A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation
Abstract
:1. Introduction
2. Materials and Methods
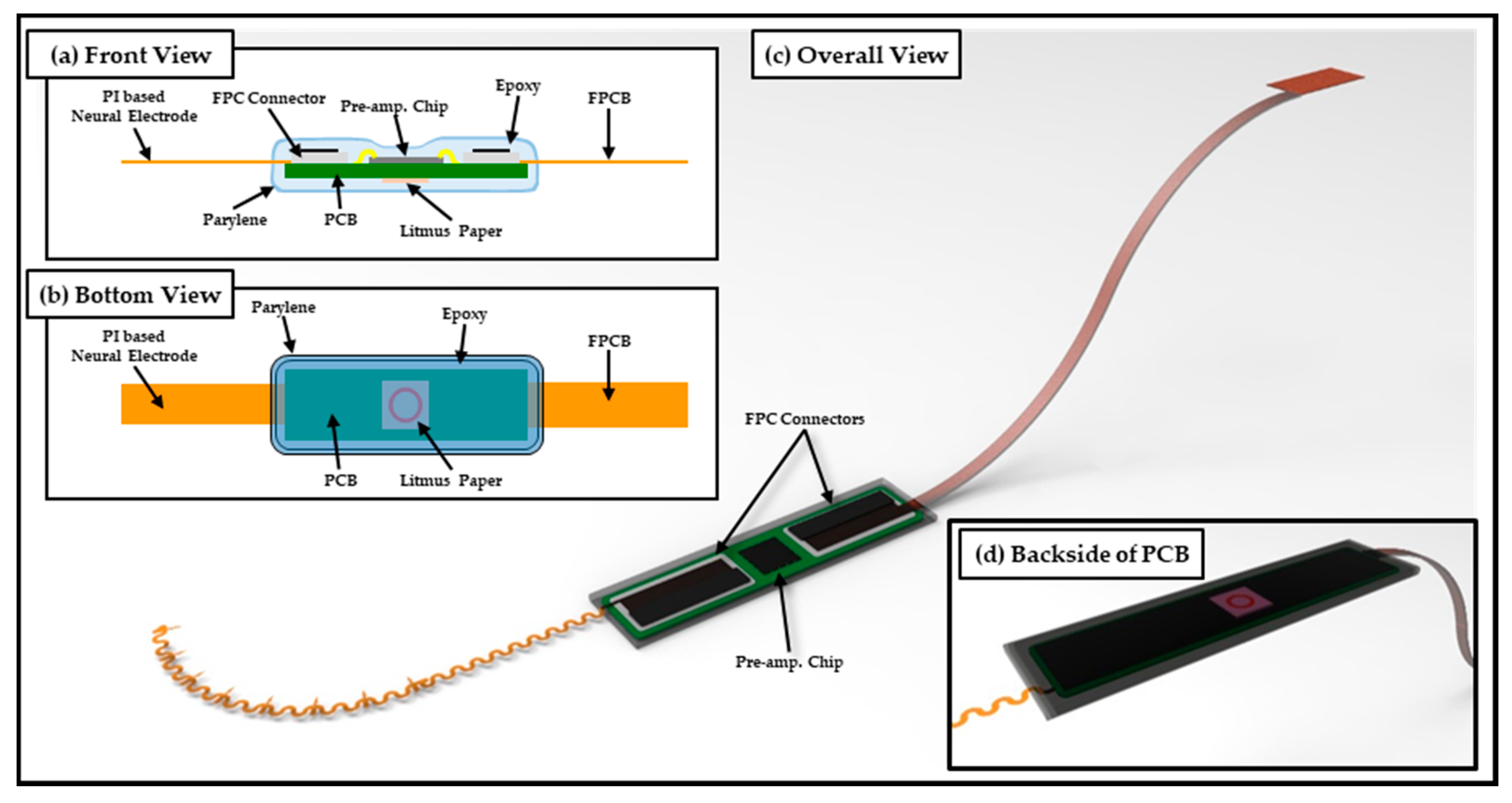
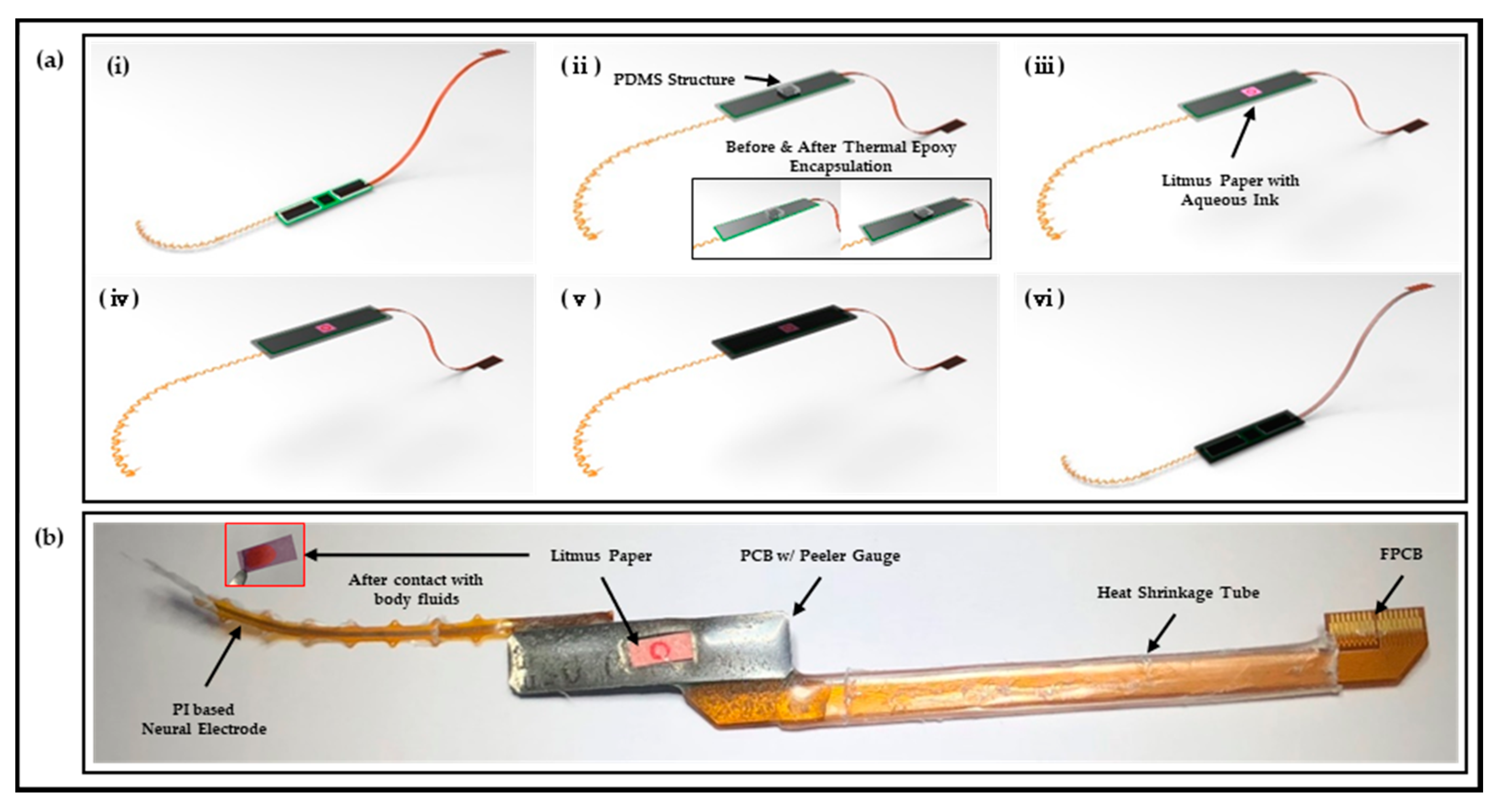
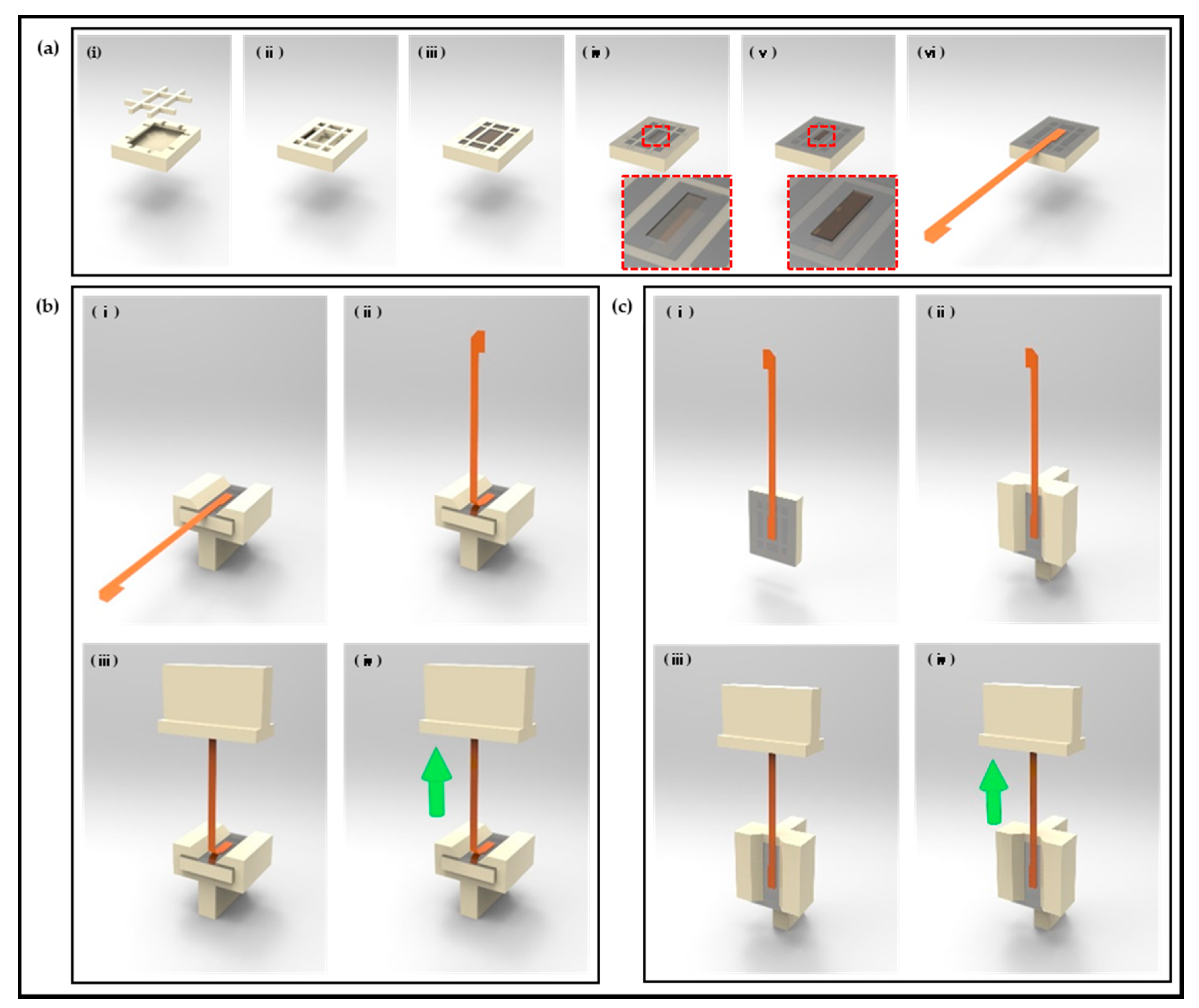
2.1. Design and Fabrication of Polymer-Based Neural Interface Packaging Prototype
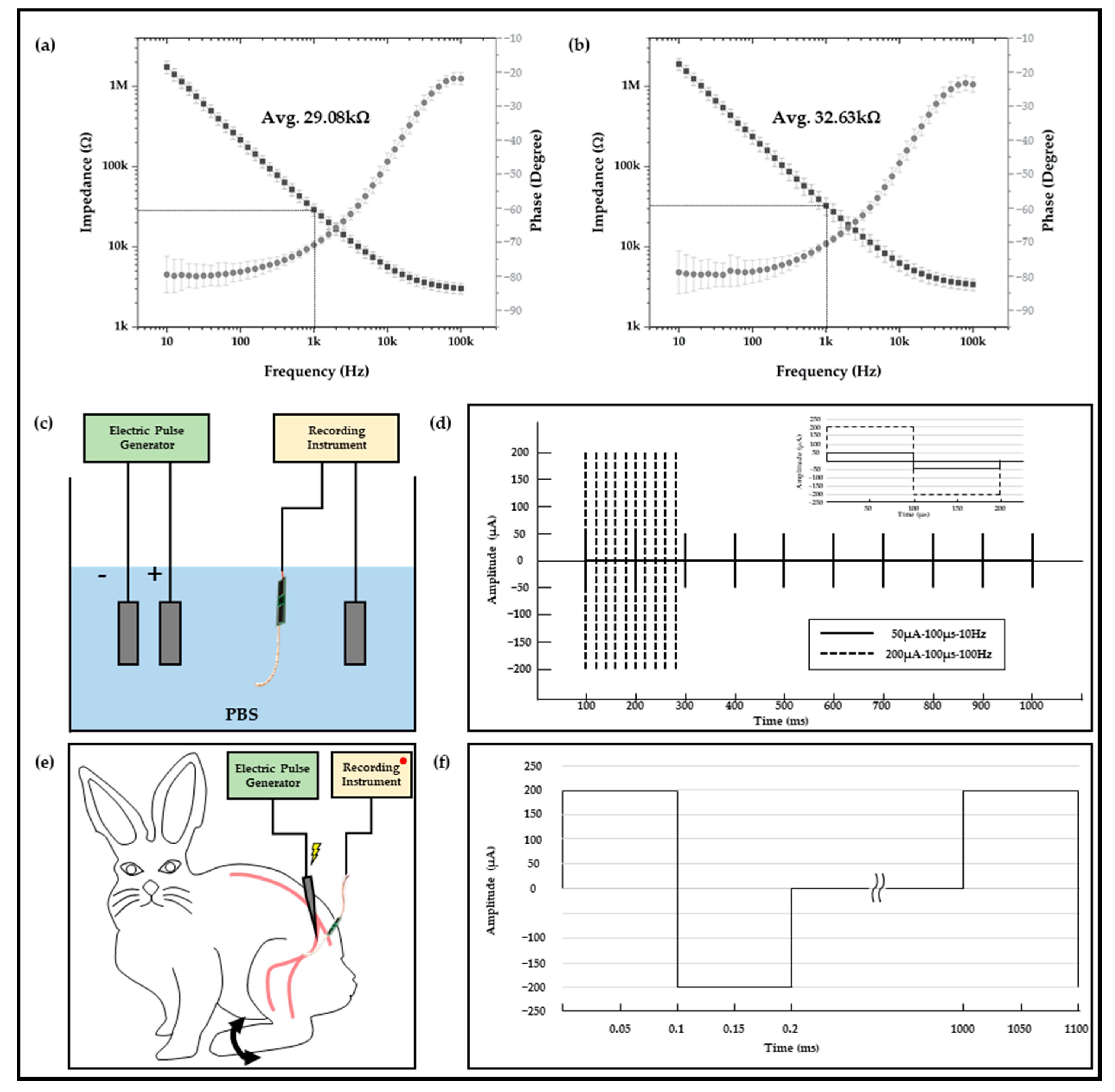
2.2. Electrophysiological Experiment Setups
2.3. In Vivo Experiment Setups
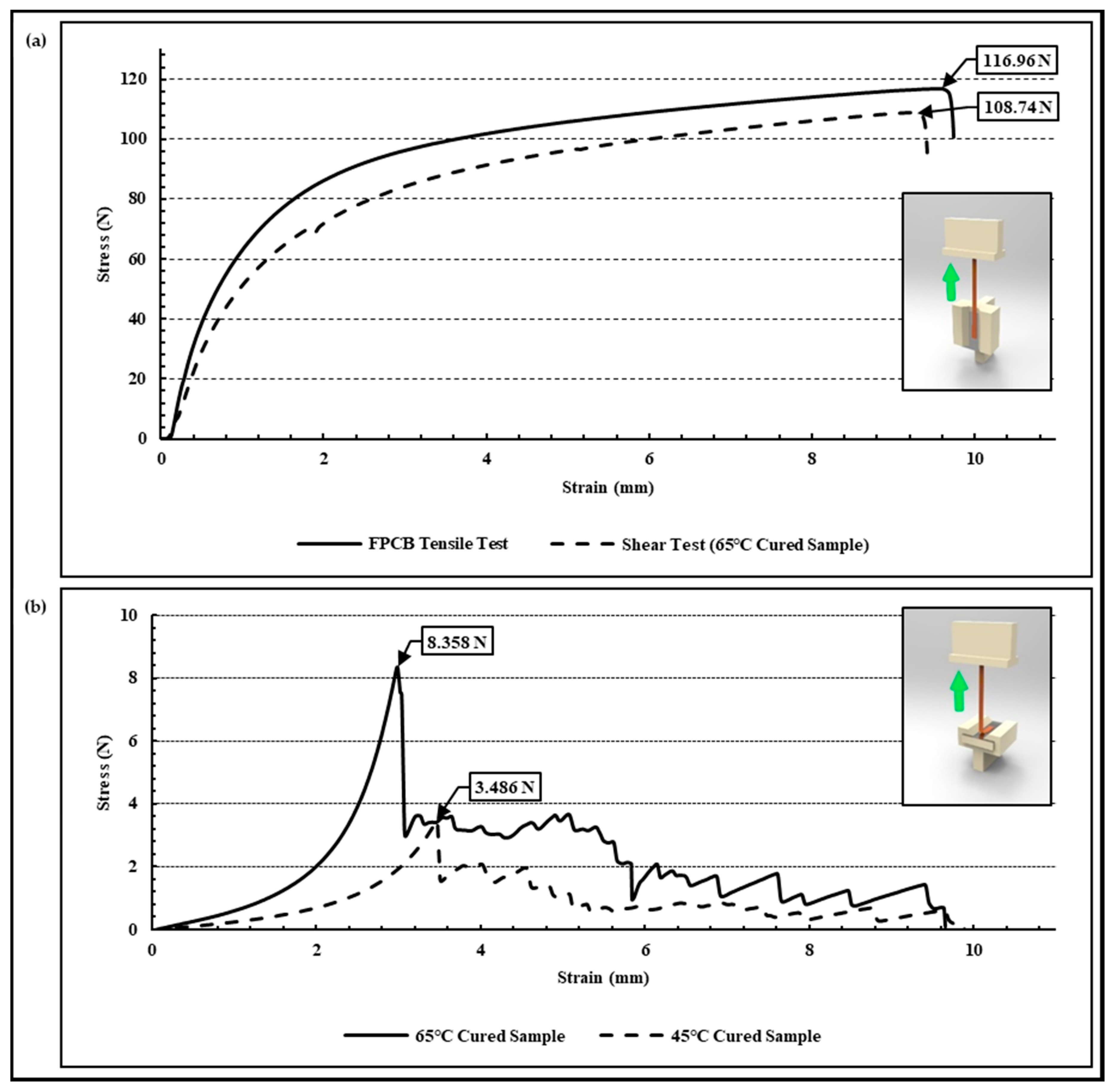
2.4. Test Sample Preparation for Polyimide (FPCB)-Epoxy Peel-Off Test
2.5. Peel-Off Test Setup for Polyimide (FPCB) Epoxy
2.6. Acceleration Test Setup
2.7. In Vivo Chronic Implantation
3. Results
3.1. In Vitro and In Vivo Characterizations of Polymer-Based Neural Interface Packaging Prototype
3.2. Adhesion Evaluation of Peel-Off Test
3.3. Lifetime and Characteristics of Acceleration Test
3.4. In Vivo Chronic Implantation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Patschger, A.; Hopf, A.; Güpner, M.; Bliedtner, J. Laser Material Processing of Medical Titanium: Titanium boxes for hermetic transport of medical implants. Laser Tech. J. 2016, 13, 24–27. [Google Scholar] [CrossRef]
- Yang, H.; Wu, T.; Zhao, S.; Xiong, S.; Peng, B.; Humayun, M.S. Chronically implantable package based on alumina ceramics and titanium with high-density feedthroughs for medical implants. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 3382–3385. [Google Scholar]
- Jiang, G.; Zhou, D.D. Technology advances and challenges in hermetic packaging for implantable medical devices. In Implantable Neural Prostheses 2; Springer: New York, NY, USA, 2009; pp. 27–61. [Google Scholar]
- Dewald, H.A.; Lukyanenko, P.; Lambrecht, J.M.; Anderson, J.R.; Tyler, D.J.; Kirsch, R.F.; Williams, M.R. Stable, three degree-of-freedom myoelectric prosthetic control via chronic bipolar intramuscular electrodes: A case study. J. Neuroeng. Rehabil. 2019, 16, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, J.E.; Carlson, J.D.; Rosenberg, W.S.; Slavin, K.V.; Deer, T.R. Peripheral nerve stimulation for pain in extremities: An update. Stimul. Peripher. Nerv. Syst. 2016, 29, 139–157. [Google Scholar]
- Tan, D.W.; Schiefer, M.A.; Keith, M.W.; Anderson, J.R.; Tyler, J.; Tyler, D.J. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014, 6, ra138–ra257. [Google Scholar] [CrossRef] [Green Version]
- Oddo, C.M.; Raspopovic, S.; Artoni, F.; Mazzoni, A.; Spigler, G.; Petrini, F.; Micera, S. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 2016, 5, e09148. [Google Scholar] [CrossRef]
- Koopman, F.A.; Schuurman, P.R.; Vervoordeldonk, M.J.; Tak, P.P. Vagus nerve stimulation: A new bioelectronics approach to treat rheumatoid arthritis? Best Pract. Res. Clin. Rheumatol. 2014, 28, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Haidar, A.; Legault, L.; Messier, V.; Mitre, T.M.; Leroux, C.; Rabasa-Lhoret, R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: An open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015, 3, 17–26. [Google Scholar] [CrossRef]
- Güemes, A.; Georgiou, P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron. Med. 2018, 4, 9. [Google Scholar] [CrossRef]
- Sung, C.; Jeon, W.; Nam, K.S.; Kim, Y.; Butt, H.; Park, S. Multimaterial and multifunctional neural interfaces: From surface-type and implantable electrodes to fiber-based devices. J. Mater. Chem. B 2020, 8, 6624–6666. [Google Scholar] [CrossRef]
- Coker, R.A.; Zellmer, E.R.; Moran, D.W. Micro-channel sieve electrode for concurrent bidirectional peripheral nerve interface. Part B: Stimulation. J. Neural Eng. 2019, 16, 026002. [Google Scholar] [CrossRef]
- Christensen, M.B.; Pearce, S.M.; Ledbetter, N.M.; Warren, D.J.; Clark, G.A.; Tresco, P.A. The foreign body response to the Utah Slant Electrode Array in the cat sciatic nerve. Acta Biomater. 2014, 10, 4650–4660. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Harreby, K.R.; Yoshida, K.; Boretius, T.; Stieglitz, T.; Jensen, W. Stimulation selectivity of the “thin-film longitudinal intrafascicular electrode”(tfLIFE) and the “transverse intrafascicular multi-channel electrode”(TIME) in the large nerve animal model. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rieth, L.; Tathireddy, P.; Harrison, R.; Oppermann, H.; Klein, M.; Solzbacher, F. Long term in vitro functional stability and recording longevity of fully integrated wireless neural interfaces based on the Utah Slant Electrode Array. J. Neural Eng. 2011, 8, 045004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shon, A.; Chu, J.U.; Jung, J.; Kim, H.; Youn, I. An implantable wireless neural interface system for simultaneous recording and stimulation of peripheral nerve with a single cuff electrode. Sensors 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.N.; Chou, N.; Jang, J.W.; Byun, D.; Kang, H.; Moon, D.J.; Kim, S. An intrafascicular neural interface with enhanced interconnection for recording of peripheral nerve signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.E.; Kuntaegowdanahalli, S.S.; Abbas, J.J.; Patrick, J.; Horch, K.W.; Jung, R. Mechanical fatigue resistance of an implantable branched lead system for a distributed set of longitudinal intrafascicular electrodes. J. Neural Eng. 2017, 14, 066014. [Google Scholar] [CrossRef]
- Skok, T.; Tabakow, P.; Chmielak, K. Methods of integrating the human nervous system with electronic circuits. Adv. Clin. Exp. Med. 2019, 28, 1125–1135. [Google Scholar] [CrossRef] [Green Version]
- Rijnbeek, E.H.; Eleveld, N.; Olthuis, W. Update on peripheral nerve electrodes for closed-loop neuroprosthetics. Front. Neurosci. 2018, 12, 350. [Google Scholar] [CrossRef]
- George, J.A.; Page, D.M.; Davis, T.S.; Duncan, C.C.; Hutchinson, D.T.; Rieth, L.W.; Clark, G.A. Long-term performance of Utah slanted electrode arrays and intramuscular electromyographic leads implanted chronically in human arm nerves and muscles. J. Neural Eng. 2020, 17, 056042. [Google Scholar] [CrossRef]
- Kaijankoski, H.; Nissen, M.; Ikäheimo, T.M.; von Und Zu Fraunberg, M.; Airaksinen, O.; Huttunen, J. Effect of spinal cord stimulation on early disability pension in 198 failed back surgery syndrome patients: Case-control study. Neurosurgery 2019, 84, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Seok, S. Polymer-Based Biocompatible Packaging for Implantable Devices: Packaging Method, Materials, and Reliability Simulation. Micromachines 2021, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Bae, S.H.; Seo, J.M.; Chung, H.; Kim, S.J. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J. Neural Eng. 2016, 13, 025004. [Google Scholar] [CrossRef]
- Kim, O.; Choi, W.; Jung, W.; Jung, S.; Park, H.; Jeong, J.; Chu, J.U.; Park, J.W.; Kim, J. Spirally Arrayed Electrode for Spatially Selective and Minimally Displacive Peripheral Nerve Interface. J. Microelectromechanical Syst. 2020, 29, 514–521. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly (dimethylsiloxane)(PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, Y.; Zhou, S.; Chen, Y.; Liang, M.; Zou, H. Enhanced mechanical and adhesive properties of PDMS based on novel PDMS-epoxy IPN structure. J. Polym. Res. 2021, 28, 171. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, L.; Liang, H.; Chen, L.; Huang, S. Synthesis of a novel polysiloxane containing phosphorus, and boron and its effect on flame retardancy, mechanical, and thermal properties of epoxy resin. Polym. Compos. 2018, 39, 807–814. [Google Scholar] [CrossRef]
- Amerian, M.; Amerian, M.; Sameti, M.; Seyedjafari, E. Improvement of PDMS surface biocompatibility is limited by the duration of oxygen plasma treatment. J. Biomed. Mater. Res. Part A 2019, 107, 2806–2813. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Newbold, C.; Richardson, R.; Millard, R.; Seligman, P.; Cowan, R.; Shepherd, R. Electrical stimulation causes rapid changes in electrode impedance of cell-covered electrodes. J. Neural Eng. 2011, 8, 036029. [Google Scholar] [CrossRef] [Green Version]
- Günter, C.; Delbeke, J.; Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. Neuroeng. Rehabil. 2019, 16, 13. [Google Scholar] [CrossRef]
- Li, J.; Kang, L.; Yu, Y.; Long, Y.; Jeffery, J.J.; Cai, W.; Wang, X. Study of long-term biocompatibility and bio-safety of implantable nanogenerators. Nano Energy 2018, 51, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.C.; Wu, T.C.; Wu, C.F.; Wang, W.C. Bioperformance analysis of parylene C coating for implanted nickel titanium alloy. Mater. Today Commun. 2021, 27, 102306. [Google Scholar] [CrossRef]
- Iacovacci, V.; Naselli, I.; Salgarella, A.R.; Clemente, F.; Ricotti, L.; Cipriani, C. Stability and in vivo safety of gold, titanium nitride and parylene C coatings on NdFeB magnets implanted in muscles towards a new generation of myokinetic prosthetic limbs. RSC Adv. 2021, 11, 6766–6775. [Google Scholar] [CrossRef]
- Rusdi, M.S.; Abdullah, M.Z.; Aziz, M.S.A.; Abdullah, M.K.; Chellvarajoo, S.; Husin, A.; Rethinasamy, P.; Veerasamy, S. Multiphase flow in solder paste stencil printing process using CFD approach. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 46, 147–152. [Google Scholar]
- Lapique, F.; Redford, K. Curing effects on viscosity and mechanical properties of a commercial epoxy resin adhesive. Int. J. Adhes. Adhes. 2002, 22, 337–346. [Google Scholar] [CrossRef]
- Donaldson, P.E.K.; Sayer, E. A technology for implantable hermetic packages. Part 2: An implementation. Med. Biol. Eng. Comput. 1981, 19, 403–405. [Google Scholar] [CrossRef]
- Perge, J.A.; Homer, M.L.; Malik, W.Q.; Cash, S.; Eskandar, E.; Friehs, G.; Donoghue, J.; Hochberg, L.R. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J. Neural Eng. 2013, 10, 036004. [Google Scholar] [CrossRef] [Green Version]



| Samples | Stimulation Parameter | Signal Amplitude (μV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Max. | Positive Min. | Negative Max. | Negative Min. | Positive Avg. | Positive Std. Dev. | Negative Avg. | Negative Std. Dev. | Total Amplitude Avg. | Total Amplitude Std. Dev. | ||
| In vitro sample | 50 μA-100 μs-10 Hz | 183.3 | 15.3 | −63.9 | −189.8 | 128.3 | 50.8 | −165.5 | 26.5 | 293.8 | 54.0 |
| 200 μA-100 μs-10 Hz | 896.5 | 554.5 | −592.0 | −897.6 | 641.7 | 89.7 | −816.2 | 90.8 | 1457.6 | 123.0 | |
| In vivo sample | 200 μA-100 μs-1 Hz | 884.8 | 650.8 | −811.8 | −899.6 | 797.9 | 80.8 | −848.2 | 33.9 | 1646.0 | 107.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Choi, W.; Oh, S.; Kim, Y.-J.; Seok, S.; Kim, J. A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation. Micromachines 2022, 13, 516. https://doi.org/10.3390/mi13040516
Park H, Choi W, Oh S, Kim Y-J, Seok S, Kim J. A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation. Micromachines. 2022; 13(4):516. https://doi.org/10.3390/mi13040516
Chicago/Turabian StylePark, HyungDal, Wonsuk Choi, Seonghwan Oh, Yong-Jun Kim, Seonho Seok, and Jinseok Kim. 2022. "A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation" Micromachines 13, no. 4: 516. https://doi.org/10.3390/mi13040516
APA StylePark, H., Choi, W., Oh, S., Kim, Y.-J., Seok, S., & Kim, J. (2022). A Study on Biocompatible Polymer-Based Packaging of Neural Interface for Chronic Implantation. Micromachines, 13(4), 516. https://doi.org/10.3390/mi13040516






