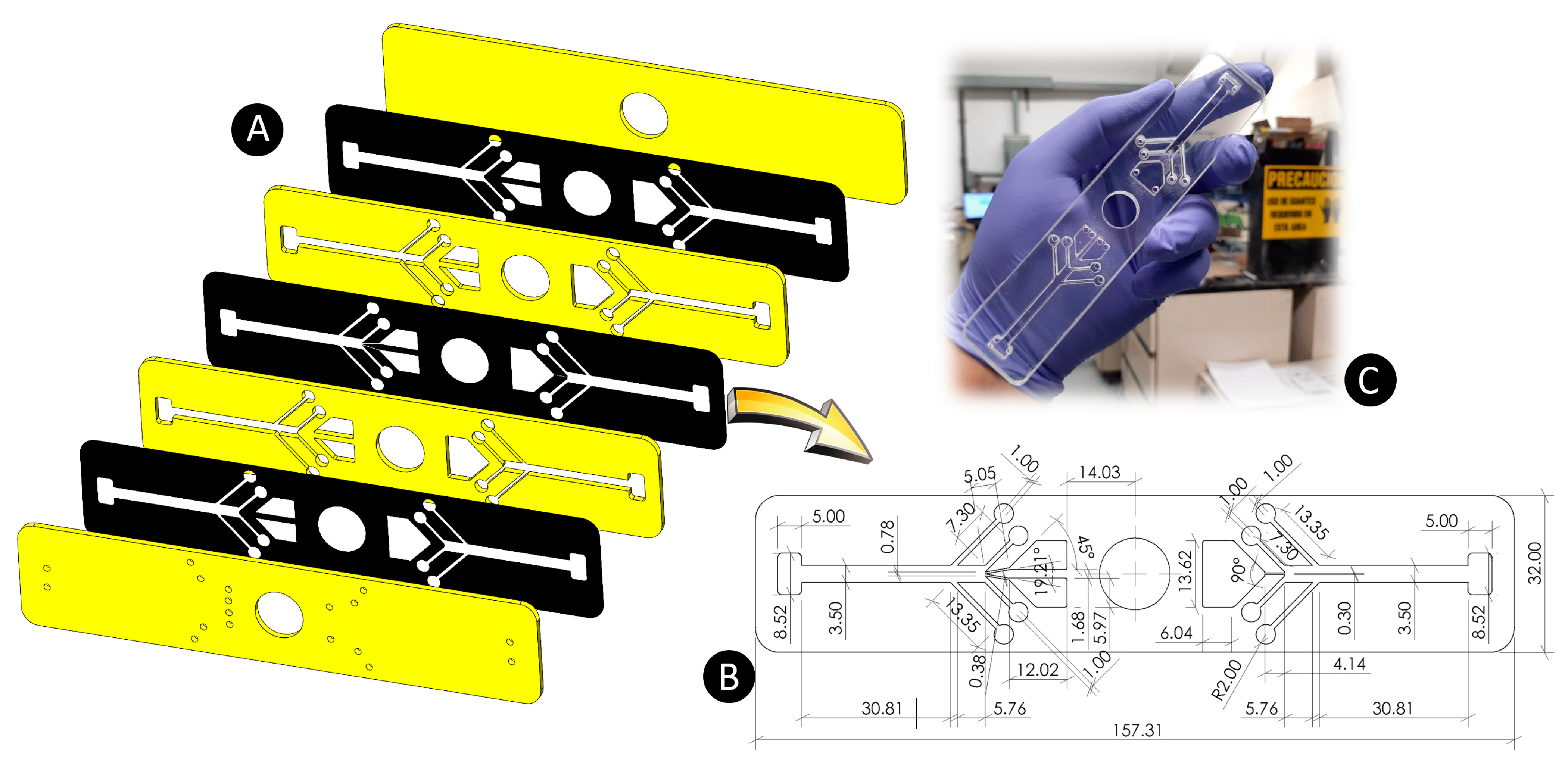
3.2. Fabrication of Microfluidic Devices
3.3. Experimental Procedure
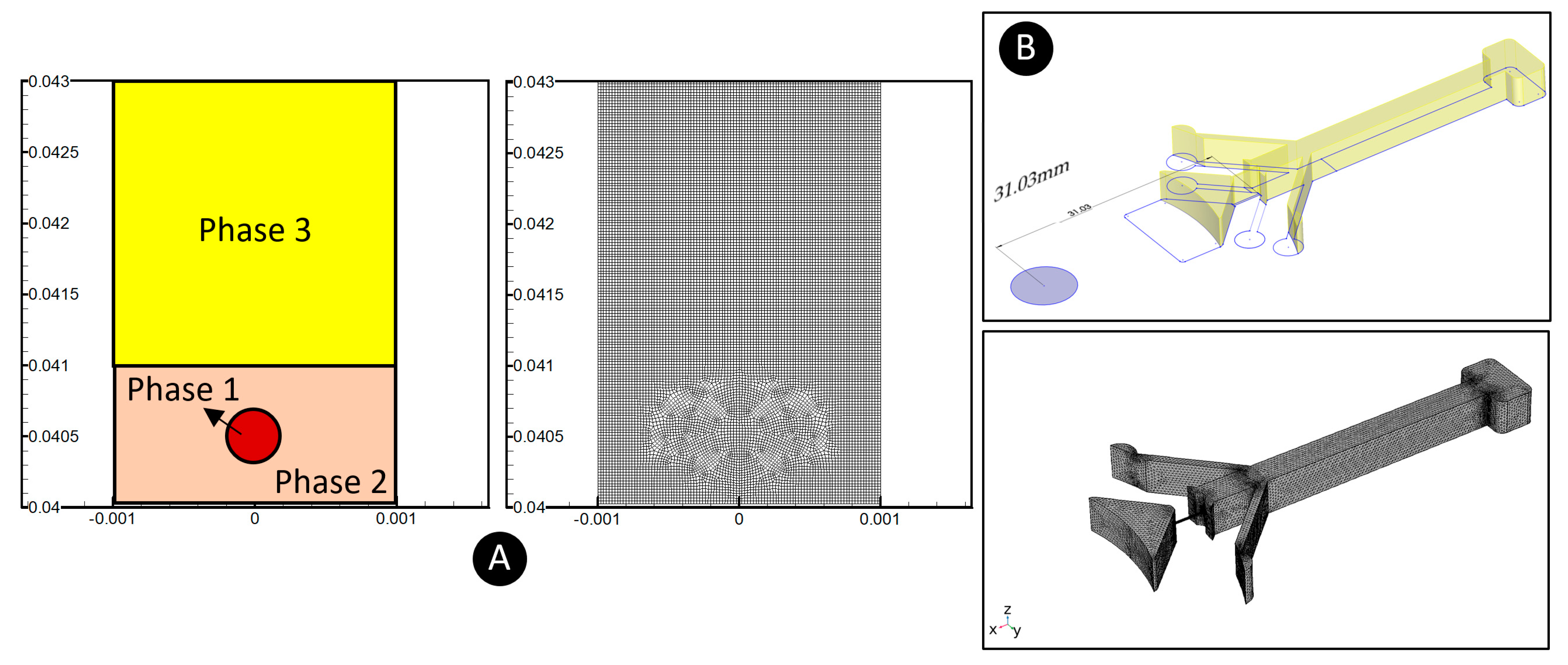
3.4. Modeling Methodology
3.5. Data Analysis
4. Results and Discussion
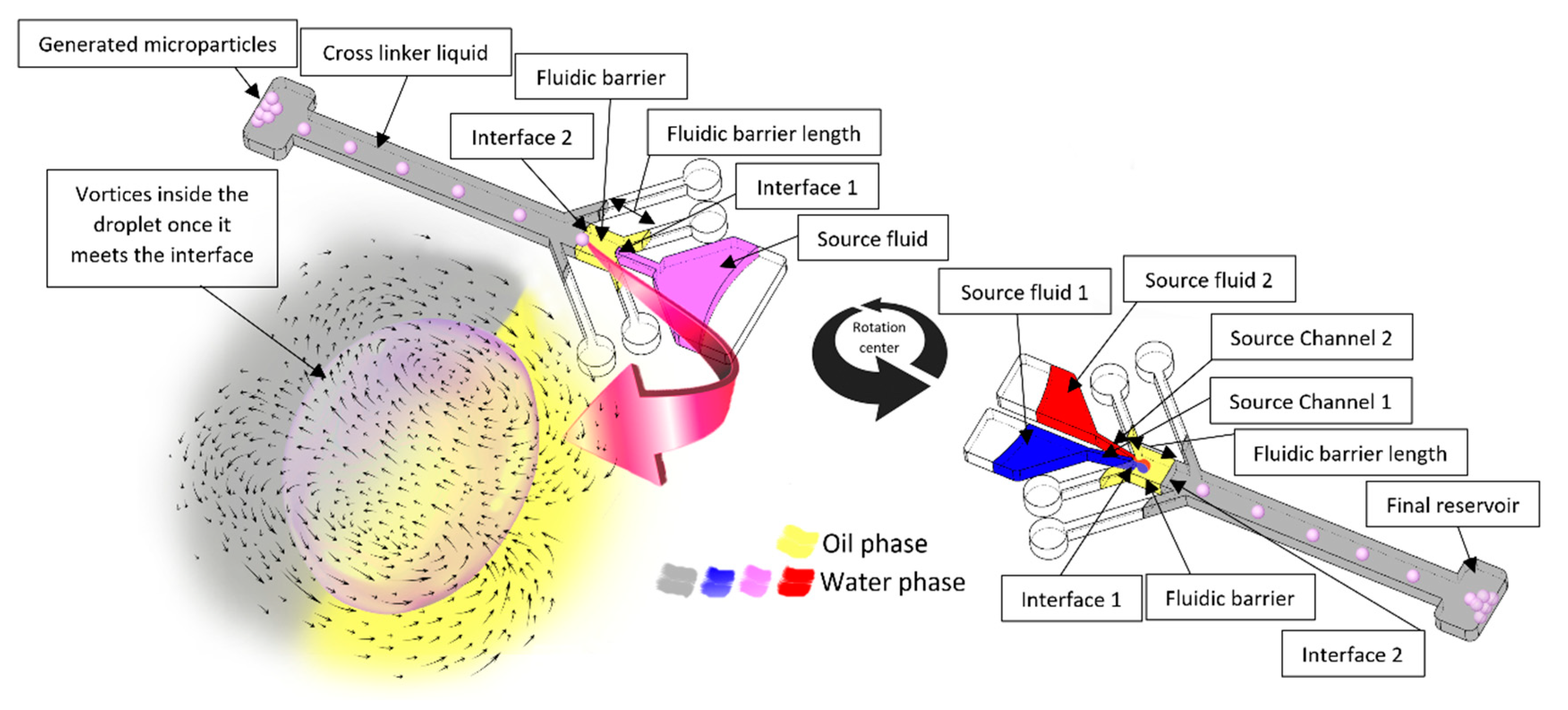
4.1. What Happens When a Droplet Crosses a Fluidic Barrier?
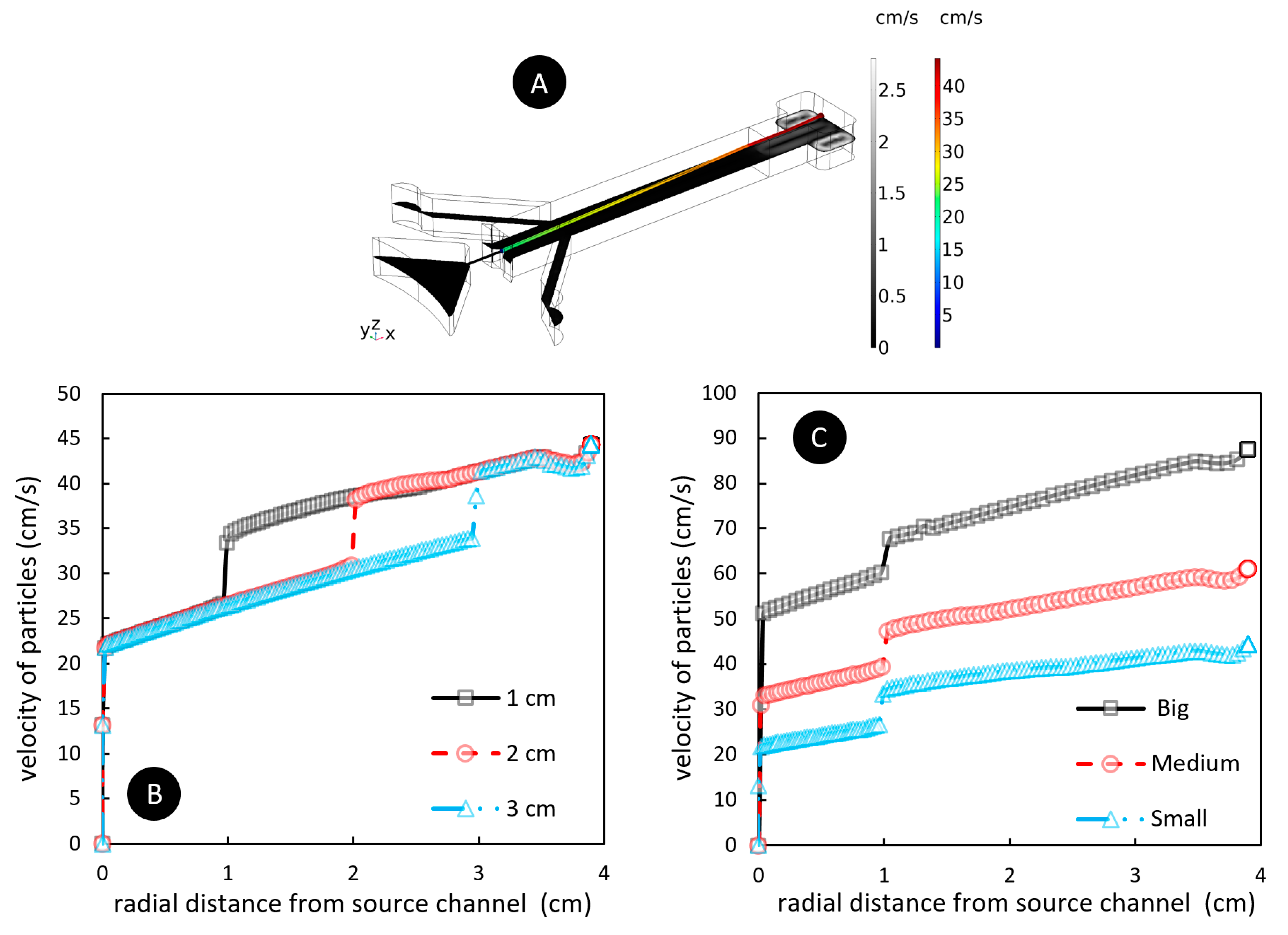
4.2. The Importance of Barrier Length and Source Channel Width on Particles Velocity
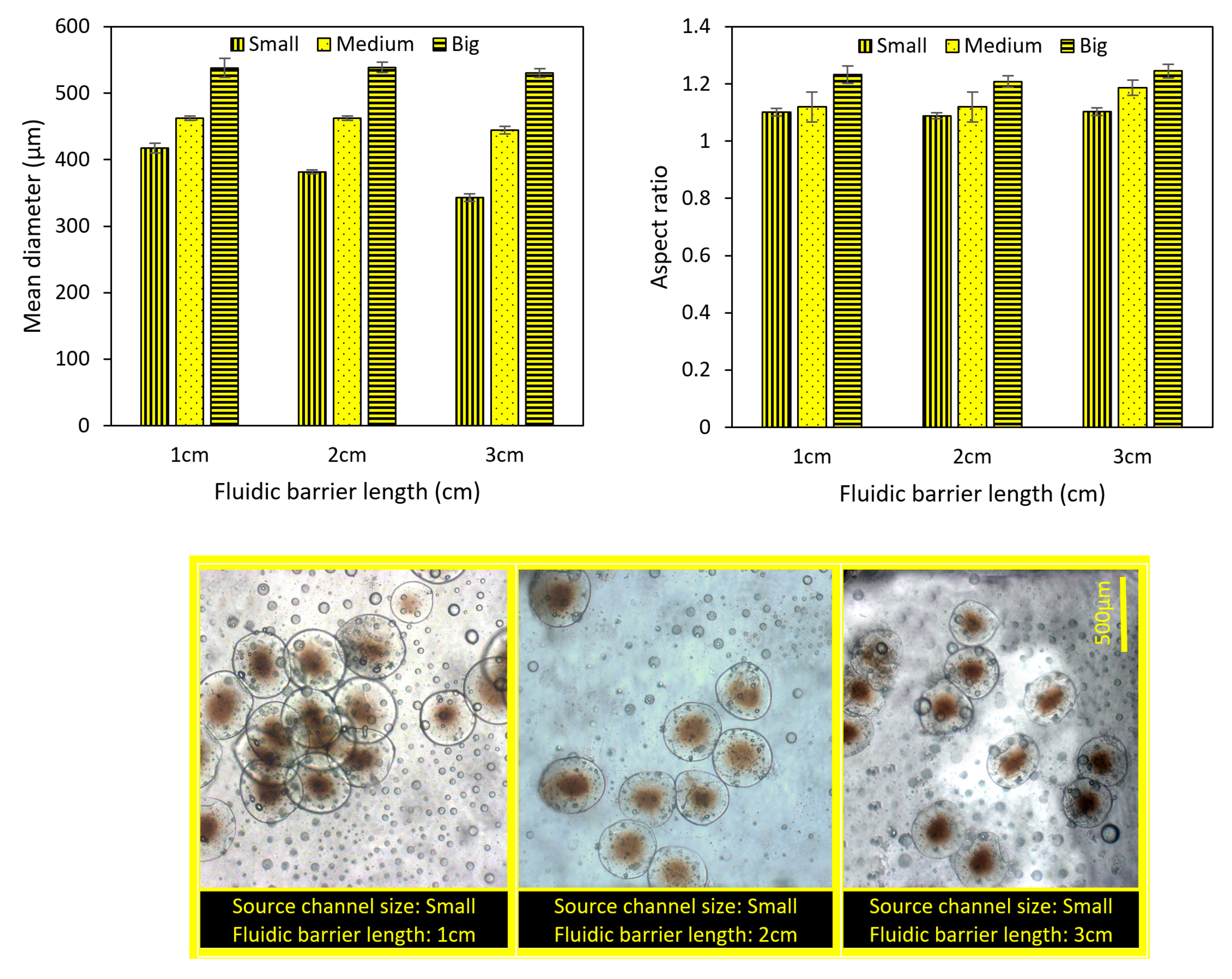
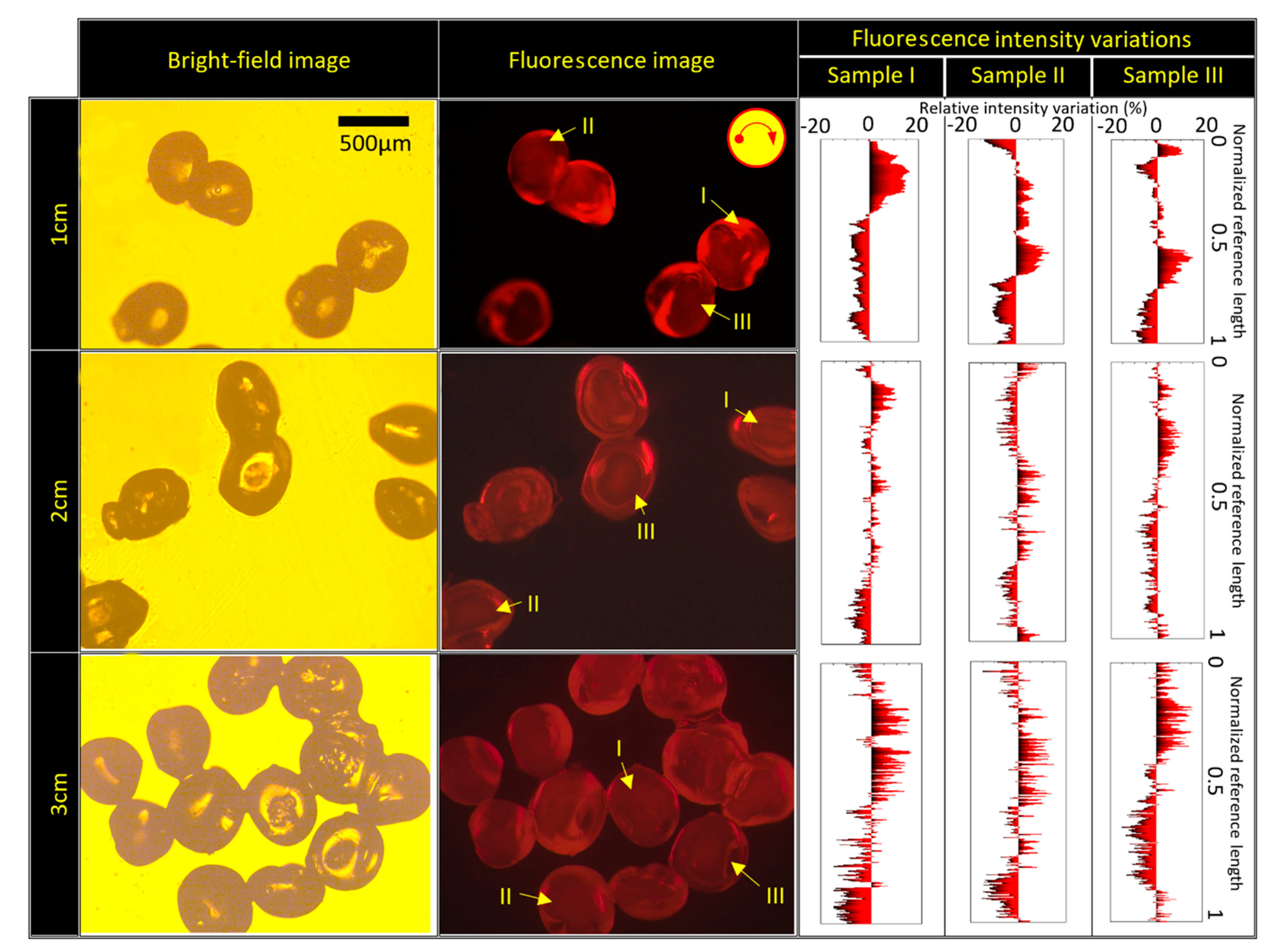
4.3. The Importance of Fluidic Farrier Length and Source Channel Width on Particles Characteristics
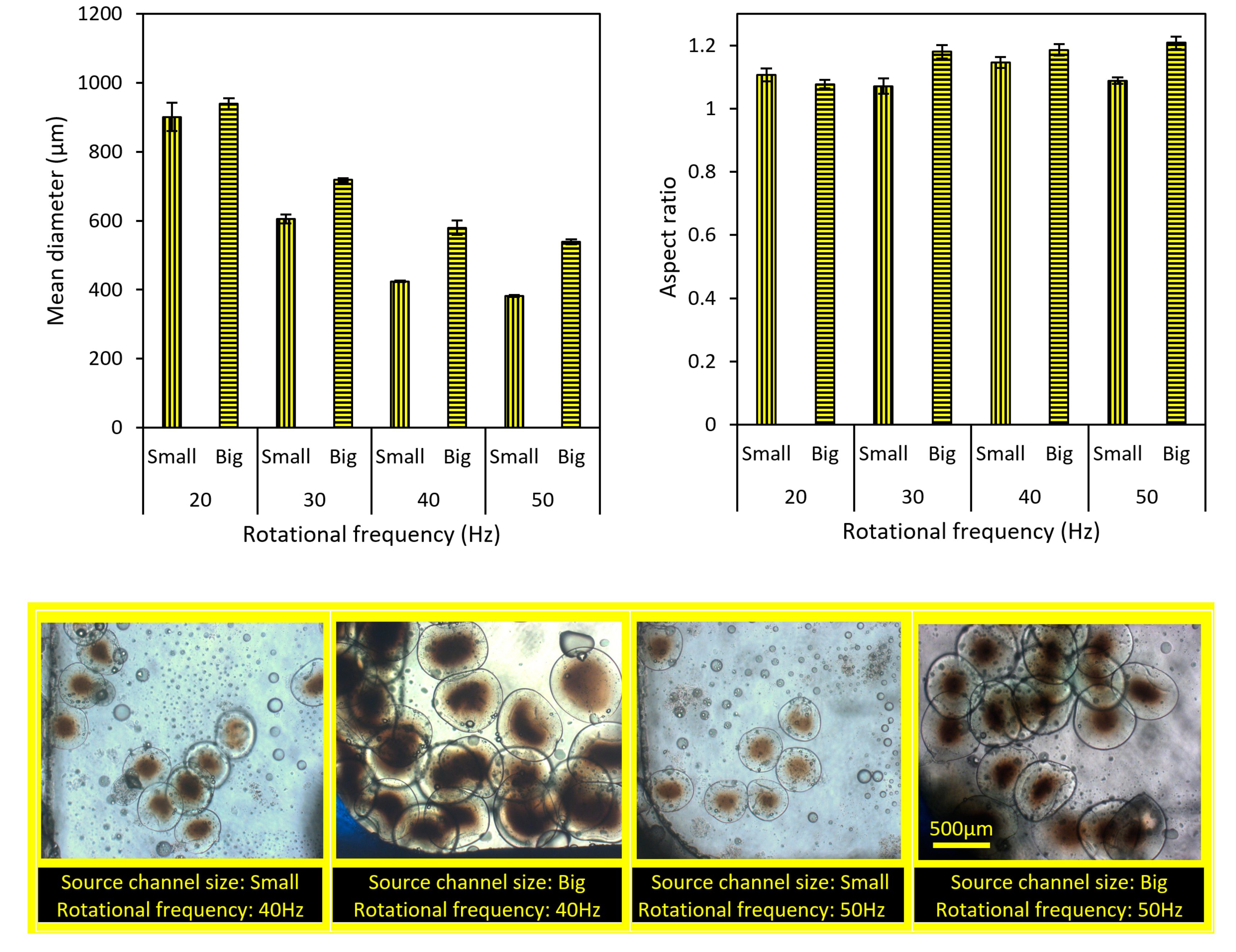
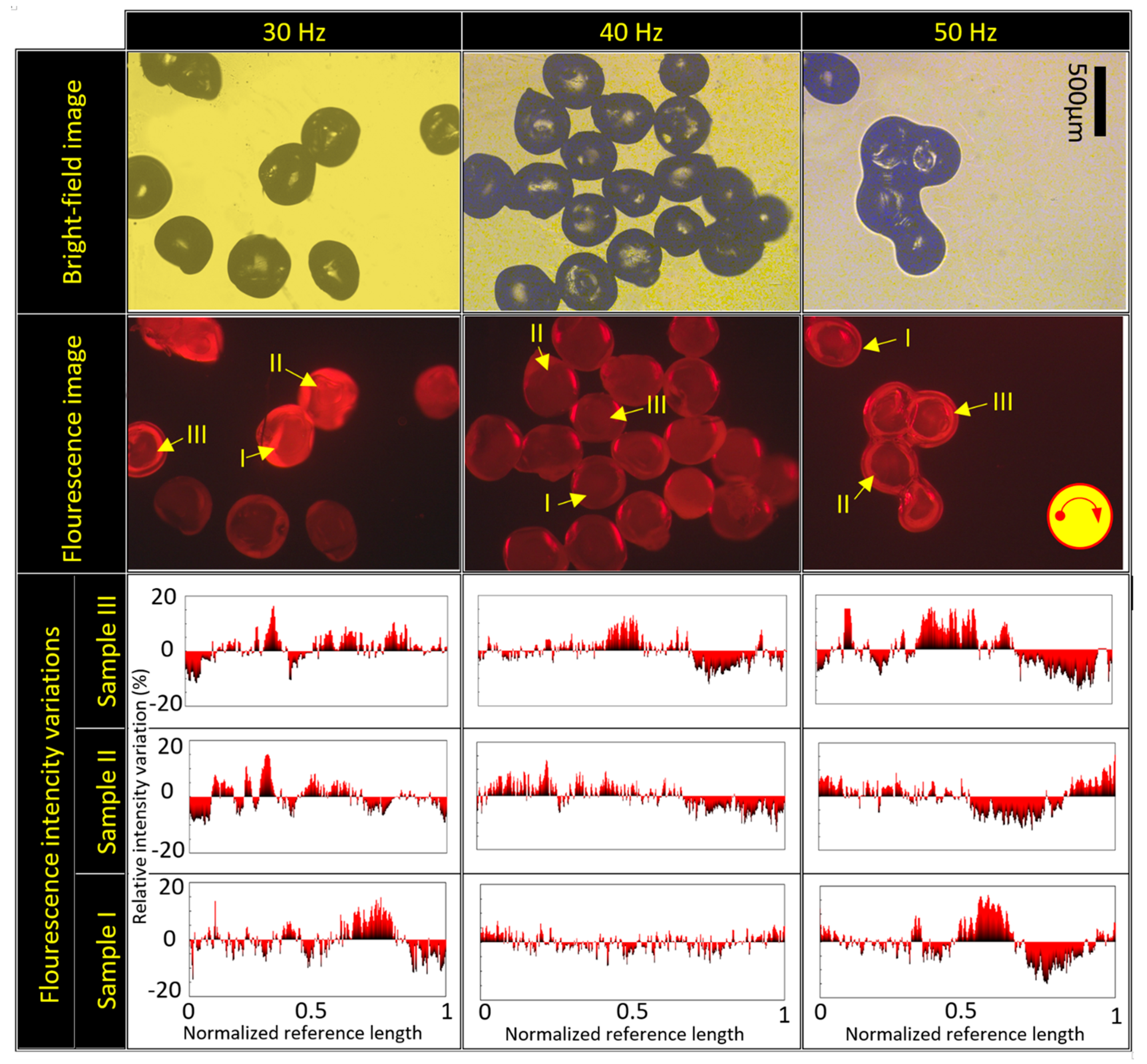
4.4. The Importance of Rotational Frequency on Particles Size and Aspect Ratio
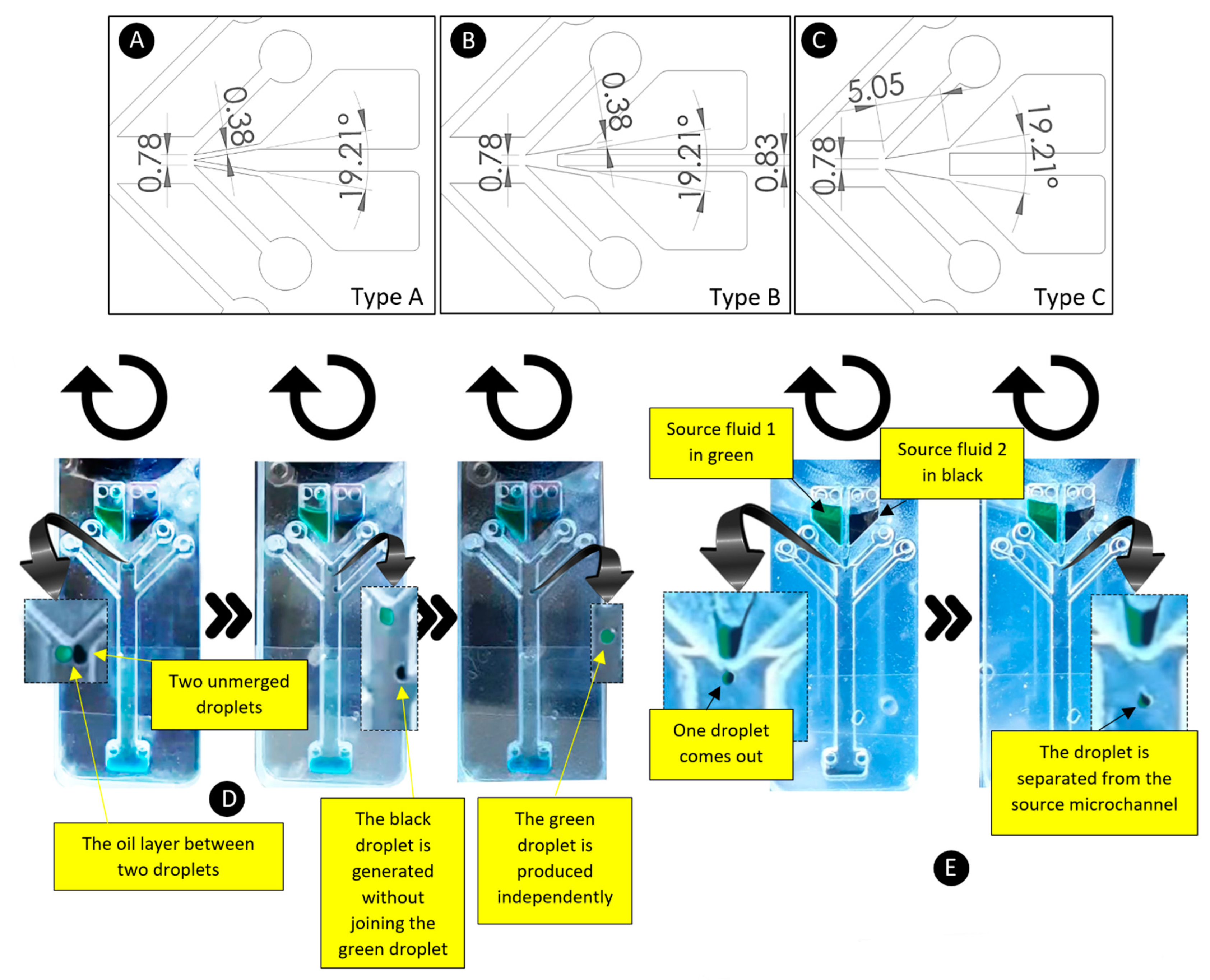
4.5. The Capability of Using More Than One Source Fluid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, C.; Fan, J.; Chen, D.; Xu, Y.; Wang, T. Microfluidics-generated graphene oxide microspheres and their application to removal of perfluorooctane sulfonate from polluted water. Nano Res. 2016, 9, 866–875. [Google Scholar] [CrossRef]
- Parsa, S.F.; Vafajoo, A.; Rostami, A.; Salarian, R.; Rabiee, M.; Rabiee, N.; Rabiee, G.; Tahriri, M.; Yadegari, A.; Vashaee, D.; et al. Early diagnosis of disease using microbead array technology: A review. Anal. Chim. Acta 2018, 1032, 1–17. [Google Scholar] [CrossRef]
- Verlinden, E.; Madadelahi, M.; Sarajlic, E.; Shamloo, A.; Engel, A.; Staufer, U.; Ghatkesar, M.K. Volume and concentration dosing in picolitres using a two-channel microfluidic AFM cantilever. Nanoscale 2020, 12, 10292–10305. [Google Scholar] [CrossRef]
- Madadelahi, M.; Kalan, K.; Shamloo, A. Designing a Polymerase Chain Reaction Device Working with Radiation and Convection Heat Transfer. IOP Conf. Series Mater. Sci. Eng. 2018, 350, 012010. [Google Scholar] [CrossRef] [Green Version]
- Burger, R.; Ducrée, J. Handling and analysis of cells and bioparticles on centrifugal microfluidic platforms. Expert Rev. Mol. Diagn. 2012, 12, 407–421. [Google Scholar] [CrossRef]
- Azimi-Boulali, J.; Madadelahi, M.; Madou, M.J.; Martinez-Chapa, S.O. Droplet and Particle Generation on Centrifugal Microfluidic Platforms: A Review. Micromachines 2020, 11, 603. [Google Scholar] [CrossRef]
- Chou, W.-L.; Lee, P.-Y.; Yang, C.-L.; Huang, W.-Y.; Lin, Y.-S. Recent Advances in Applications of Droplet Microfluidics. Micromachines 2015, 6, 1249–1271. [Google Scholar] [CrossRef] [Green Version]
- Madadelahi, M.; Shamloo, A. Newtonian and generalized Newtonian reacting flows in serpentine microchannels: Pressure driven and centrifugal microfluidics. J. Non-Newtonian Fluid Mech. 2018, 251, 88–96. [Google Scholar] [CrossRef]
- Ghazimirsaeed, E.; Madadelahi, M.; Dizani, M.; Shamloo, A. Secondary Flows, Mixing, and Chemical Reaction Analysis of Droplet-Based Flow inside Serpentine Microchannels with Different Cross Sections. Langmuir 2021, 37, 5118–5130. [Google Scholar] [CrossRef]
- Madadelahi, M.; Madou, M.J.; Nokoorani, Y.D.; Shamloo, A.; Martinez-Chapa, S.O. Fluidic barriers in droplet-based centrifugal microfluidics: Generation of multiple emulsions and microspheres. Sens. Actuators B Chem. 2020, 311, 127833. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.; Yang, L.-Y.; Ouyang, X.-K. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eral, H.B.; Safai, E.R.; Keshavarz, B.; Kim, J.J.; Lee, J.; Doyle, P.S. Governing Principles of Alginate Microparticle Synthesis with Centrifugal Forces. Langmuir 2016, 32, 7198–7209. [Google Scholar] [CrossRef] [PubMed]
- Debroy, D.; Li-Oakey, K.D.; Oakey, J. Engineering functional hydrogel microparticle interfaces by controlled oxygen-inhibited photopolymerization. Colloids Surfaces B Biointerfaces 2019, 180, 371–375. [Google Scholar] [CrossRef]
- Salahuddin, B.; Warren, H.; Spinks, G.M. Thermally actuated hydrogel bead based braided artificial muscle. Smart Mater. Struct. 2020, 29, 055042. [Google Scholar] [CrossRef]
- Lim, J.I. Fabrication of 3D Self-Assembled Porous Microbead-Type Poly(l-Lactide-co-ε-Caprolactone) Scaffolds for Tissue Engineering. 3D Print. Addit. Manuf. 2019, 6, 204–209. [Google Scholar] [CrossRef]
- Madadelahi, M.; Shamloo, A.; Salehi, S.S. Numerical Simulation of Bio-Chemical Diffusion in Bone Scaffolds. Int. J. Med. Health Sci. 2017, 11, 211–214. [Google Scholar]
- Lalegül-Ülker, Ö.; Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Encapsulation of bone marrow-MSCs in PRP-derived fibrin microbeads and preliminary evaluation in a volumetric muscle loss injury rat model: Modular muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 47, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Younis, S.A.; Motawea, E.A.; Moustafaab, Y.M.; Leed, J.; Kimc, K.H. A strategy for the efficient removal of chlorophenols in petrochemical wastewater by organophilic and aminated silica@alginate microbeads: Taguchi optimization and isotherm modeling based on partition coefficient. J. Hazard. Mater. 2020, 397, 122792. [Google Scholar] [CrossRef]
- Yu, D.; Dong, Z.; Lim, H.; Chen, Y.; Ding, Z.; Sultana, N.; Wu, J.; Qin, B.; Cheng, J.; Li, W. Microfluidic preparation, shrinkage, and surface modification of monodispersed alginate microbeads for 3D cell culture. RSC Adv. 2019, 9, 11101–11110. [Google Scholar] [CrossRef] [Green Version]
- Mark, D.; Haeberle, S.; Zengerle, R.; Ducrée, J.; Vladisavljevic, G. Manufacture of chitosan microbeads using centrifugally driven flow of gel-forming solutions through a polymeric micronozzle. J. Colloid Interface Sci. 2009, 336, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Yao, T.; Ji, X.; Zeng, C.; Wang, C.; Zhang, L. Versatile Preparation of Nonspherical Multiple Hydrogel Core PAM/PEG Emulsions and Hierarchical Hydrogel Microarchitectures. Angew. Chem. Int. Ed. 2014, 53, 7504–7509. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.-H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef]
- Tani, M.; Kawano, R.; Kamiya, K.; Okumura, K. Towards combinatorial mixing devices without any pumps by open-capillary channels: Fundamentals and applications. Sci. Rep. 2015, 5, srep10263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajgiran, K.R.; Cordova, A.S.; Elkhanoufi, R.; Dorman, J.A.; Melvin, A.T. Simultaneous Droplet Generation with In-Series Droplet T-Junctions Induced by Gravity-Induced Flow. Micromachines 2021, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, Y.-A.; Skipper, P.L.; Han, J. Electrohydrodynamic Generation and Delivery of Monodisperse Picoliter Droplets Using a Poly(dimethylsiloxane) Microchip. Anal. Chem. 2006, 78, 8011–8019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Yoshida, K.; Kim, J.-W. Developing O/O (oil-in-oil) droplet generators on a chip by using ECF (electro-conjugate fluid) micropumps. Sensors Actuators B Chem. 2019, 296, 126669. [Google Scholar] [CrossRef]
- Varma, V.; Ray, A.; Wang, Z.M.; Ramanujan, R.V. Droplet Merging on a Lab-on-a-Chip Platform by Uniform Magnetic Fields. Sci. Rep. 2016, 6, 37671. [Google Scholar] [CrossRef]
- Haghighi, M.A.; Emdad, H.; Jafarpur, K.; Ziaei, A.N. Numerical study of MHD effects on drop formation in a t-shaped microchannel. Int. J. Mech. Mechatron. Eng. 2012, 6, 180–183. [Google Scholar]
- Qian, S.; Bau, H.H. Magneto-hydrodynamics based microfluidics. Mech. Res. Commun. 2009, 36, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Haeberle, S.; Naegele, L.; Burger, R.; Von Stetten, F.; Zengerle, R.; Ducrée, J. Alginate bead fabrication and encapsulation of living cells under centrifugally induced artificial gravity conditions. J. Microencapsul. 2008, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Onuki, M.; Takeuchi, S. Mass Production of Cell-Laden Calcium Alginate Particles with Centrifugal Force. Adv. Healthc. Mater. 2017, 6, 1601375. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, X.T.; Yang, C.G.; Xu, Z.R. On-chip preparation of calcium alginate particles based on droplet templates formed by using a centrifugal microfluidic technique. J. Colloid Interface Sci. 2015, 466, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Cao, Y.; Tian, C.; Li, Y.; Wang, M.; Zhao, Y.; Zhao, G. Centrifugal microfluidics for ultra-rapid fabrication of versatile hydrogel microcarriers. Appl. Mater. Today 2018, 13, 116–125. [Google Scholar] [CrossRef]
- Madadelahi, M.; Shamloo, A. Droplet-based flows in serpentine microchannels: Chemical reactions and secondary flows. Int. J. Multiph. Flow 2017, 97, 186–196. [Google Scholar] [CrossRef]
- Sugiura, S.; Oda, T.; Izumida, Y.; Aoyagi, Y.; Satake, M.; Ochiai, A.; Ohkohchi, N.; Nakajima, M. Size control of calcium alginate beads containing living cells using micro-nozzle array. Biomaterials 2004, 26, 3327–3331. [Google Scholar] [CrossRef]
- de Vos, P.; van Hoogmoed, C.G.; van Zanten, J.; Netter, S.; Strubbe, J.H.; Busscher, H.J. Long-term biocompatibility, chemistry, and function of microencapsulated pancreatic islets. Biomaterials 2002, 24, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Tice, J.D.; Song, H.; Lyon, A.D.; Ismagilov, R.F. Formation of Droplets and Mixing in Multiphase Microfluidics at Low Values of the Reynolds and the Capillary Numbers. Langmuir 2003, 19, 9127–9133. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madadelahi, M.; Azimi-Boulali, J.; Madou, M.; Martinez-Chapa, S.O. Characterization of Fluidic-Barrier-Based Particle Generation in Centrifugal Microfluidics. Micromachines 2022, 13, 881. https://doi.org/10.3390/mi13060881
Madadelahi M, Azimi-Boulali J, Madou M, Martinez-Chapa SO. Characterization of Fluidic-Barrier-Based Particle Generation in Centrifugal Microfluidics. Micromachines. 2022; 13(6):881. https://doi.org/10.3390/mi13060881
Chicago/Turabian StyleMadadelahi, Masoud, Javid Azimi-Boulali, Marc Madou, and Sergio Omar Martinez-Chapa. 2022. "Characterization of Fluidic-Barrier-Based Particle Generation in Centrifugal Microfluidics" Micromachines 13, no. 6: 881. https://doi.org/10.3390/mi13060881
APA StyleMadadelahi, M., Azimi-Boulali, J., Madou, M., & Martinez-Chapa, S. O. (2022). Characterization of Fluidic-Barrier-Based Particle Generation in Centrifugal Microfluidics. Micromachines, 13(6), 881. https://doi.org/10.3390/mi13060881








