Hydrogen Sensor Based on NTC Thermistor with Pt-Loaded WO3/SiO2 Coating
Abstract
1. Introduction
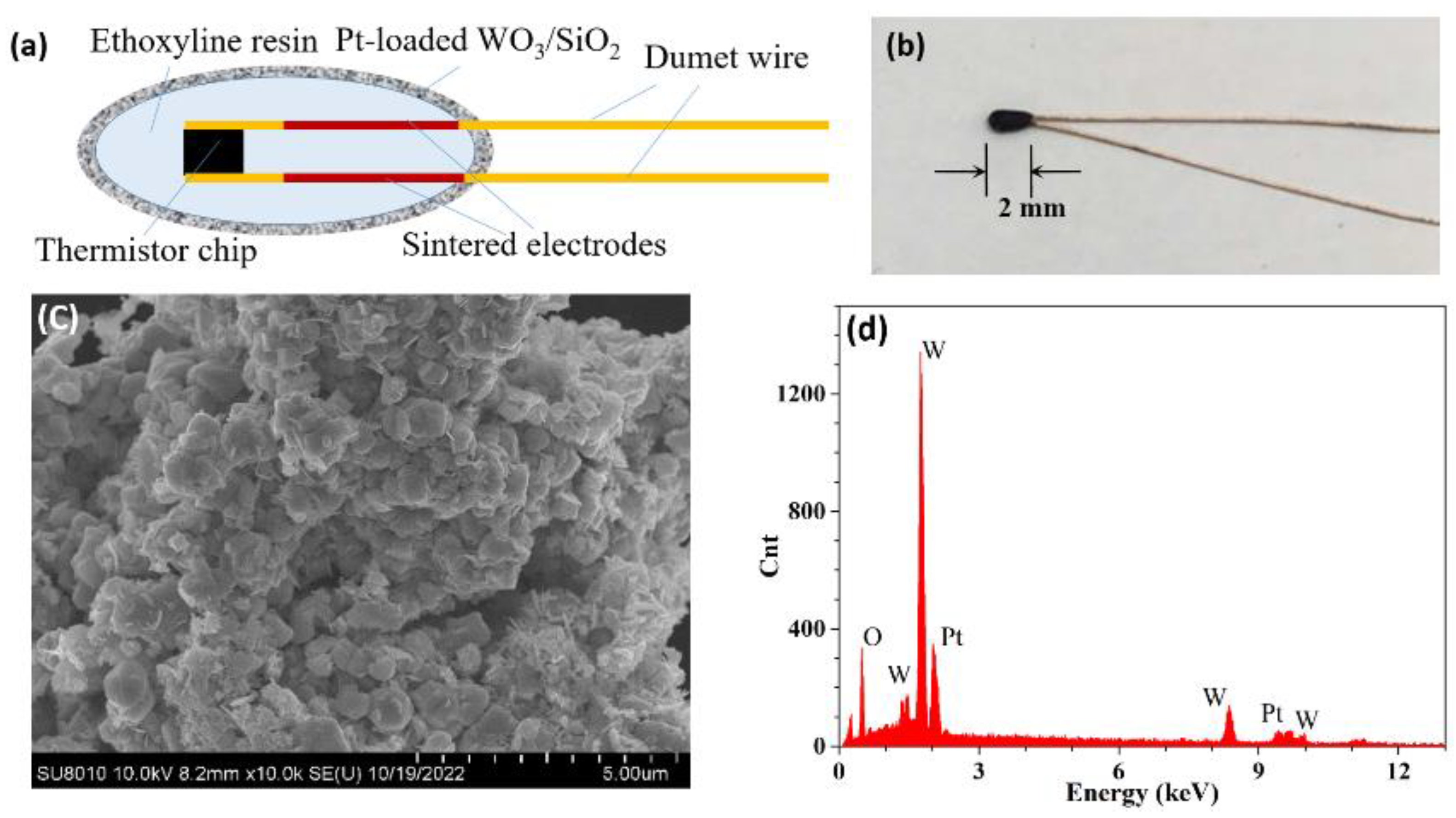
2. Operation Principle
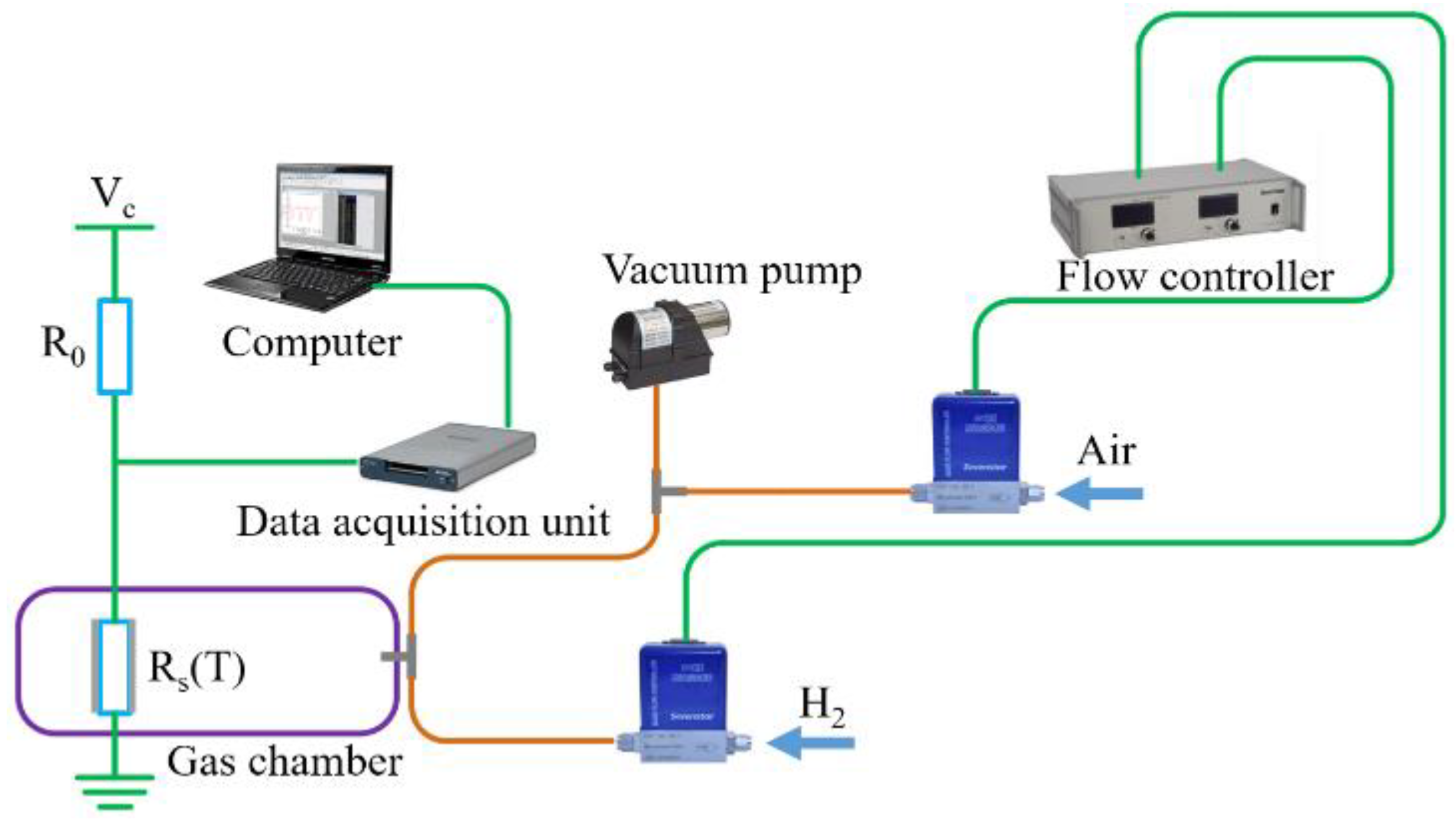
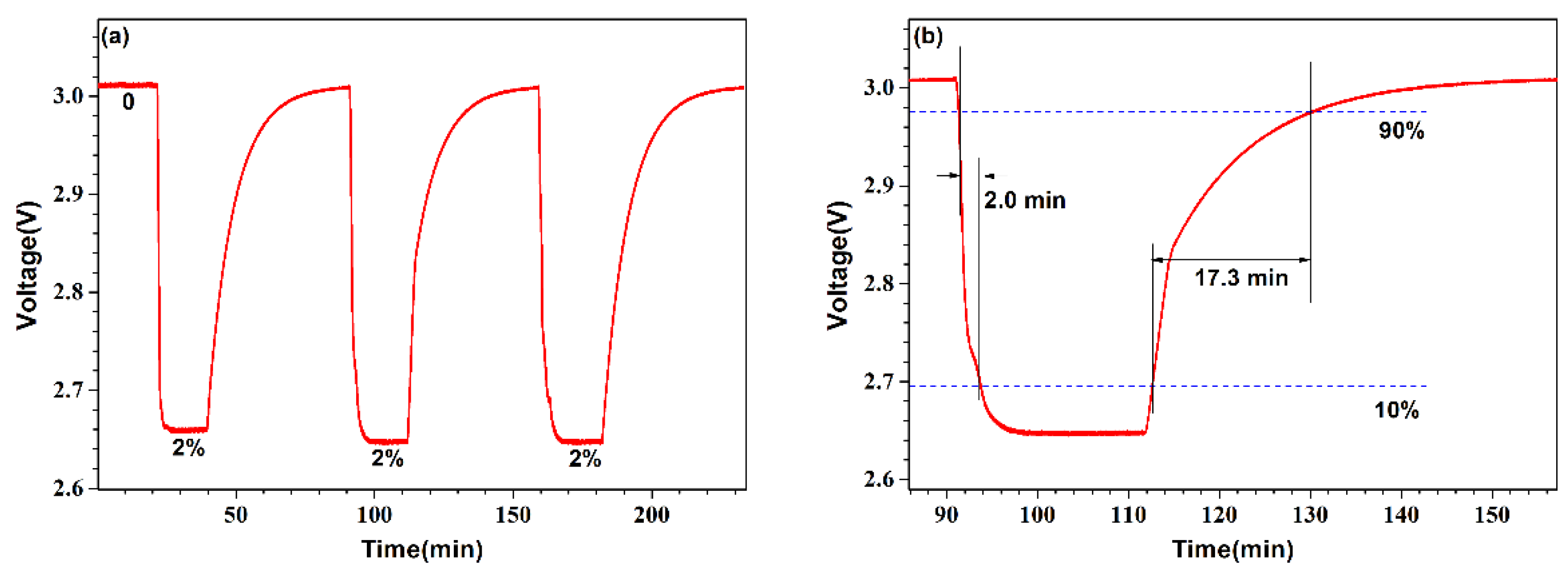
3. Experimental Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Dai, J.; Yang, M. Fiber-optic hydrogen sensors: A review. IEEE Sens. J. 2021, 21, 12706–12718. [Google Scholar] [CrossRef]
- Yang, M.H.; Dai, J. Fiber optic hydrogen sensors: A review. Photonic Sens. 2014, 4, 300–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B Chem. 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Lee, E.B.; Hwang, I.S.; Cha, J.H.; Lee, H.-J.; Lee, W.-B.; Pak, J.J.; Lee, J.-H.; Ju, B.-K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B Chem. 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Han, C.H.; Hong, D.W.; Han, S.D.; Gwak, J.; Singh, K.C. Catalytic combustion type hydrogen gas sensor using TiO2 and UV-LED. Sens. Actuators B Chem. 2007, 125, 224–228. [Google Scholar] [CrossRef]
- Gu, J.T.; Zhang, Y.D.; Jiang, J.G. Design and Experimentation with Sandwich Microstructure for Catalytic Combustion-Type Gas Sensors. Sensors 2014, 14, 5183–5197. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Intern. J. Hydrog. Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Rashid, T.R.; Phan, D.T.; Chung, G.S. A flexible hydrogen sensor based on Pd nanoparticles decorated ZnO nanorods grown on polyimide tape. Sens. Actuators B Chem. 2013, 185, 777–784. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.-B.; Hong, S.-H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticles decoration. Sens. Actuators B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Hunze, A. Improving the Sensitivity of Palladium-Based Fiber Optic Hydrogen Sensors. J. Light. Technol. 2018, 36, 2166–2174. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, P.; Chen, Y.; Zhong, J.; Wang, Y. Highly sensitive hydrogen sensor based on an optical driven nanofilm resonator. ACS Appl. Mater. Interfaces 2022, 14, 29357–29365. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Coelho, L.; Almeida, M.J.; Frazao, O.; Santos, J.L.; Malcata, F.X.; Becker, M.; Rothhardt, M.; Bartelt, H. H2 sensing based on a Pd-coated tapered-FBG fabricated by DUV Femtosecond laser technique. IEEE Photonics Technol. Lett. 2013, 25, 401–403. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, C.; Liu, X.; Liu, S.; Chen, H.; Huang, Z.; Wang, Z.; Lang, T.; Zhao, C.; Zhang, Y. Pd/Au nanofilms based tilted fiber Bragg grating hydrogen sensor. Opt. Commun. 2022, 502, 127424. [Google Scholar] [CrossRef]
- Saad, S.; Hassine, L.; Elfahem, W. Hydrogen FBG sensor using Pd/Ag film with application in propulsion system fuel tank model of aerospace vehicle. Photonic Sens. 2014, 4, 254–264. [Google Scholar] [CrossRef]
- Chan, C.C.; Hsu, W.C.; Chang, C.C.; Hsu, C.-S. Preparation and characterization of gasochromic Pt/WO3 hydrogen sensor by using the Taguchi design method. Sens. Actuators B Chem. 2010, 145, 691–697. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Gasochromic WO3 nanostructures for the detection of hydrogen gas: An overview. Appl. Sci. 2019, 9, 1775. [Google Scholar] [CrossRef]
- Gao, C.; Guo, X.; Nie, L.; Wu, X.; Peng, L.; Chen, J. A review on WO3 gasochromic film: Mechanism, preparation and properties. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Zheng, H.D.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured tungsten oxide–properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Shanak, H.; Schmitt, H.; Nowoczin, J. Effect of Pt-catalyst on gasochromic WO3 films: Optical, electrical and AFM investigations. Solid State Ion. 2004, 171, 99–106. [Google Scholar] [CrossRef]
- Boudibaa, A.; Rousseld, P.; Zhanga, C.; Olivier, M.-G.; Snyders, R.; Debliquy, M. Sensing mechanism of hydrogen sensors based on palladium-loaded tungsten oxide (Pd-WO3). Sens. Actuators B Chem. 2013, 187, 84–93. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Wang, D.N.; Zhao, C.-L.; Dai, J.; Yang, M. Hydrogen sensor based on polymer-filled hollow core fiber with Pt-loaded WO3/SiO2 coating. Sens. Actuators B Chem. 2017, 245, 516–523. [Google Scholar] [CrossRef]
- Zhang, G.L.; Yang, M.H.; Wang, Y. Optical fiber-tip Fabry-Perot interferometer for hydrogen sensing. Opt. Commun. 2014, 329, 34–37. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.H.; Zhang, G.L.; Dai, J.; Zhang, Y.; Zhuang, Z. Ultra-highly sensitive hydrogen sensor based on fiber Fabry-Perot interferometer with Pt/WO3 coating. In Proceedings of the 23rd International Conference on Optical Fibre Sensors International Society for Optics and Photonics, Santander, Spain, 2–6 June 2014. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, C.L.; Yang, F.; Gong, H.; Wang, D.N.; Dai, J.; Yang, M. Sagnac interferometer hydrogen sensor based on panda fiber with Pt-loaded WO3/SiO2 coating. Opt. Lett. 2016, 41, 1594–1597. [Google Scholar] [CrossRef]
- Yang, M.H.; Wang, G.P.; Dai, J.X.; Yang, Z.; Li, Z.; Wang, Y.; Zhang, Y.; Zhuang, Z. Fiber Bragg grating sensors with Pt-loaded WO3 coatings for hydrogen concentration detection down to 200 ppm. Meas. Sci. Technol. 2014, 25, 114004. [Google Scholar] [CrossRef]
- Jack, K.E.; Nwangwu, E.O.; Etu, I.A.; Osuagwu, E.U. A simple thermistor design for industrial temperature measurement. IOSR J. Electr. Electron. Eng. 2016, 11, 57–66. [Google Scholar] [CrossRef]
- Gao, G.H.; Wu, J.D.; Wu, G.M.; Zhang, Z.; Feng, W.; Shen, J.; Zhou, B. Phase transition effect on durability of WO3 hydrogen sensing films: An insight by experiment and first-principle method. Sens. Actuators B Chem. 2012, 171–172, 1288–1291. [Google Scholar] [CrossRef]
- Dai, J.X.; Yang, M.H.; Yang, Z.; Li, Z.; Wang, Y.; Wang, G.; Zhang, Y.; Zhuang, Z. Performance of fiber Bragg grating hydrogen sensor coated with Pt-loaded WO3 coating. Sens. Actuators B Chem. 2014, 190, 657–663. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Xu, B.; Li, P. Hydrogen Sensor Based on NTC Thermistor with Pt-Loaded WO3/SiO2 Coating. Micromachines 2022, 13, 2219. https://doi.org/10.3390/mi13122219
Sun C, Xu B, Li P. Hydrogen Sensor Based on NTC Thermistor with Pt-Loaded WO3/SiO2 Coating. Micromachines. 2022; 13(12):2219. https://doi.org/10.3390/mi13122219
Chicago/Turabian StyleSun, Changwei, Ben Xu, and Ping Li. 2022. "Hydrogen Sensor Based on NTC Thermistor with Pt-Loaded WO3/SiO2 Coating" Micromachines 13, no. 12: 2219. https://doi.org/10.3390/mi13122219
APA StyleSun, C., Xu, B., & Li, P. (2022). Hydrogen Sensor Based on NTC Thermistor with Pt-Loaded WO3/SiO2 Coating. Micromachines, 13(12), 2219. https://doi.org/10.3390/mi13122219





