Feature Extraction of 3T3 Fibroblast Microtubule Based on Discrete Wavelet Transform and Lucy–Richardson Deconvolution Methods
Abstract
1. Introduction
1.1. Research Background
1.2. Previous Works
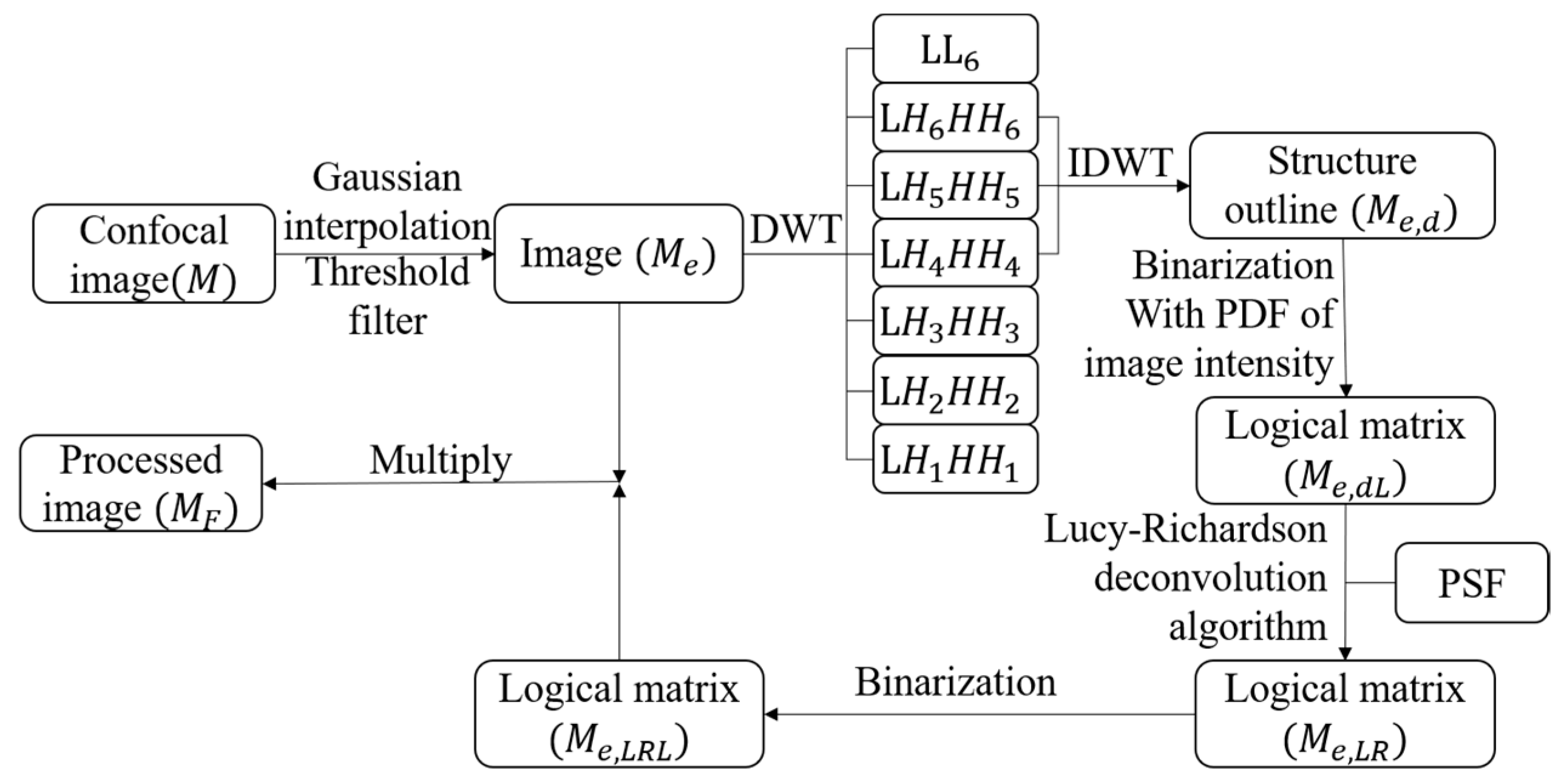
2. Methodological Principles and Process
2.1. Target of Image Process
2.2. Methods and Process
2.2.1. Expansion of Image by Gaussian Interpolation
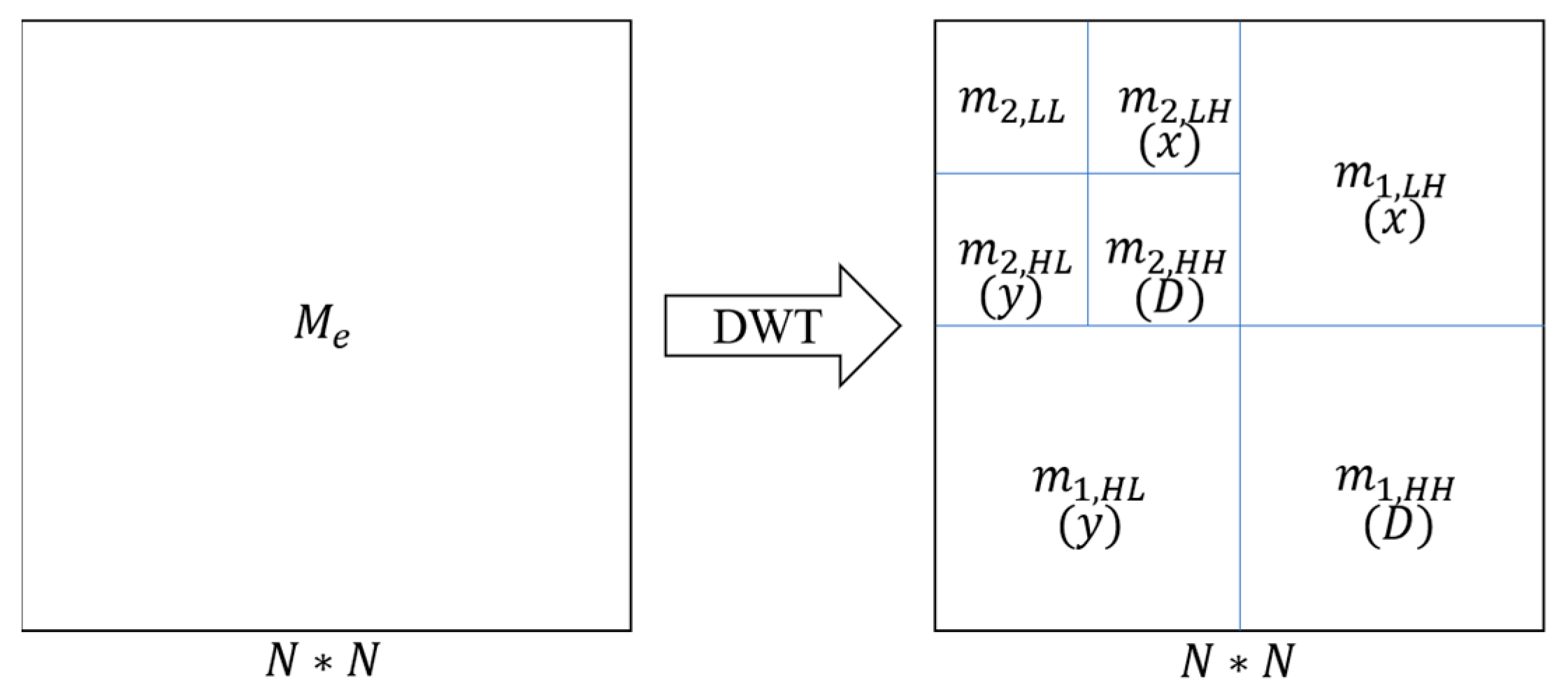
2.2.2. Extraction of Microtubule Structures by DWT
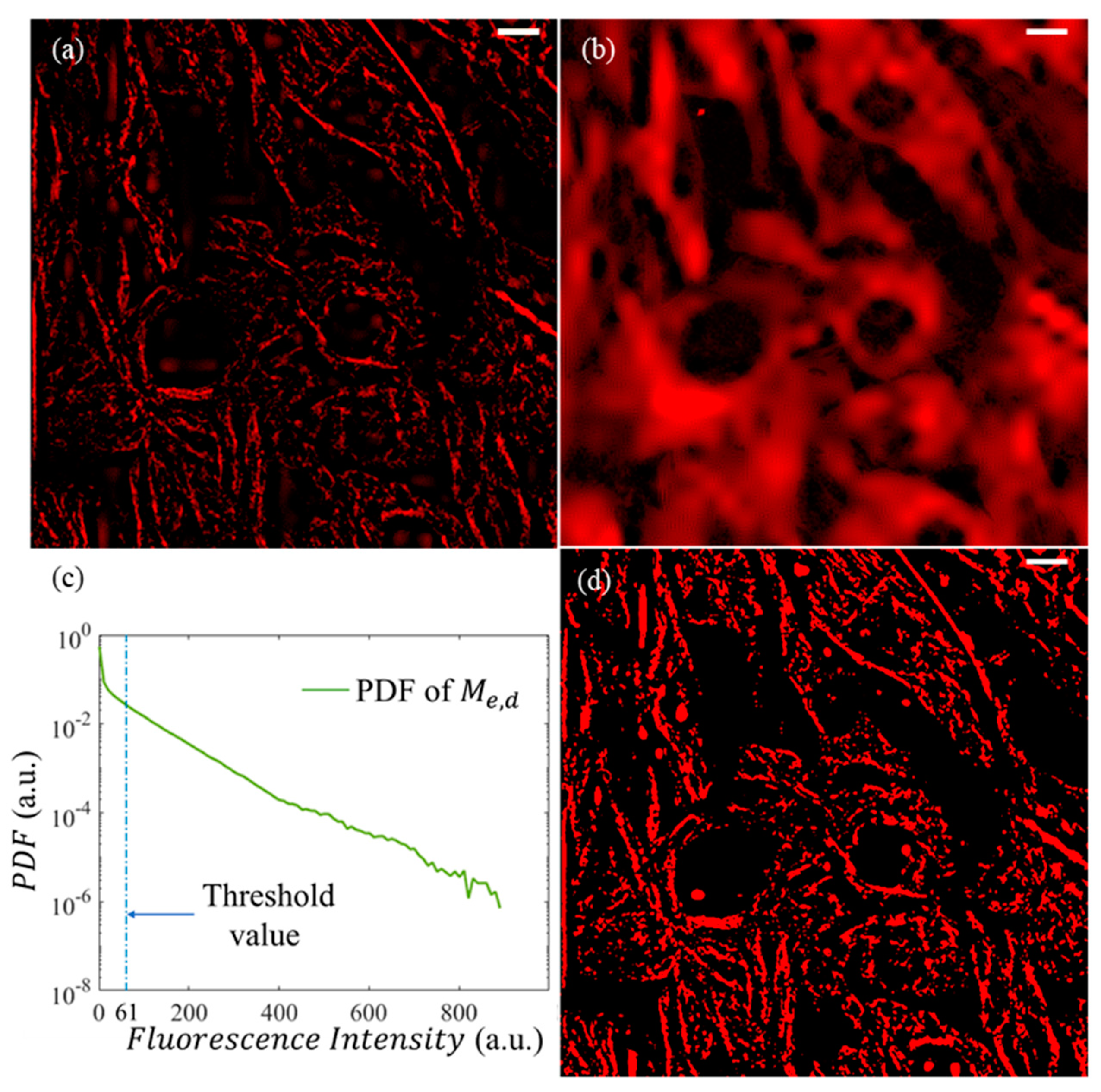
2.2.3. Binarization of Image
2.2.4. Resolution Improvement by Lucy–Richardson (LR) Deconvolution Method
2.2.5. Post-Processing
3. Experimental Results
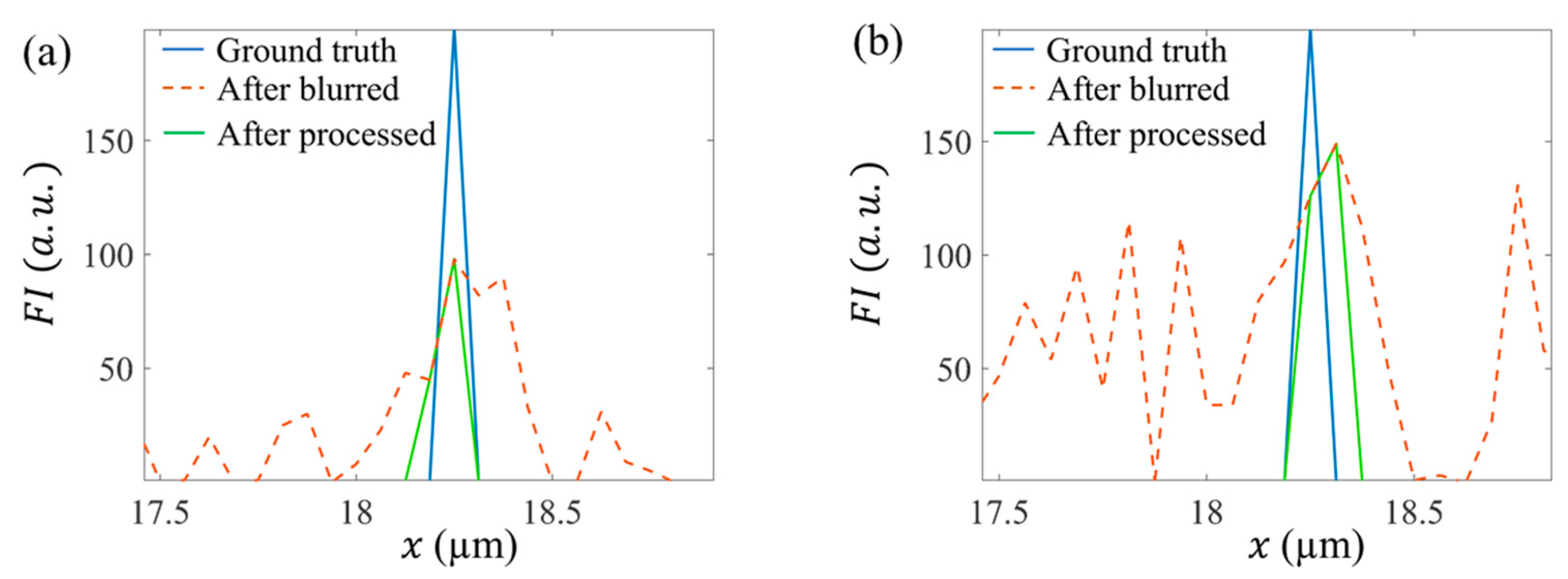
3.1. Ground Truth Verification
3.2. Expansion of Image by Gaussian Interpolation
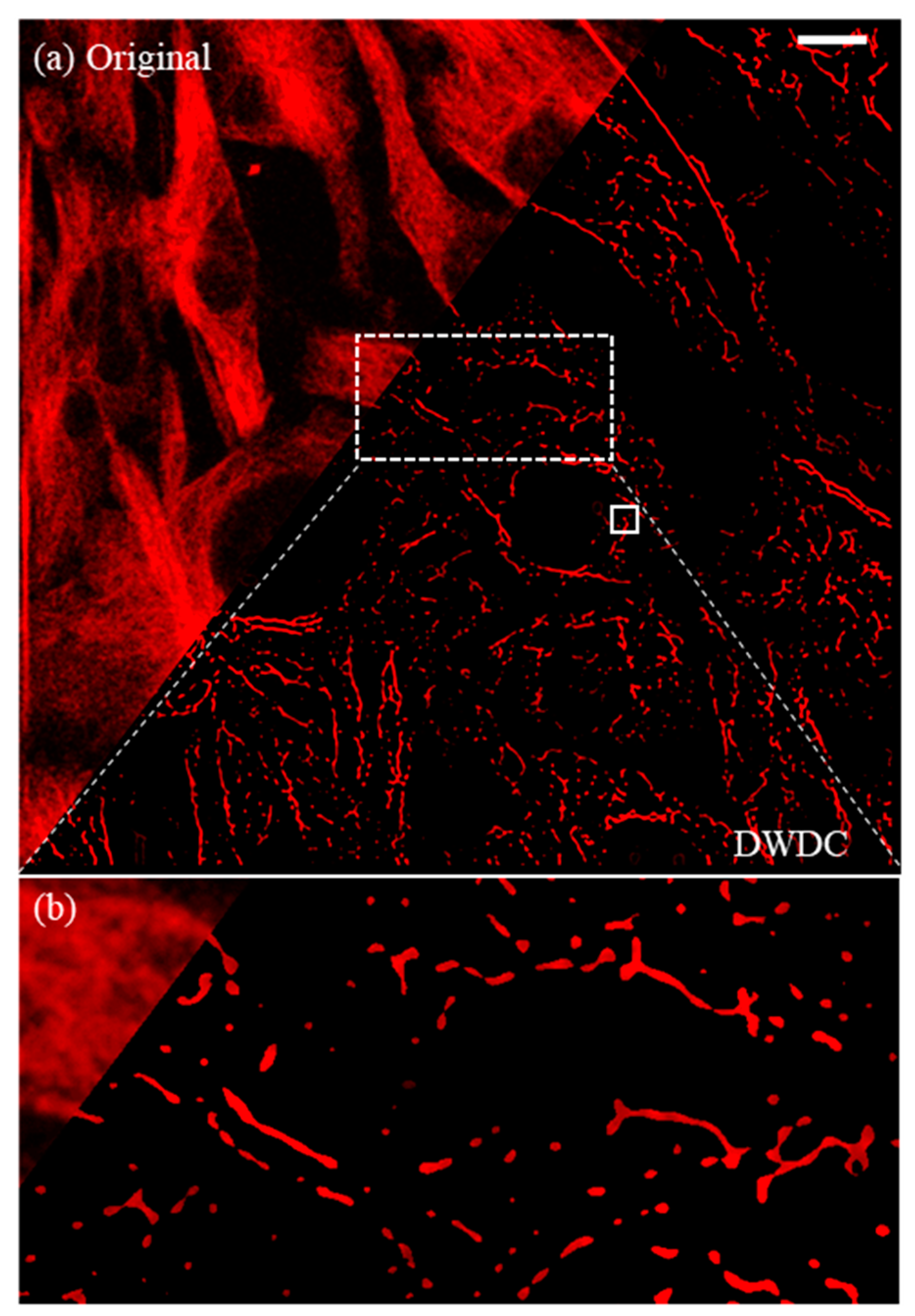
3.3. Extraction of Microtubule Structures by DWT
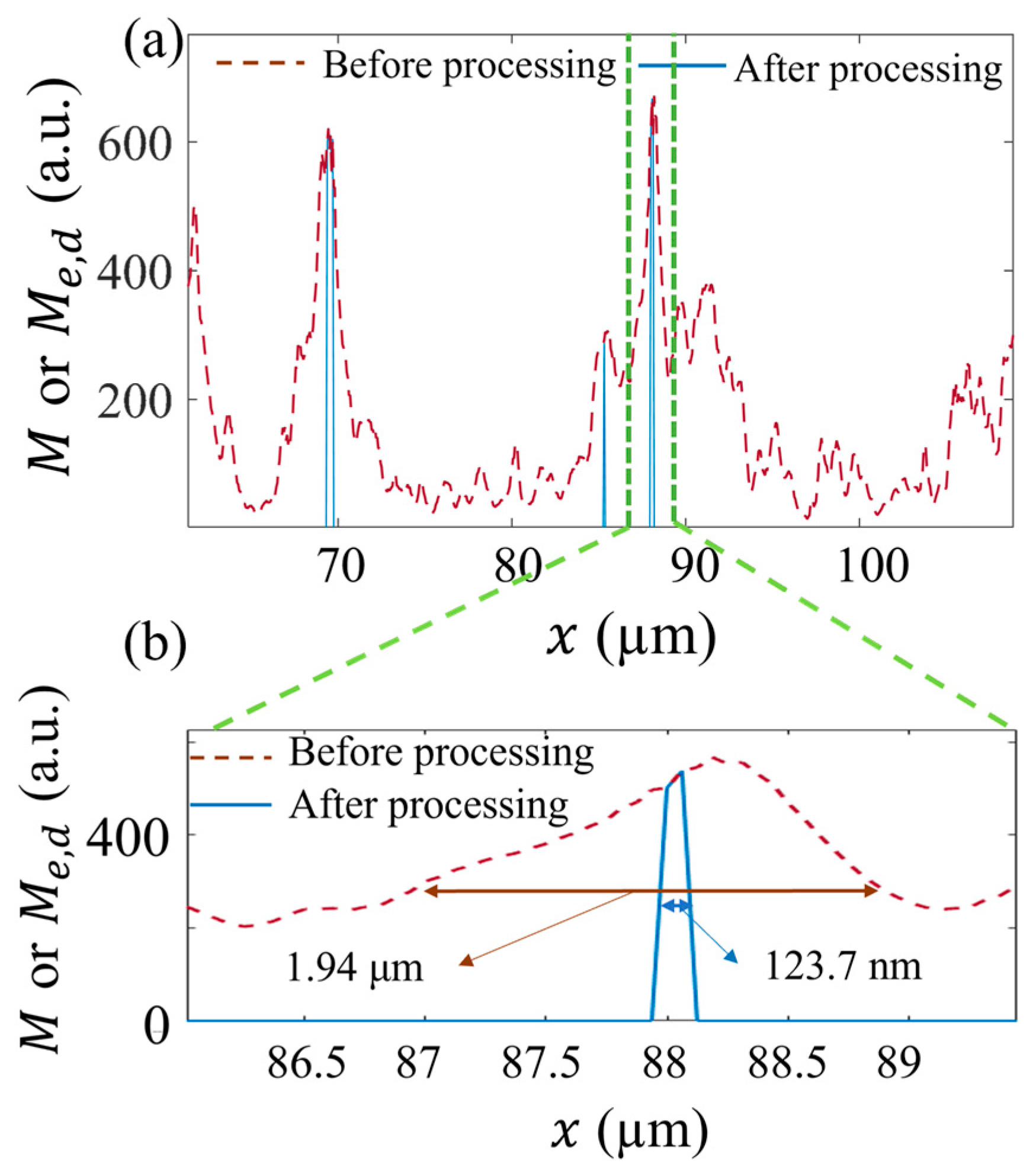
3.4. Resolution Improvement by LR Deconvolution
3.5. Image after Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In Vivo Confocal Scanning Laser Microscopy of Human Skin: Melanin Provides Strong Contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef]
- Hein, B.; Willig, K.I.; Hell, S.W. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc. Natl. Acad. Sci. USA 2008, 105, 14271–14276. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Zanghirati, G.; Cavicchioli, R.; Zanni, L.; Boccacci, P.; Bertero, M.; Vicidomini, G. Towards real-time image deconvolution: Application to confocal and STED microscopy. Sci. Rep. 2013, 3, 2523. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yan, K.; Lan, H.; Badal, J.; Qin, Z. Adaptive step-size fast iterative shrinkage-thresholding algorithm and sparse-spike deconvolution. Comput. Geosci. 2020, 134, 104343. [Google Scholar] [CrossRef]
- Sage, D.; Donati, L.; Soulez, F.; Fortun, D.; Schmit, G.; Seitz, A.; Guiet, R.; Vonesch, C.; Unser, M. DeconvolutionLab2: An Open-Source Software for Deconvolution Microscopy. Methods 2017, 115, 28–41. [Google Scholar] [CrossRef]
- Periasamy, A.; Skoglund, P.; Noakes, C.; Keller, R. An evaluation of two-photon excitation versus confocal and digital deconvolution fluorescence microscopy imaging in Xenopus morphogenesis. Microsc. Res. Tech. 2015, 47, 172–181. [Google Scholar] [CrossRef]
- Khetkeeree, S. Optimization of Lucy-Richardson Algorithm Using Modified Tikhonov Regularization for Image Deblurring. J. Phys. Conf. Ser. 2020, 1438, 012014. [Google Scholar] [CrossRef]
- Zhao, L.N.; Hu, W.B.; Cui, L.H. Face Recognition Feature Comparison Based SVD and FFT. J. Signal Inf. Process. 2012, 3, 259–262. [Google Scholar] [CrossRef][Green Version]
- Cameron, D.G.; Moffatt, D.J.; Mantsch, H.H.; Kauppinen, J.K. Fourier Self-Deconvolution: A Method for Resolving Intrinsically Overlapped Bands. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Ali, M.N. A wavelet-based method for MRI liver image denoising. Biomed. Eng. Biomed. Tech. 2019, 64, 699–709. [Google Scholar] [CrossRef]
- Ali, M.; Chang, W.A. Comments on “Optimized gray-scale image watermarking using DWT-SVD and Firefly Algorithm”. Expert Syst. Appl. 2015, 42, 2392–2394. [Google Scholar] [CrossRef]
- Dumic, E. New image-quality measure based on wavelets. J. Electron. Imaging 2010, 19, 011018. [Google Scholar] [CrossRef]
- Dumic, E.; Grgic, S.; Grgic, M. IQM2: New image quality measure based on steerable pyramid wavelet transform and structural similarity index. Signal Image Video Process. 2014, 8, 1159–1168. [Google Scholar] [CrossRef]
- Abdulrahman, A.A.; Rasheed, M.; Shihab, S. The Analytic of Image Processing Smoothing Spaces Using Wavelet. J. Phys. Conf. Ser. 2021, 1879, 022118. [Google Scholar] [CrossRef]
- Liby, J.J.; Jaya, T. Data Hiding Scheme for Video Watermarking Using Horizontal and Vertical Coefficients of Single Level Discrete Wavelet Transform Method. J. Circuits Syst. Comput. 2022, 31, 2250044. [Google Scholar] [CrossRef]
- Wang, H.; Rivenson, Y.; Jin, Y.; Wei, Z.; Ozcan, A. Cross-Modality Deep Learning Achieves Super-Resolution in Fluorescence Microscopy. In Proceedings of the CLEO: Science and Innovations, San Jose, CA, USA, 1 January 2019. [Google Scholar]
- Shajkofci, A.; Liebling, M. Spatially-Variant CNN-Based Point Spread Function Estimation for Blind Deconvolution and Depth Estimation in Optical Microscopy. IEEE Trans. Image Process. 2020, 29, 5848–5861. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Cai, Y.; Wang, X. Small Waterbody Extraction With Improved U-Net Using Zhuhai-1 Hyperspectral Remote Sensing Images. IEEE Geosci. Remote Sens. Lett. 2021, 19, 5502705. [Google Scholar] [CrossRef]
- Schaap, I.A.T.; Carrasco, C.; de Pablo, P.J.; MacKintosh, F.C.; Schmidt, C.F. Elastic response, buckling, and instability of microtubules under radial indentation. Biophys. J. 2006, 91, 1521–1531. [Google Scholar] [CrossRef]
- Nagorni, M.; Hell, S.W. 4Pi-Confocal Microscopy Provides Three-Dimensional Images of the Microtubule Network with 100- to 150-nm Resolution. J. Struct. Biol. 1998, 123, 236–247. [Google Scholar] [CrossRef]
- Ogier, A.; Dorval, T.; Genovesio, A. Inhomogeneous deconvolution in a biological images context. In Proceedings of the 2008 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 17 May 2008. [Google Scholar]
- Li, J.; Luisier, F.; Blu, T. PURE-LET image deconvolution. IEEE Trans. Image Process. 2017, 27, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Carlavan, M.; Blanc-Feraud, L. Sparse Poisson Noisy Image Deblurring. IEEE Trans. Image Process. 2012, 21, 1834–1846. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Liu, S.; Liu, T.; Yan, L.; Zhang, T. Richardson–Lucy blind deconvolution of spectroscopic data with wavelet regularization. Appl. Opt. 2015, 54, 1770–1775. [Google Scholar] [CrossRef]
- Sroubek, F.; Milanfar, P. Robust Multichannel Blind Deconvolution via Fast Alternating Minimization. IEEE Trans. Image Process. 2012, 21, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Javaran, T.A.; Hassanpour, H.; Abolghasemi, V. Non-blind image deconvolution using a regularization based on re-blurring process. Comput. Vis. Image Underst. 2017, 154, 16–34. [Google Scholar] [CrossRef]
- Gong, D.; Tan, M.; Shi, Q.; van den Hengel, A.; Zhang, Y. MPTV: Matching Pursuit-Based Total Variation Minimization for Image Deconvolution. IEEE Trans. Image Process 2019, 28, 1851–1865. [Google Scholar] [CrossRef]
- Willig, K.I.; Kellner, R.R.; Medda, R.; Hein, B.; Jakobs, S.; Hell, S.W. Nanoscale resolution in GFP-based microscopy. Nat. Methods 2006, 3, 721–723. [Google Scholar] [CrossRef]
- Hell, S.W. Far-Field Optical Nanoscopy. Science 2007, 316, 1153–1158. [Google Scholar] [CrossRef]
- Bioucas-Dias, J.M. Bayesian wavelet-based image deconvolution: A GEM algorithm exploiting a class of heavy-tailed priors. IEEE Trans Image Process. 2006, 15, 937–951. [Google Scholar] [CrossRef]
- Vonesch, C.; Unser, M. A fast thresholded landweber algorithm for wavelet-regularized multidimensional deconvolution. IEEE Trans Image Process. 2008, 17, 539–549. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Nowak, R.D. An EM algorithm for wavelet-based image restoration. IEEE Trans. Image Process. 2003, 12, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.A.T.; Nowak, R.D. A bound optimization approach to wavelet-based image deconvolution. In Proceedings of the IEEE International Conference on Image Processing, Genova, Italy, 14 September 2005; p. II-782. [Google Scholar]
- Peng, Y.; Li, Q. Wavelet transform-based feature extraction for ultrasonic flaw signal classification. Neural Comput. Appl. 2014, 9, 817–826. [Google Scholar] [CrossRef]
- Tam, N.W.P.; Lee, J.-S.; Hu, C.-M.; Liu, R.-S.; Chen, J.-C. A Haar-wavelet-based Lucy–Richardson algorithm for positron emission tomography image restoration. Nucl. Instrum. M ethods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 648, S122–S127. [Google Scholar] [CrossRef]
- Lussana, C.; Nipen, T.N.; Seierstad, I.A.; Elo, C.A. Ensemble-based statistical interpolation with Gaussian anamorphosis for the spatial analysis of precipitation. Nonlinear Process. Geophys. 2021, 28, 61–91. [Google Scholar] [CrossRef]
- Platte, R.B.; Driscoll, T.A. Polynomials and Potential Theory for Gaussian Radial Basis Function Interpolation. Siam J. Numer. Anal. 2006, 43, 750–766. [Google Scholar] [CrossRef]
- Hell, S.W. Increasing the Resolution of Far-Field Fluorescence Light Microscopy by Point-Spread-Function Engineering. Top. Fluoresc. Spectrosc. 2002, 5, 361–426. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef]
- Pustelnik, N.; Benazza-Benhayia, A.; Zheng, Y.; Pesquet, J.-C. Wavelet-Based Image Deconvolution and Reconstruction. In Wiley Encyclopedia of Electrical and Electronics Engineering; American Cancer Society: Washington, DC, USA, 2017; pp. 1–34. [Google Scholar]
- Richardson, W.H. Bayesian-Based Iterative Method of Image Restoration. J. Opt. Soc. Am. 1972, 62, 55–59. [Google Scholar] [CrossRef]
- Sfakianakis, N.; Peurichard, D.; Brunk, A.; Schmeiser, C. Modelling cell-cell collision and adhesion with the filament based lamellipodium model. BioMath 2018, 7, 1811097. [Google Scholar] [CrossRef]
- Bonforti, A.; Duran-Nebreda, S.; MontañEz, R.; Solé, R. Spatial self-organization in hybrid models of multicellular adhesion. Chaos 2016, 26, 1102–1109. [Google Scholar] [CrossRef]


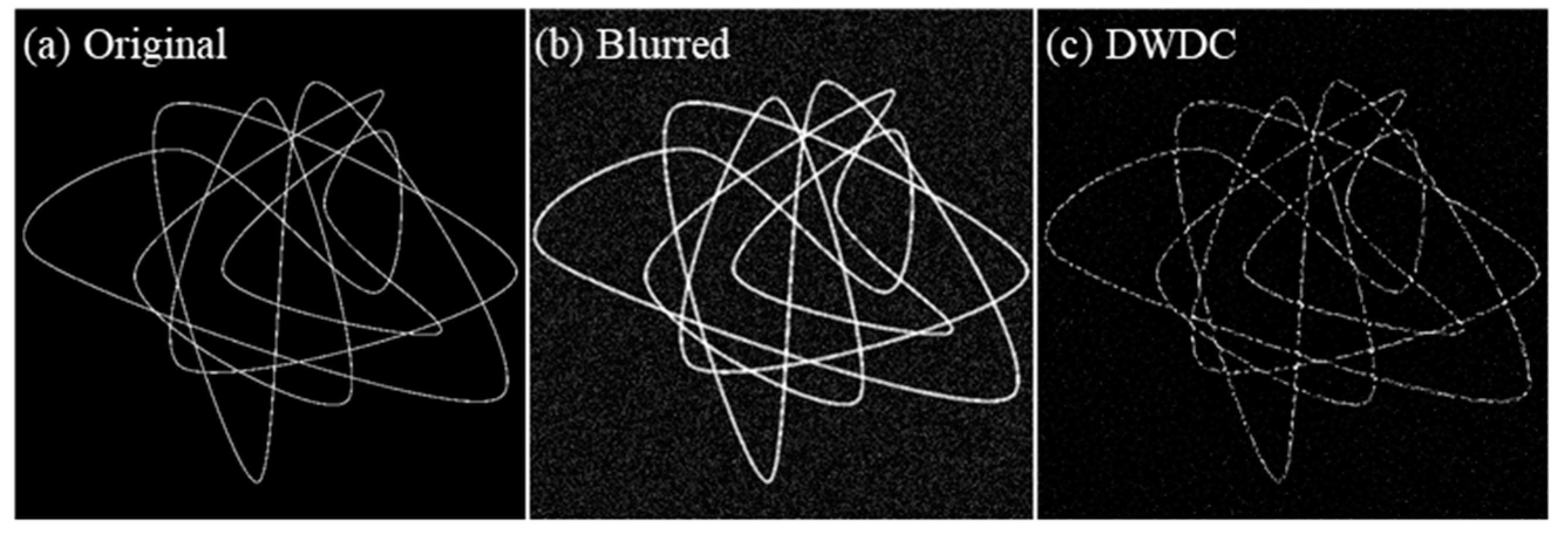
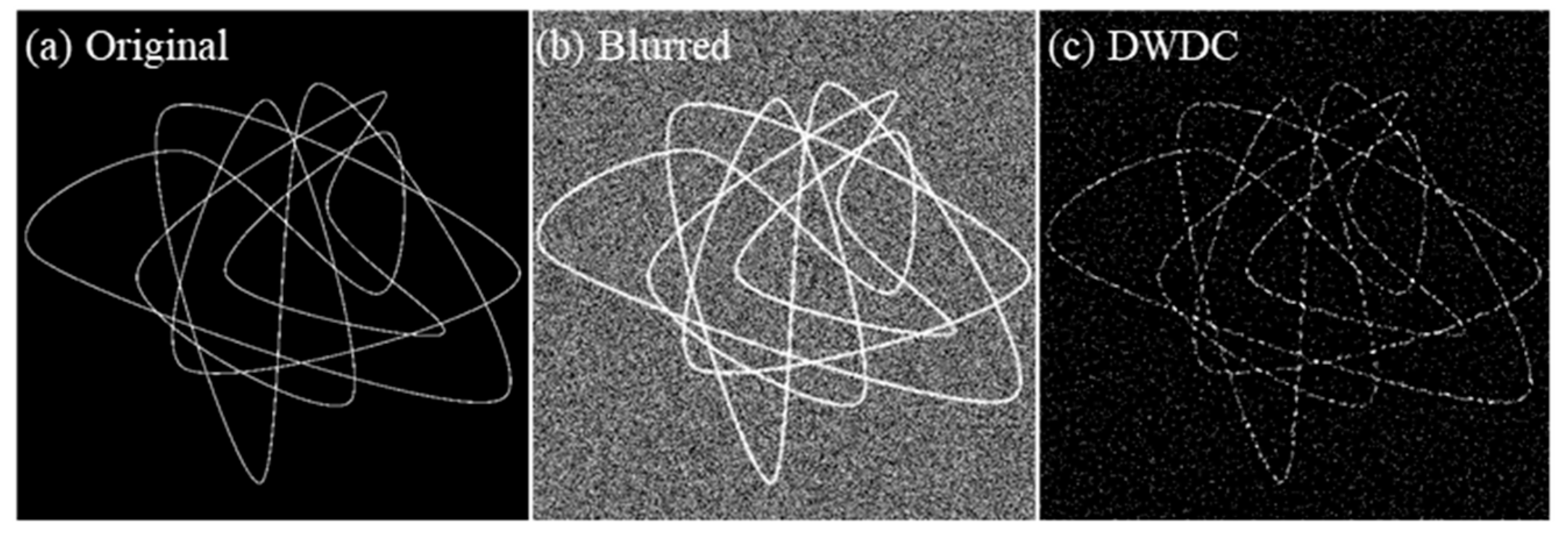



| Gaussian Noise | PSNR before Processing (dB) | PSNR after Processing (dB) | SSIM before Processing | SSIM after Processing | |
|---|---|---|---|---|---|
| Average | STD | ||||
| 0 | 2 | 19.5773 | 20.6765 | 0.0887 | 0.7082 |
| 20 | 4 | 15.9681 | 19.7943 | 0.0425 | 0.4916 |
| 40 | 6 | 12.8995 | 18.7648 | 0.0330 | 0.3908 |
| 40 | 10 | 12.1049 | 17.8139 | 0.0297 | 0.3251 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Che, B.; Zhao, T.; Zhao, W.; Wang, K.; Zhang, C.; Bai, J. Feature Extraction of 3T3 Fibroblast Microtubule Based on Discrete Wavelet Transform and Lucy–Richardson Deconvolution Methods. Micromachines 2022, 13, 824. https://doi.org/10.3390/mi13060824
Bai H, Che B, Zhao T, Zhao W, Wang K, Zhang C, Bai J. Feature Extraction of 3T3 Fibroblast Microtubule Based on Discrete Wavelet Transform and Lucy–Richardson Deconvolution Methods. Micromachines. 2022; 13(6):824. https://doi.org/10.3390/mi13060824
Chicago/Turabian StyleBai, Haoxin, Bingchen Che, Tianyun Zhao, Wei Zhao, Kaige Wang, Ce Zhang, and Jintao Bai. 2022. "Feature Extraction of 3T3 Fibroblast Microtubule Based on Discrete Wavelet Transform and Lucy–Richardson Deconvolution Methods" Micromachines 13, no. 6: 824. https://doi.org/10.3390/mi13060824
APA StyleBai, H., Che, B., Zhao, T., Zhao, W., Wang, K., Zhang, C., & Bai, J. (2022). Feature Extraction of 3T3 Fibroblast Microtubule Based on Discrete Wavelet Transform and Lucy–Richardson Deconvolution Methods. Micromachines, 13(6), 824. https://doi.org/10.3390/mi13060824







