Fabrication of a New Electrochemical Sensor Based on Bimetal Oxide for the Detection of Furazolidone in Biological Samples
Abstract
:1. Introduction
2. Experiments
2.1. Reagents
2.2. Material Characterization
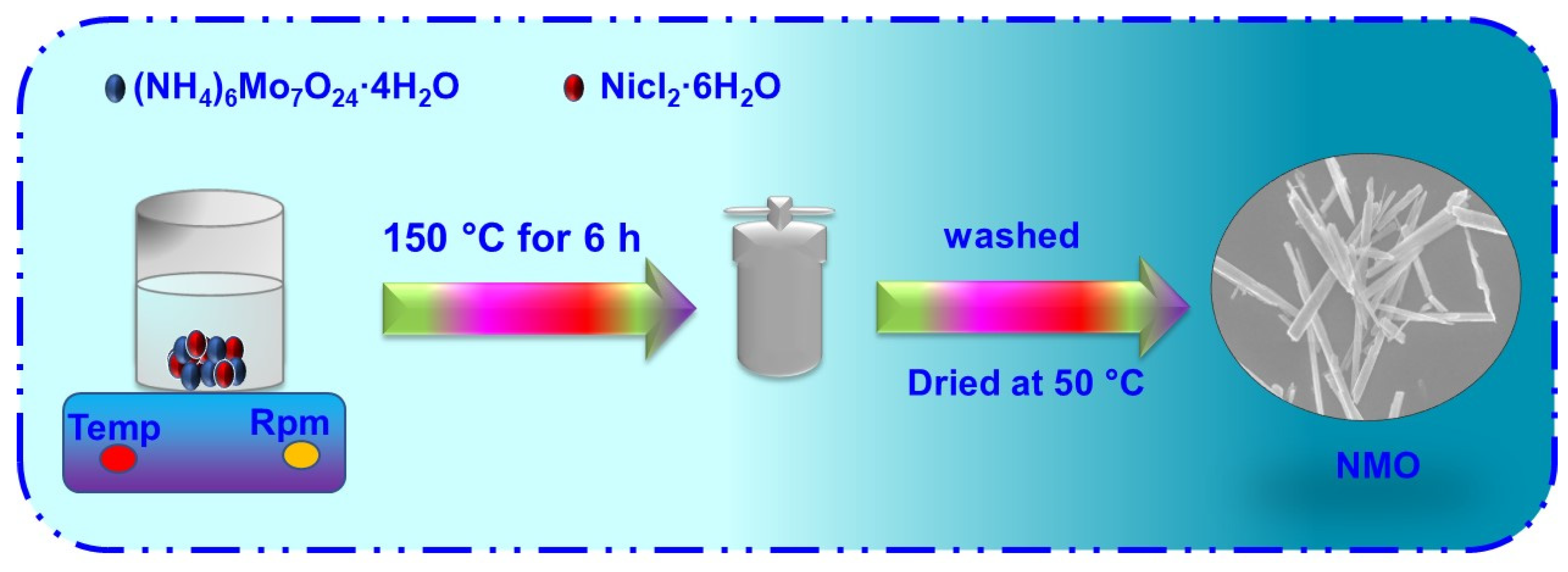
2.3. Synthesis of NMO Rods
2.4. Fabrication of Electrodes
2.5. Choice of Material
3. Result and Discussion
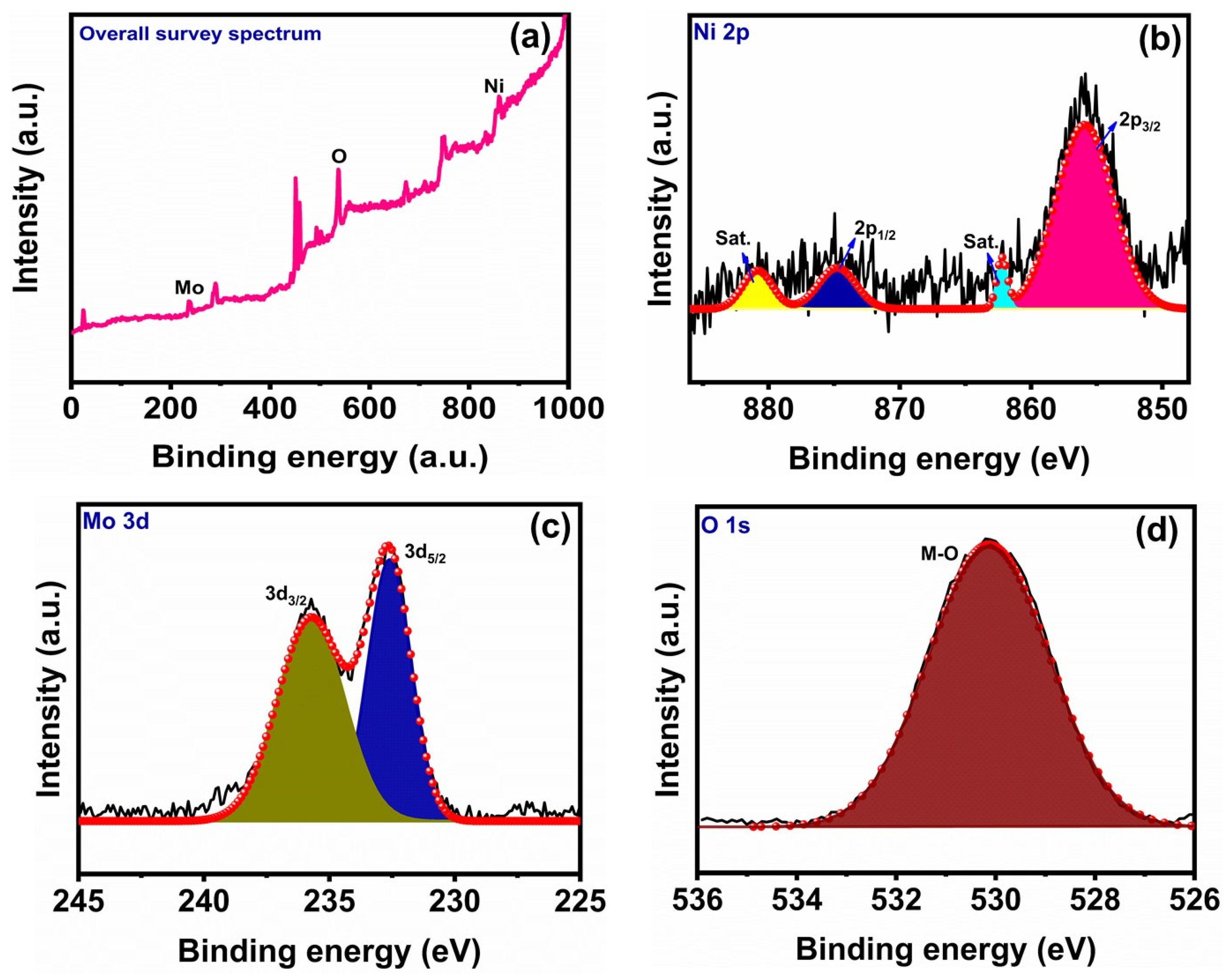
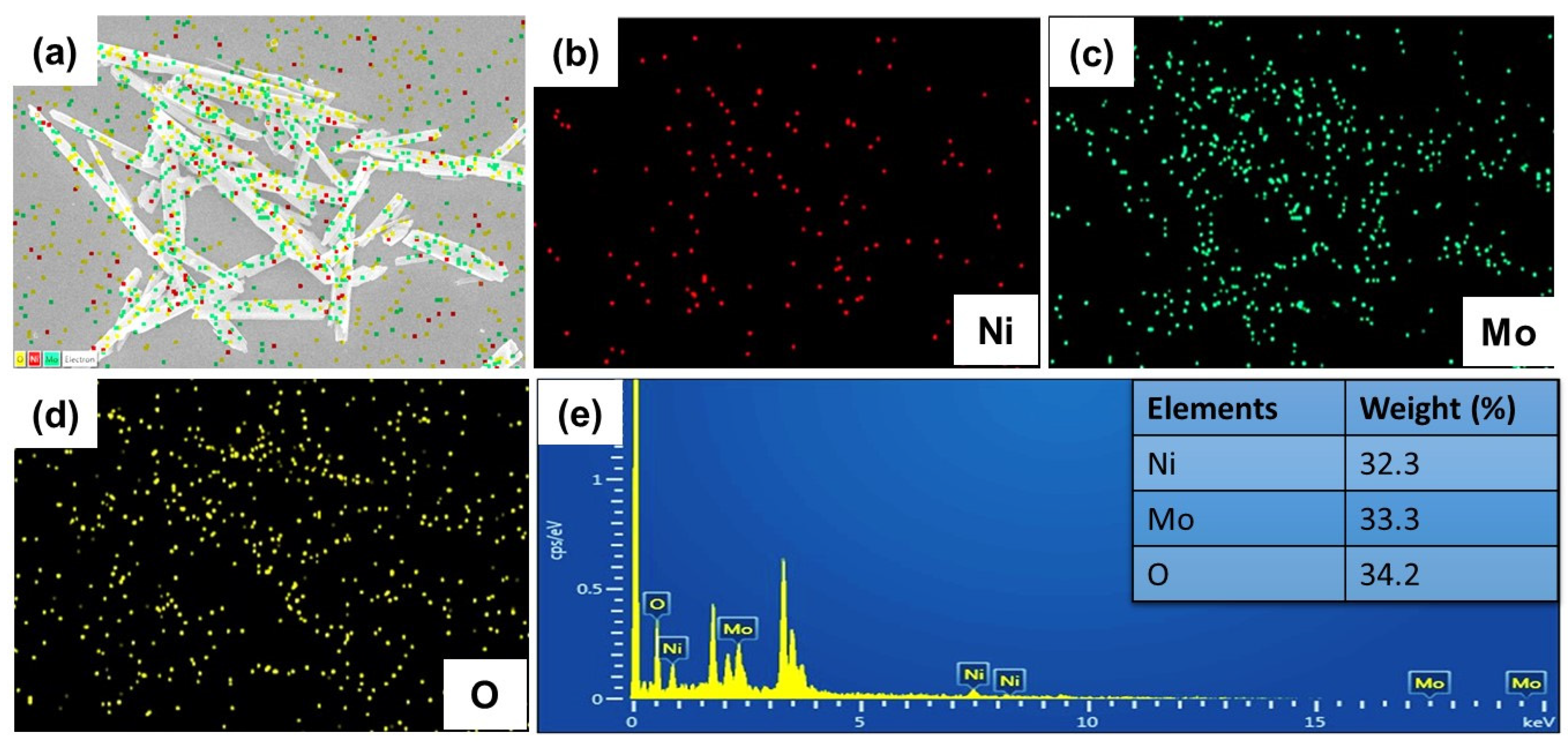
3.1. Structure and Morphology of NMO Rods
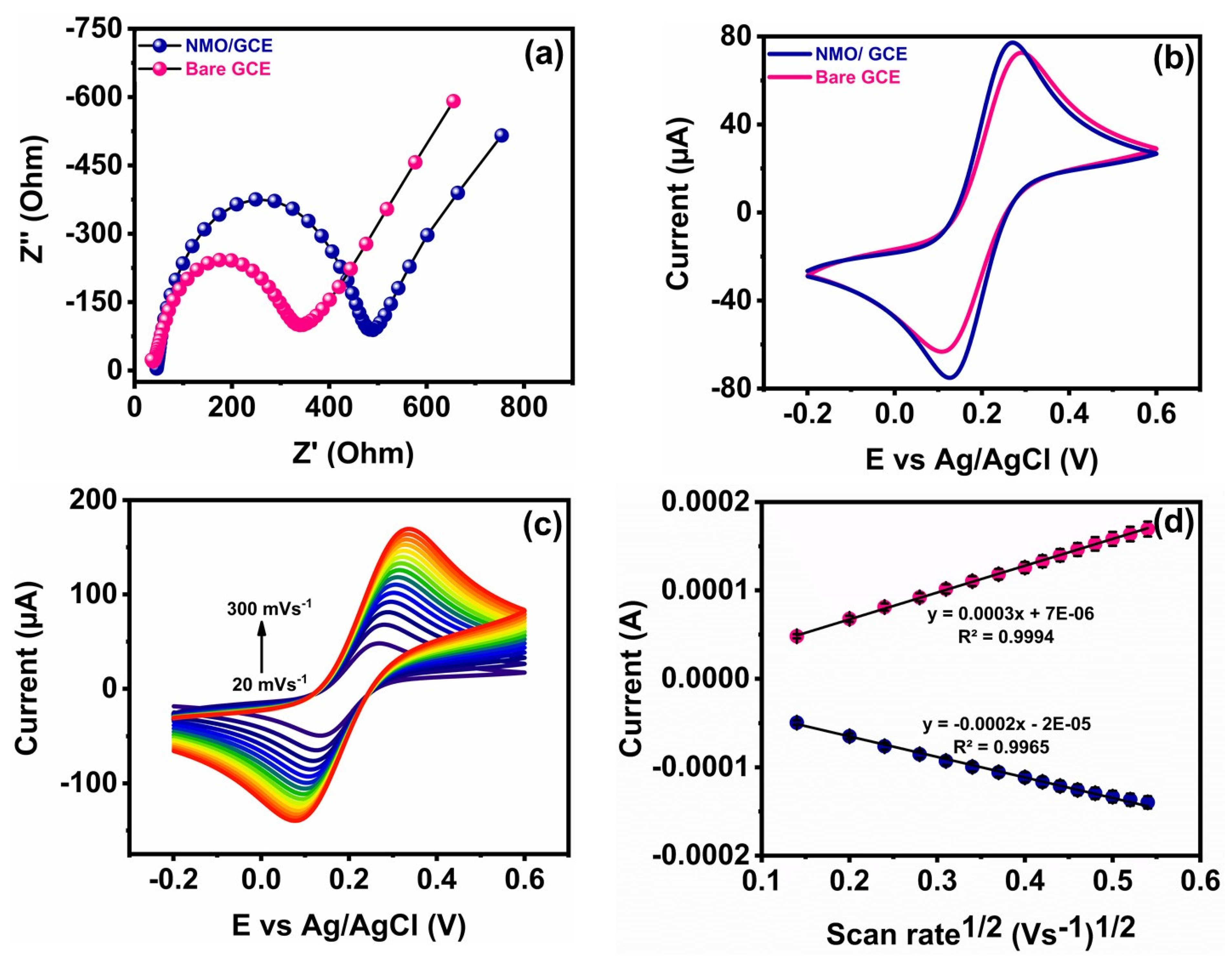
3.2. Electrochemical Impedance Study
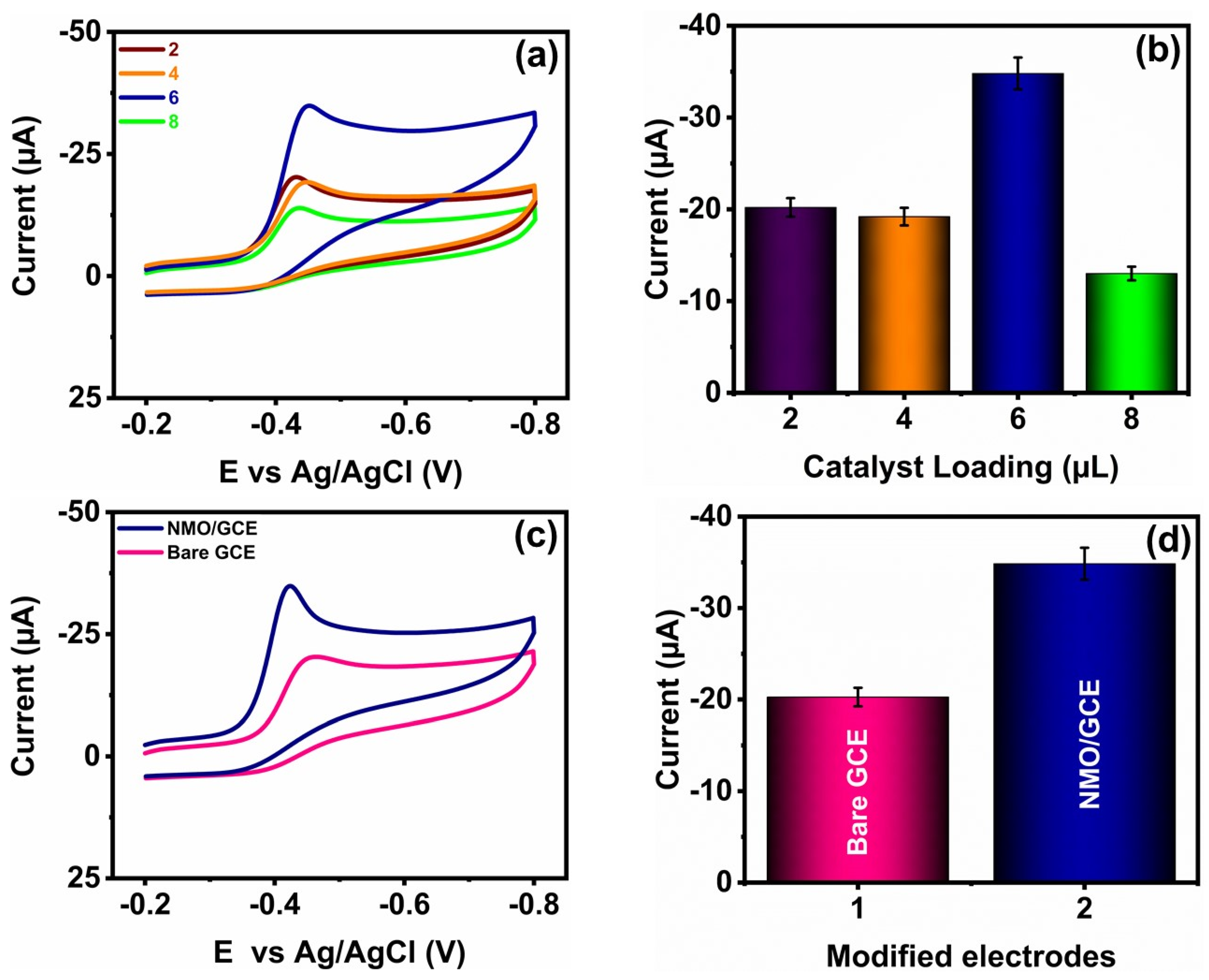
3.3. Electrochemical Behavior of GCE and Modified GCE
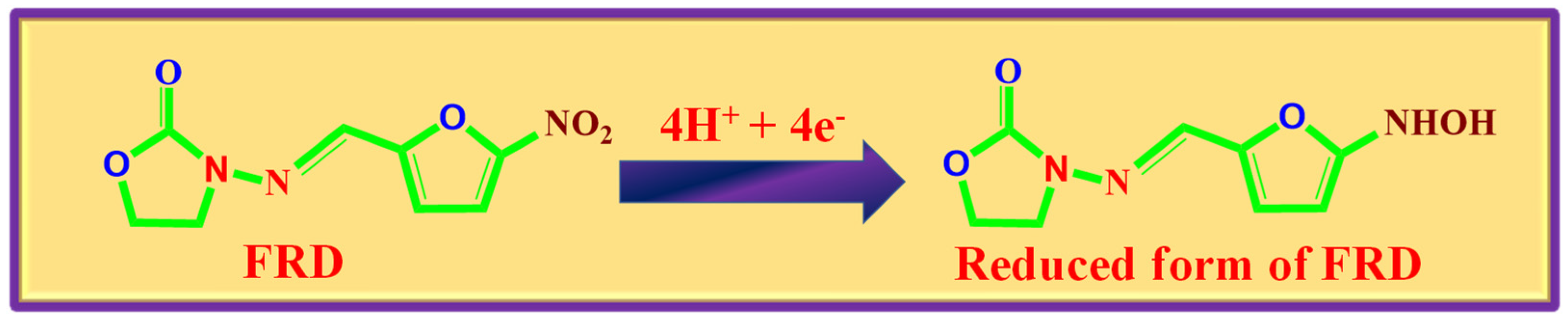
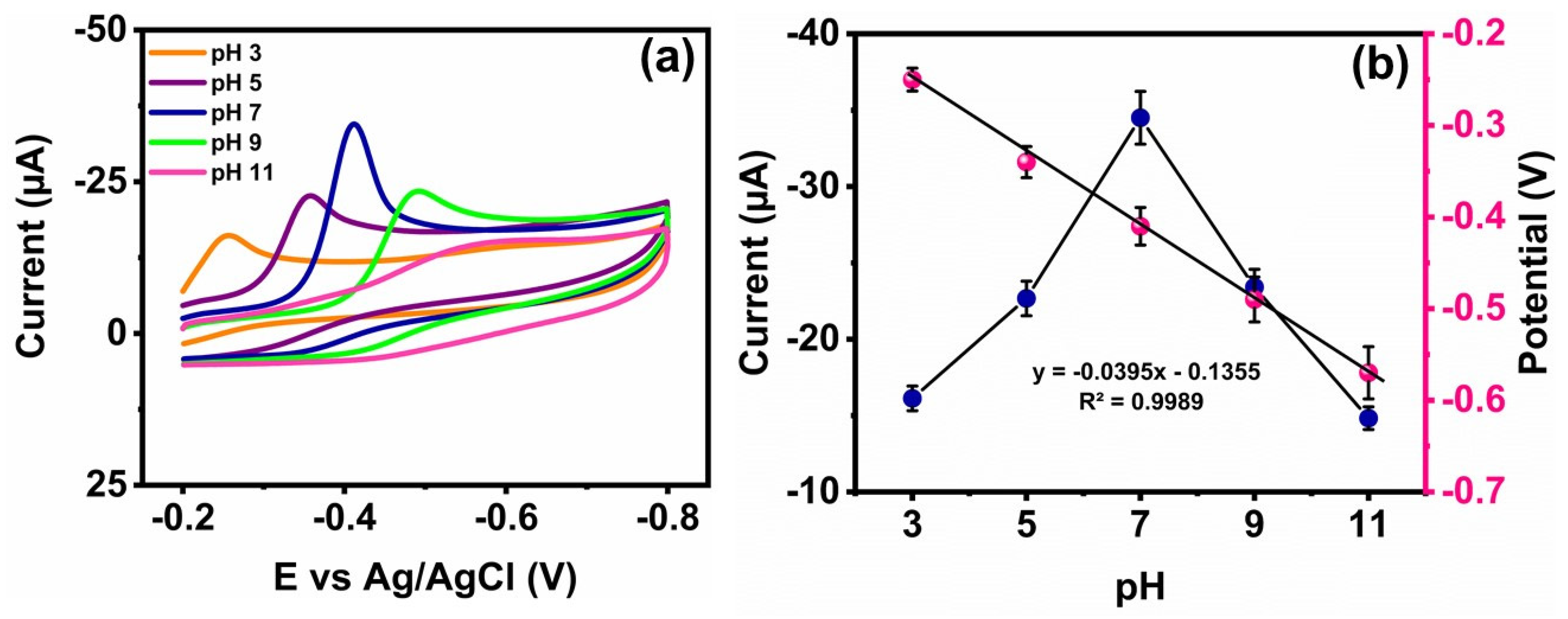
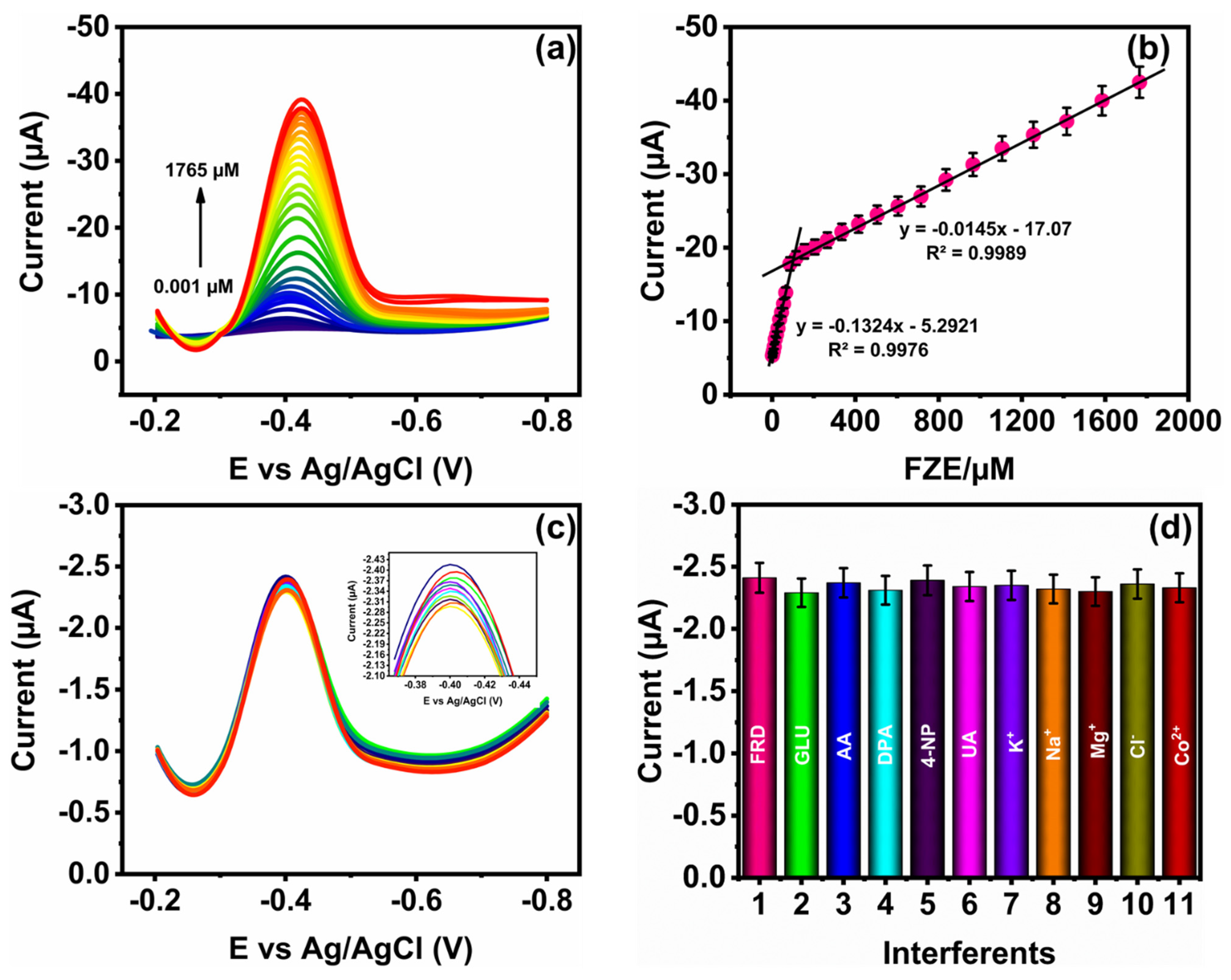
3.4. Detection of FZE
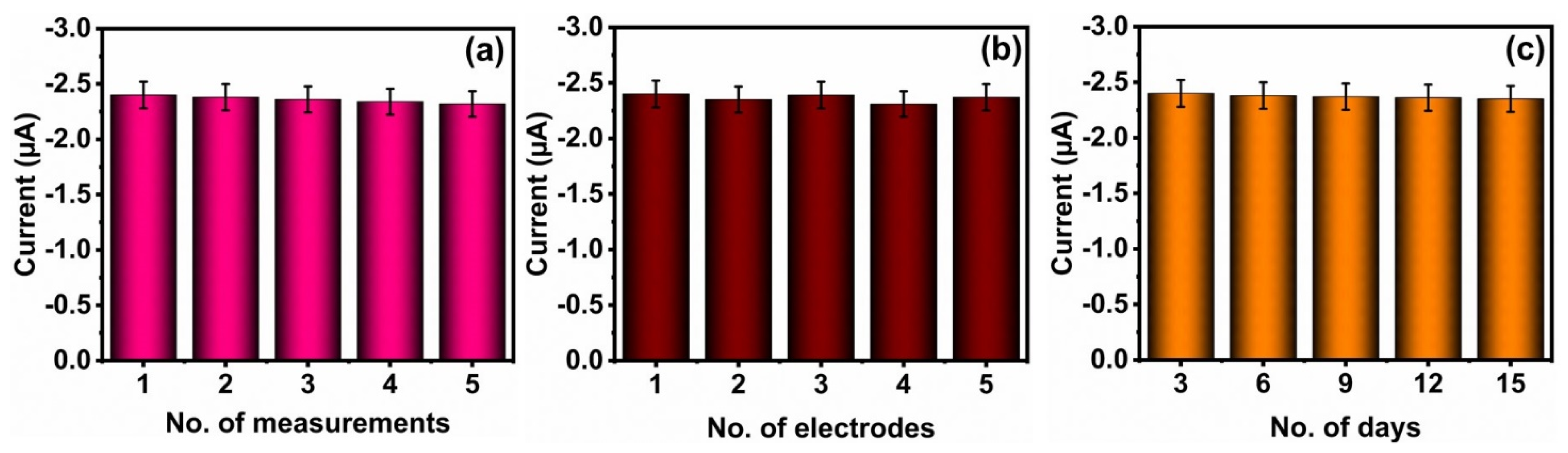
3.5. Selectivity, Repeatability, Reproducibility, Long-Term Stability Studies, and Real Sample Analysis of FZE Using NMO/GCE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, B.-S.; Du, G.-A. A simple and sensitive electrochemical detection of furazolidone based on an Au nanoparticle functionalized graphene modified electrode. Anal. Methods 2017, 9, 4341–4348. [Google Scholar] [CrossRef]
- Done, H.Y.; Venkatesan, A.K.; Halden, R.U. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J. 2015, 17, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.; Jain, N.; Jain, D.K.; Patel, V.K.; Rajak, H.; Jain, S.K. Novel UV spectrophotometer methods for quantitative estimation of metronidazole and furazolidone using mixed hydrotropy solubilization. Arab. J. Chem. 2017, 10, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Stehly, G.R.; Plakas, S.M.; El Said, K.R. Liquid chromatographic determination of furazolidone in shrimp. J. AOAC Int. 1994, 77, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Toudeshki, R.M.; Dadfarnia, S.; Shabani, A.M.H. Chemiluminescence determination of furazolidone in poultry tissues and water samples after selective solid phase microextraction using magnetic molecularly imprinted polymers. New J. Chem. 2018, 42, 10751–10760. [Google Scholar] [CrossRef]
- Rodziewicz, L. Determination of nitrofuran metabolites in milk by liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2008, 864, 156–160. [Google Scholar] [CrossRef]
- Maheshwaran, S.; Balaji, R.; Chen, S.-M.; Chang, Y.-S.; Tamilalagan, E.; Chandrasekar, N.; Ethiraj, S.; Samuel, M.S.; Kumar, M. Ultrasensitive electrochemical detection of furazolidone in biological samples using 1D-2D BiVO4@ MoS2 hierarchical nano-heterojunction composites armed electrodes. Environ. Res. 2022, 205, 112515. [Google Scholar] [CrossRef]
- Bhuvaneswari, C.; Babu, S.G. Nanoarchitecture and surface engineering strategy for the construction of 3D hierarchical CuS-rGO/g-C3N4 nanostructure: An ultrasensitive and highly selective electrochemical sensor for the detection of furazolidone drug. J. Electroanal. Chem. 2022, 907, 116080. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Huang, H.; Li, Q.; Wang, T. Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 12905–12910. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Rapid degradation of naproxen by AgBr-α-NiMoO4 composite photocatalyst in visible light: Mechanism and pathways. Chem. Eng. J. 2018, 347, 836–848. [Google Scholar] [CrossRef]
- Park, J.-S.; Cho, J.S.; Kang, Y.C. Scalable synthesis of NiMoO4 microspheres with numerous empty nanovoids as an advanced anode material for Li-ion batteries. J. Power Sources 2018, 379, 278–287. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Ji, S.; Wang, X.; Zhou, P.; Huo, S.; Linkov, V.; Wang, R. A high faraday efficiency NiMoO4 nanosheet array catalyst by adjusting the hydrophilicity for overall water splitting. Chem.–A Eur. J. 2020, 26, 12067–12074. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, R.; Mariyappan, V.; Chen, S.-M.; Keerthi, M.; Ramachandran, R. Electrochemical sensor for detection of tryptophan in the milk sample based on MnWO4 nanoplates encapsulated RGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126889. [Google Scholar] [CrossRef]
- Kannan, P.; Chen, F.; Jiang, H.; Wang, H.; Wang, R.; Subramanian, P.; Ji, S.J.A. Hierarchical core–shell structured Ni3S2/NiMoO4 nanowires: A high-performance and reusable electrochemical sensor for glucose detection. Analyst 2019, 144, 4925–4934. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.; Yu, X.; Li, Q.; Wang, T. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Sankar, K.V.; Selvan, R.K.; Danielle, M.; Manickam, M. Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. Rsc Adv. 2013, 3, 352–357. [Google Scholar] [CrossRef]
- Yesuraj, J.; Padmaraj, O.; Suthanthiraraj, S.A. Synthesis, characterization, and improvement of supercapacitor properties of NiMoO4 nanocrystals with polyaniline. J. Inorg. Organomet. Polym. Mater. 2020, 30, 310–321. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, P.; Luo, J.; Xu, W.; Li, J.; Yuan, D. Facile synthesis of graphene@ NiMoO4 nanosheet arrays on Ni foam for a high-performance asymmetric supercapacitor. J. Mater. Sci. 2017, 52, 13909–13919. [Google Scholar] [CrossRef]
- Mariyappan, V.; Keerthi, M.; Chen, S.-M. Highly Selective Electrochemical Sensor Based on Gadolinium Sulfide Rod-Embedded RGO for the Sensing of Carbofuran. J. Agric. Food Chem. 2021, 69, 2679–2688. [Google Scholar] [CrossRef]
- Jothi, P.R.; Shanthi, K.; Salunkhe, R.R.; Pramanik, M.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y. Synthesis and Characterization of α-NiMoO4 Nanorods for Supercapacitor Application. Eur. J. Inorg. Chem. 2015, 2015, 3694–3699. [Google Scholar] [CrossRef]
- Sudha, K.; Karthikaikumar, S.; Abinaya, K.; Sundharamurthi, S.; Kalimuthu, P. Surface Modification of TiO2 Nano-Particles Coated on ITO Glass Plate by Chemisorption of Cyanoacetic Acid. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=Surface+Modification+of+TiO2+Nano-Particles+Coated+on+ITO+Glass+Plate+by+Chemisorption+of+Cyanoacetic+Acid&btnG= (accessed on 29 May 2022).
- Mariyappan, V.; Murugan, K.; Chen, S.-M. Solvothermal synthesis of carbon incorporated MnS2 Spheres; high sensing performance towards the detection of furazolidone in bio-fluids. J. Alloys Compd. 2021, 882, 160744. [Google Scholar] [CrossRef]
- Koventhan, C.; Pandiyarajan, S.; Chen, S.-M. Simple sonochemical synthesis of flake-ball shaped bismuth vanadate for voltammetric detection of furazolidone. J. Alloys Compd. 2022, 895, 162315. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Wang, S.-F.; Govindasamy, M.; George, M.; Jothiramalingam, R. Cobalt molybdate nanorods decorated on boron-doped graphitic carbon nitride sheets for electrochemical sensing of furazolidone. Microchim. Acta 2020, 187, 654. [Google Scholar] [CrossRef] [PubMed]
- Rajakumaran, R.; Krishnapandi, A.; Chen, S.-M.; Balamurugan, K.; Chang, F.M.; Sakthinathan, S. Electrochemical investigation of zinc tungstate nanoparticles; a robust sensor platform for the selective detection of furazolidone in biological samples. Microchem. J. 2021, 160, 105750. [Google Scholar] [CrossRef]



| Electrodes | Linear Range (µM) | LOD (µM) | Methods | Preparation Method | Reference |
|---|---|---|---|---|---|
| MnS2@N-CS a | 0.001–1590 | 0.0041 | DPV i | Solvothermal | [22] |
| MoS2@BiV-MRs b | 0.01–14 and 14–614 | 0.0029 | DPV | Hydrothermal | [7] |
| BV/SPCE c | 0.1–1189.8 | 0.016 | DPV | Sonochemical | [23] |
| CuS-rGO d/g-C3N4 e/GCE f | 0.1–336.4 | 0.0108 | DPV | Solvothermal | [8] |
| CoMoO4/BCN g/SPCE | 0.04–408.9 | 0.0016 | DPV | Sonochemical | [24] |
| ZW NPs h/SPCE | 0.1–849 | 0.013 | DPV | Ultrasonic-assisted co-precipitation | [25] |
| NMO/GCE | 0.001–1765 | 0.022 | DPV | Hydrothermal | This work |
| Real Sample | Added (µM) | Found (µM) | Recovery (%) | RSD * |
|---|---|---|---|---|
| 0 | - | - | - | |
| Human serum | 5 | 4.96 | 99.2 | 2.6 |
| 10 | 9.89 | 98.9 | 2.8 | |
| 15 | 14.80 | 98.6 | 2.5 | |
| 0 | - | - | - | |
| Urine | 5 | 4.97 | 99.4 | 2.3 |
| 10 | 9.85 | 98.5 | 2.6 | |
| 15 | 14.94 | 99.6 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaresan, R.; Mariyappan, V.; Chen, S.-M.; Alagarsamy, S.; Akilarasan, M. Fabrication of a New Electrochemical Sensor Based on Bimetal Oxide for the Detection of Furazolidone in Biological Samples. Micromachines 2022, 13, 876. https://doi.org/10.3390/mi13060876
Sundaresan R, Mariyappan V, Chen S-M, Alagarsamy S, Akilarasan M. Fabrication of a New Electrochemical Sensor Based on Bimetal Oxide for the Detection of Furazolidone in Biological Samples. Micromachines. 2022; 13(6):876. https://doi.org/10.3390/mi13060876
Chicago/Turabian StyleSundaresan, Ruspika, Vinitha Mariyappan, Shen-Ming Chen, Saranvignesh Alagarsamy, and Muthumariappan Akilarasan. 2022. "Fabrication of a New Electrochemical Sensor Based on Bimetal Oxide for the Detection of Furazolidone in Biological Samples" Micromachines 13, no. 6: 876. https://doi.org/10.3390/mi13060876
APA StyleSundaresan, R., Mariyappan, V., Chen, S.-M., Alagarsamy, S., & Akilarasan, M. (2022). Fabrication of a New Electrochemical Sensor Based on Bimetal Oxide for the Detection of Furazolidone in Biological Samples. Micromachines, 13(6), 876. https://doi.org/10.3390/mi13060876







