Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications
Abstract
1. Introduction
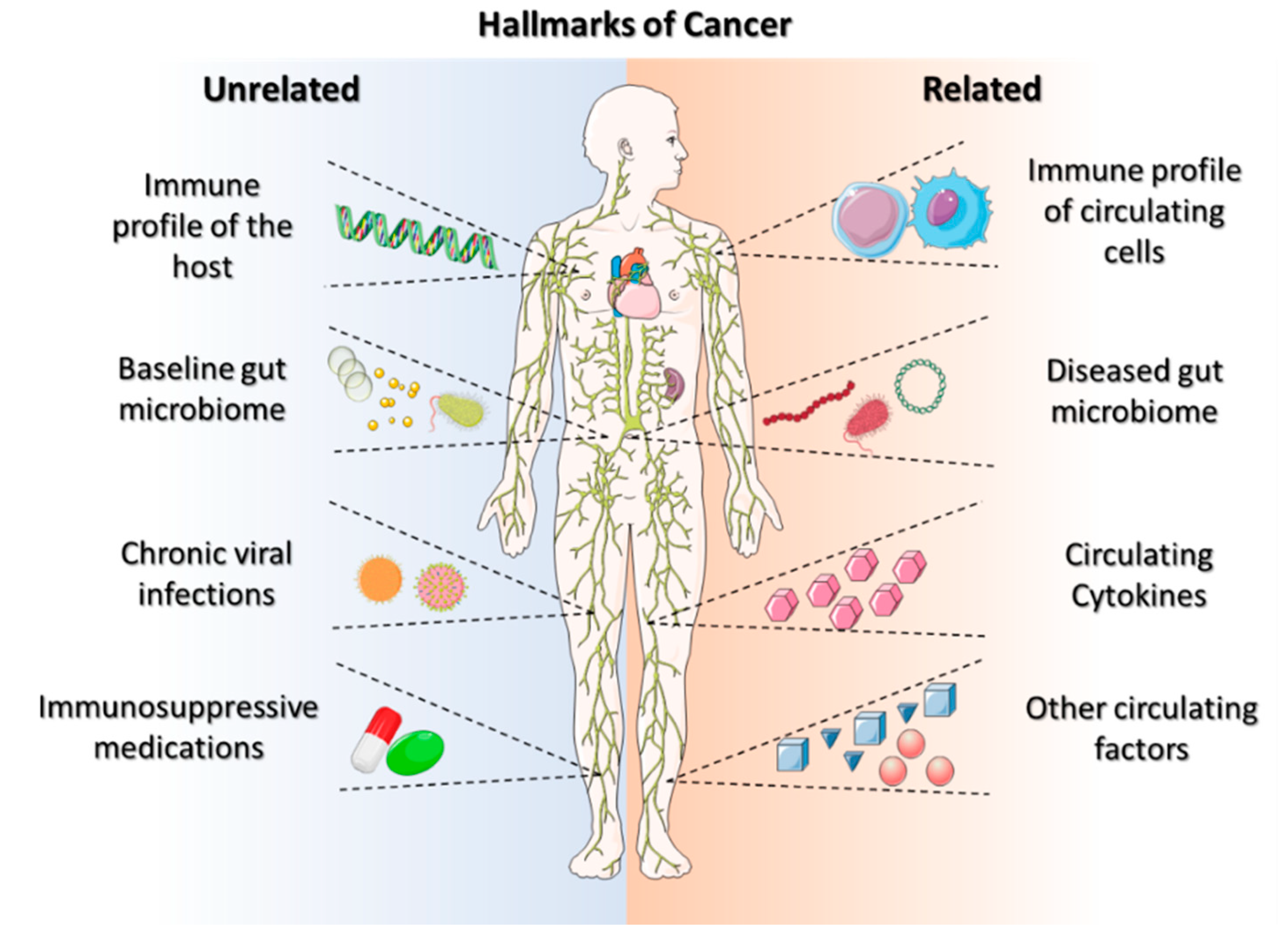
1.1. Challenges in Immunotherapy
1.2. Need for Targeted Cancer Immunotherapy
1.3. Nanotechnology in Cancer Immunotherapy
2. Immune-Targeting Nanomaterials
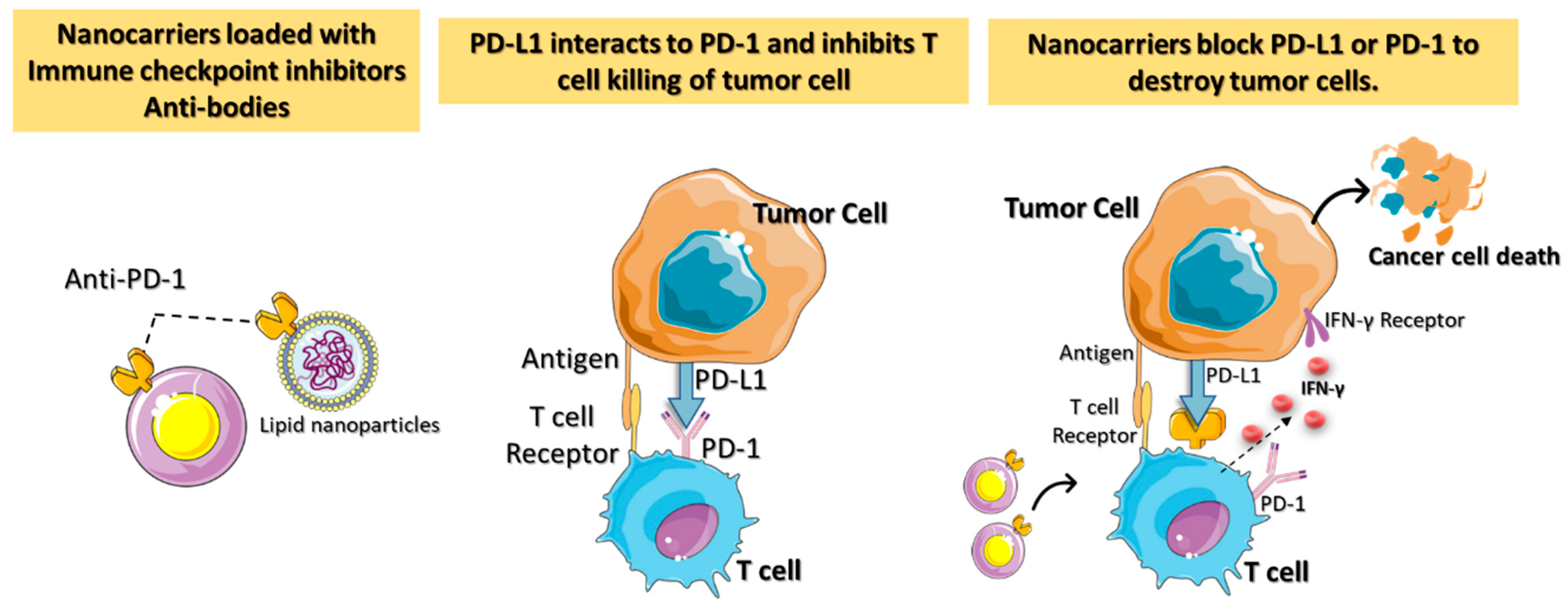
2.1. Nanocarriers for Immune Checkpoint Inhibitor (ICI) or Blockade (ICB)
2.2. Nanocarrier for Adoptive Cell Transfer (ACT)
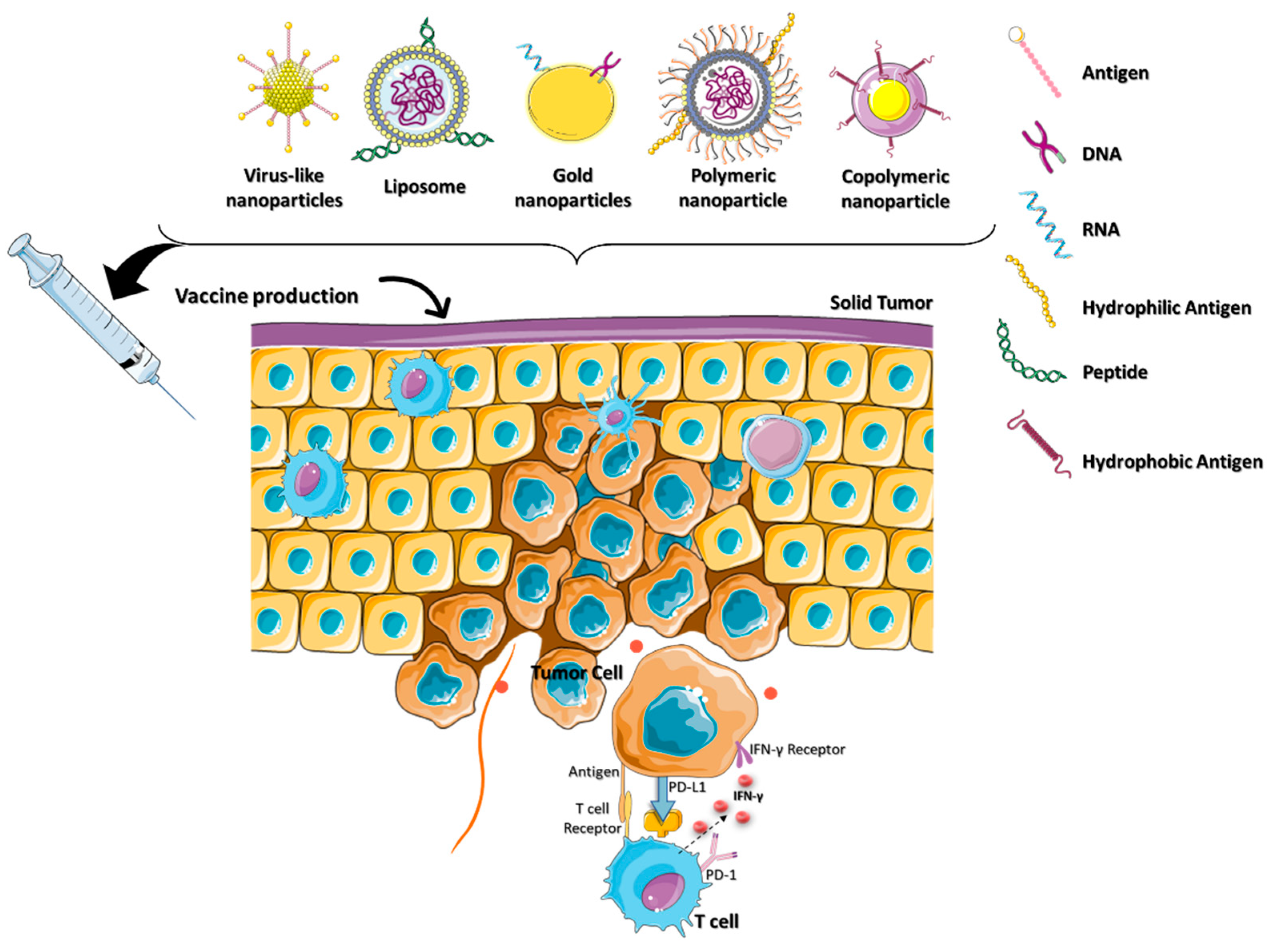
2.3. Nanocarriers for Cancer Vaccine
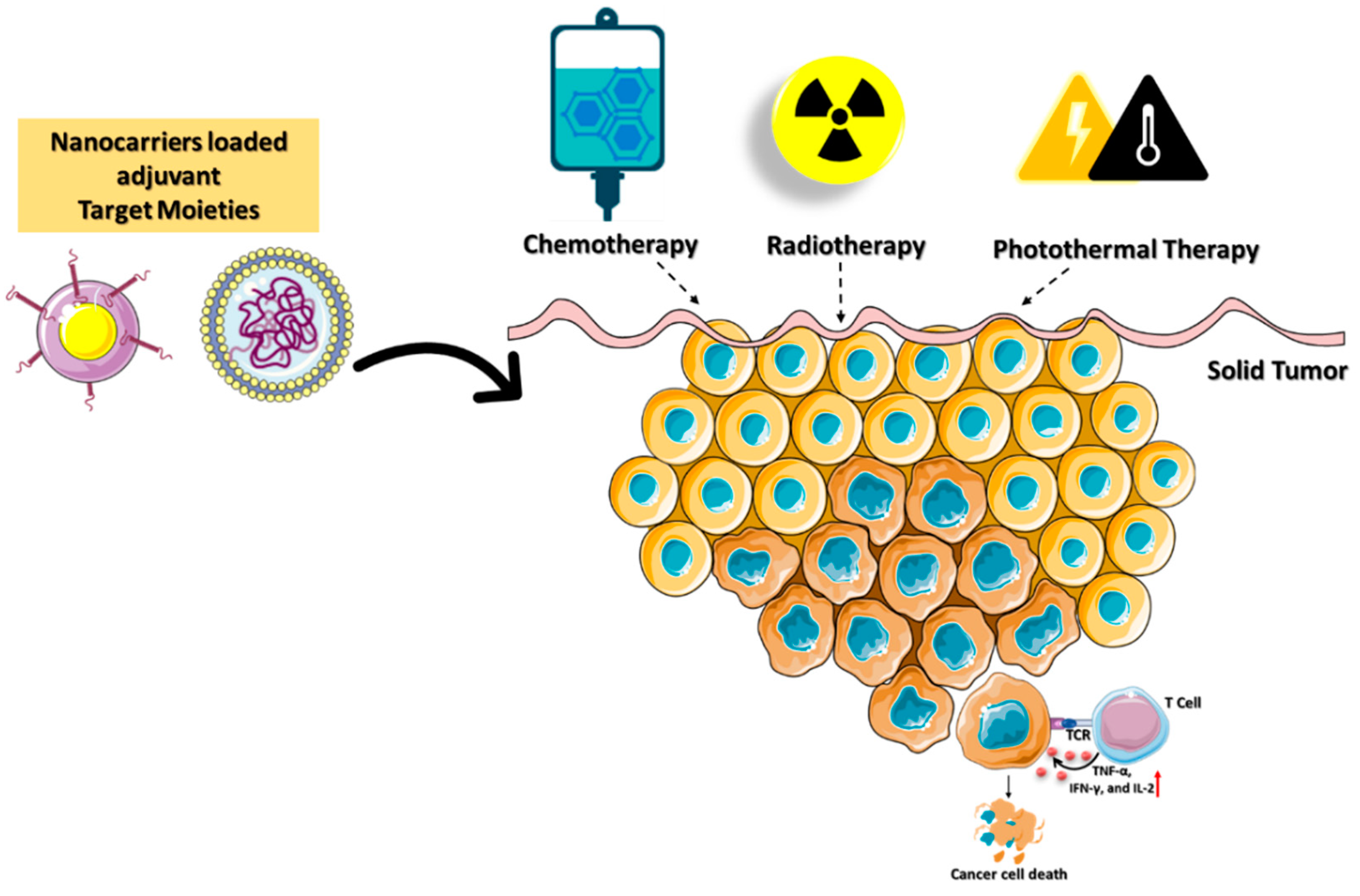
2.4. Nanocarriers for Immunogenic Cell Death (ICD)
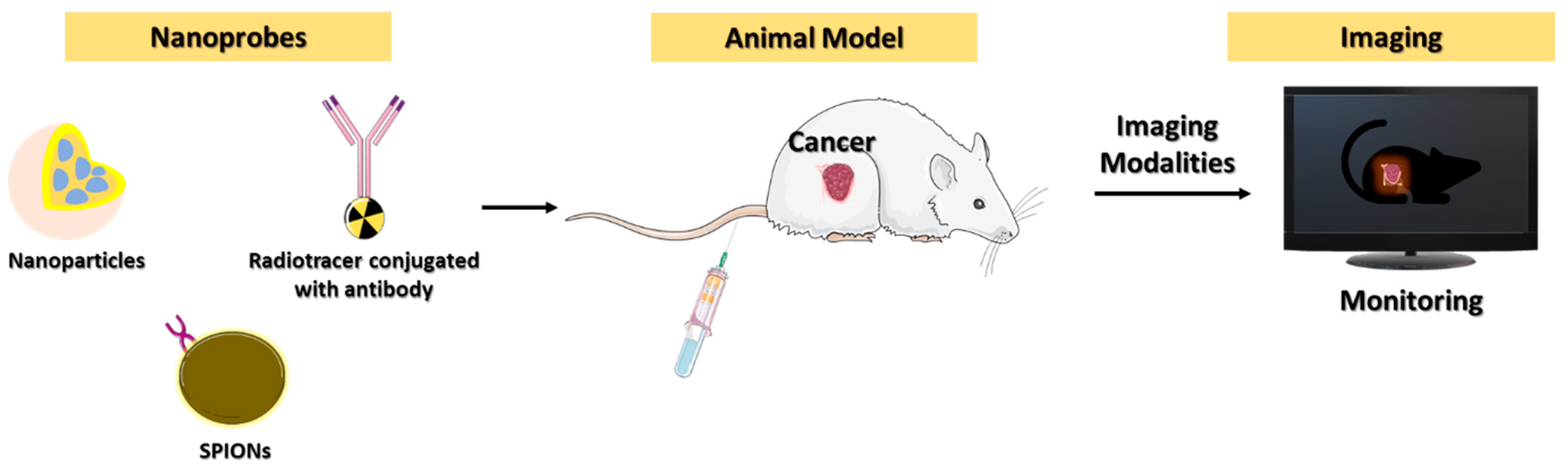
3. Diagnostic Imaging for Cancer Immunotherapy
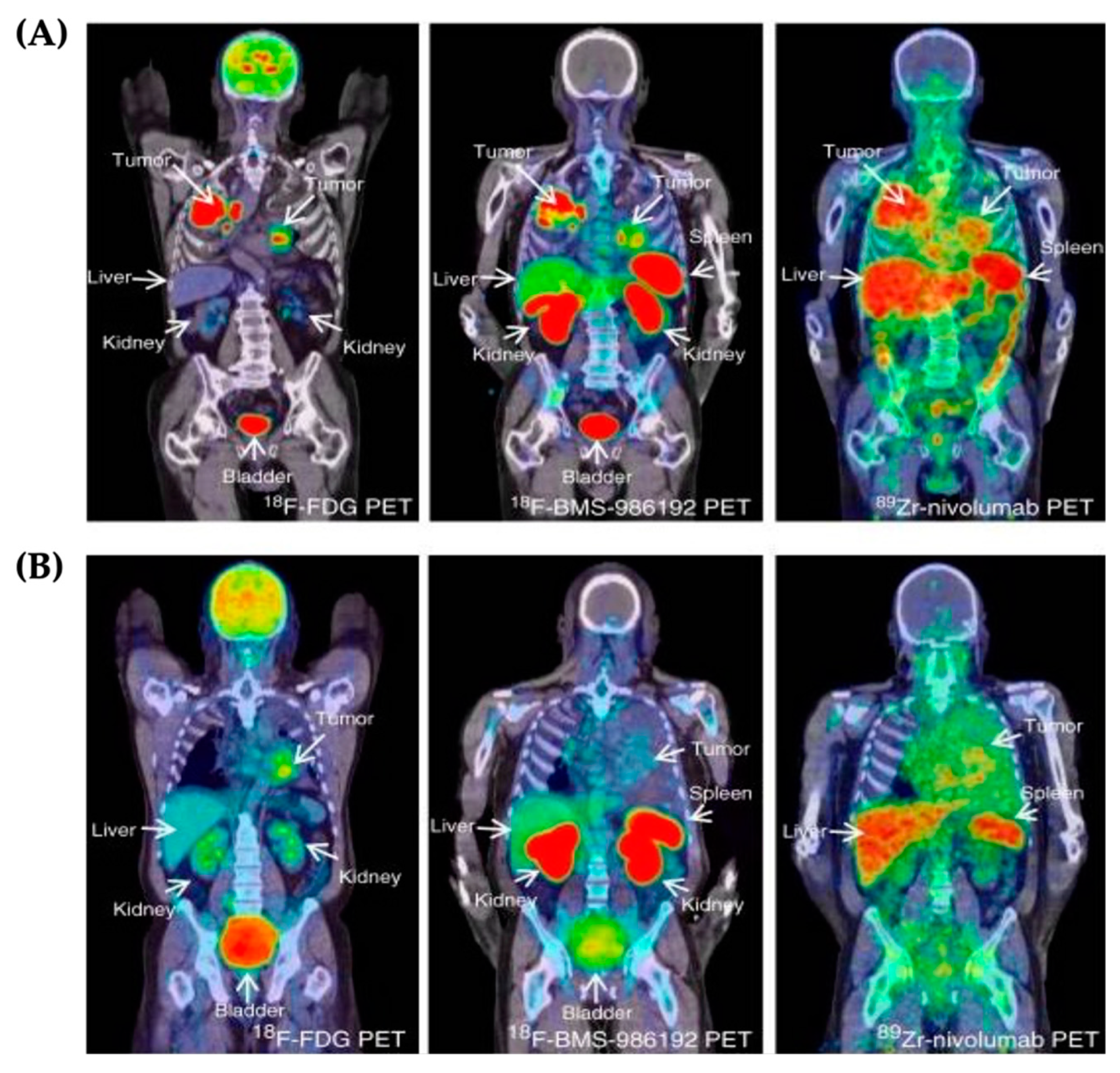
3.1. PET Imaging
3.2. Near-Infrared Imaging
3.3. Magnetic Resonance Imaging
3.4. Challenges and Perspectives of Cancer Immunotherapy Imaging
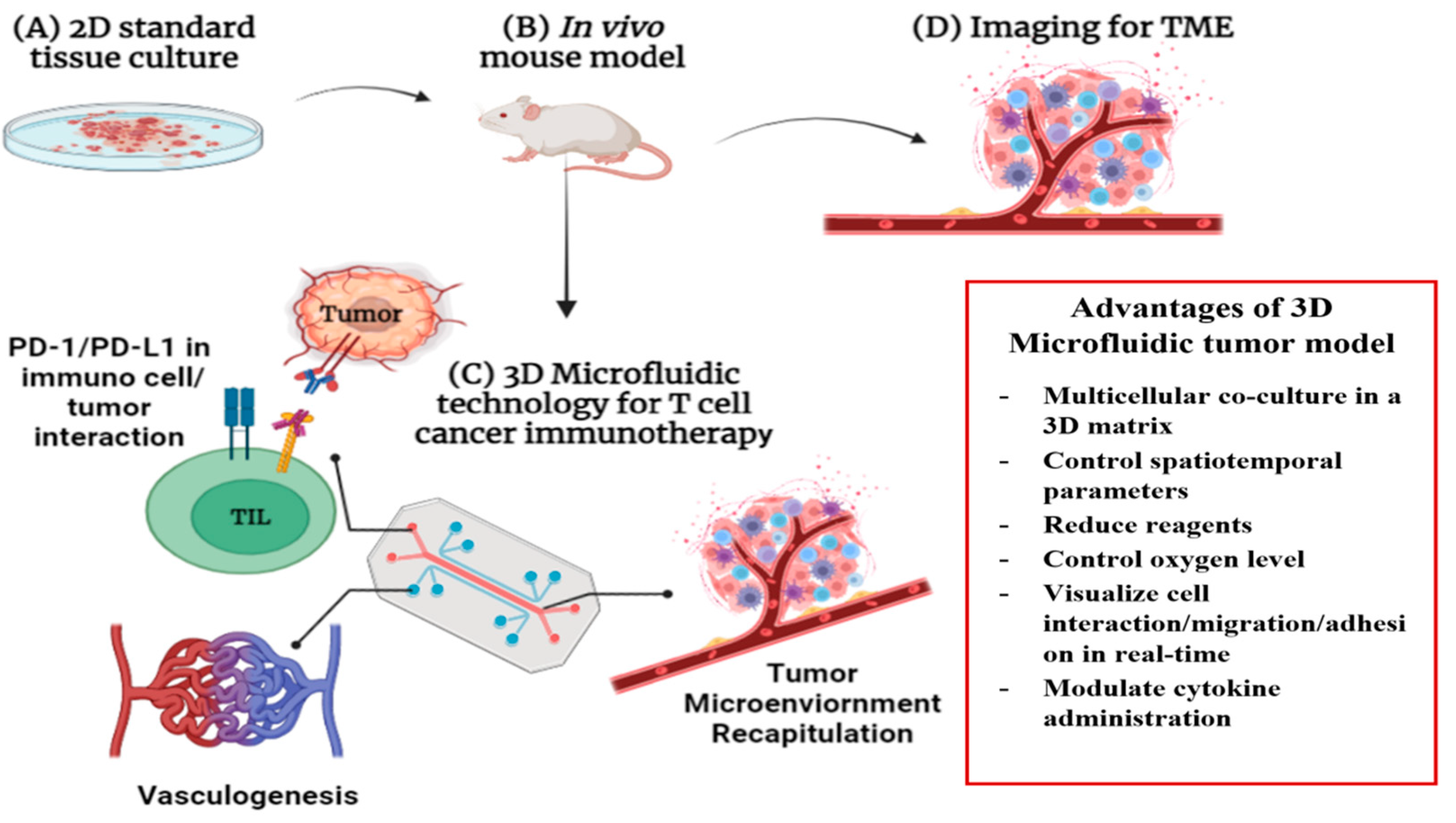
4. Lab-on-Chip-Based Theragnosis for Cancer Immunotherapy
4.1. Multilayered Blood Vessel/Tumor Tissue Chip
4.2. Chimeric Antigen Receptor (CAR)-T LOC
4.3. D/3D Cell Culture Model for Better Cancer Immunotherapy
4.4. Challenges and Perspectives of LOC
5. AI and ML in Immune-Targeted Drug Delivery
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.D.; Schreiber, R.D. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann. N. Y. Acad. Sci. 2013, 1284, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Nixon, N.A.; Blais, N.; Ernst, S.; Kollmannsberger, C.; Bebb, G.; Butler, M.; Smylie, M.; Verma, S. Current landscape of immunotherapy in the treatment of solid tumors, with future opportunities and challenges. Curr. Oncol. 2018, 25, e373–e384. [Google Scholar] [CrossRef]
- Kirilovsky, A.; Marliot, F.; El Sissy, C.; Haicheur, N.; Galon, J.; Pages, F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int. Immunol. 2016, 28, 373–382. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Fan, S.; Ren, H.; Zhao, L.; Yin, J.; Feng, G.; Wang, J.; Guan, H. Neurological immune-related adverse events associated with immune checkpoint inhibitors: A review of the literature. Asia Pac. J. Clin. Oncol. 2020, 16, 291–298. [Google Scholar] [CrossRef]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Rameshbabu, S.; Labadie, B.W.; Argulian, A.; Patnaik, A. Targeting Innate Immunity in Cancer Therapy. Vaccines 2021, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Anselmi, M.; Borbely, A.; Figueras, E.; Michalek, C.; Kemker, I.; Gentilucci, L.; Sewald, N. Linker Hydrophilicity Modulates the Anticancer Activity of RGD-Cryptophycin Conjugates. Chemistry 2021, 27, 1015–1022. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Teixidor, E.; Bosch-Barrera, J. the dark side of immunotherapy: Challenges facing the new hope in cancer treatment. Ann. Transl. Med. 2019, 7, S183. [Google Scholar] [CrossRef] [PubMed]
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Hennart, B.; Allorge, D.; van Geel, N.; van Gele, M.; et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology 2015, 4, e982382. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Sambi, M.; Qorri, B.; Baluch, N.; Ashayeri, N.; Kumar, S.; Cheng, H.M.; Yeger, H.; Das, B.; Szewczuk, M.R. The Next-Generation of Combination Cancer Immunotherapy: Epigenetic Immunomodulators Transmogrify Immune Training to Enhance Immunotherapy. Cancers 2021, 13, 3596. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Greenbaum, U.; Mahadeo, K.M.; Kebriaei, P.; Shpall, E.J.; Saini, N.Y. Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics 2022, 14, 1581. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.N.; Nowsheen, S.; Bonner, J.A.; Yang, E.S. Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors. Front. Mol. Neurosci. 2011, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, R.; Boehme, K.A.; Blattner, C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol. Cell Biol. 2005, 25, 7170–7180. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Maritz, M.F.; Thierry, B. Glycogen kinase 3 inhibitor nanoformulation as an alternative strategy to inhibit PD-1 immune checkpoint. Int. J. Pharm. 2022, 622, 121845. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Yao, H.; Shen, N.; Ji, G.; Huang, J.; Sun, J.; Wang, G.; Tang, Z.; Chen, X. Cisplatin Nanoparticles Promote Intratumoral CD8(+) T Cell Priming via Antigen Presentation and T Cell Receptor Crosstalk. Nano Lett. 2022, 22, 3328–3339. [Google Scholar] [CrossRef]
- Bansal-Pakala, P.; Halteman, B.S.; Cheng, M.H.; Croft, M. Costimulation of CD8 T cell responses by OX40. J. Immunol. 2004, 172, 4821–4825. [Google Scholar] [CrossRef]
- Liu, Z.; Han, C.; Fu, Y.X. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol. Immunol. 2020, 17, 13–26. [Google Scholar] [CrossRef]
- Dane, E.L.; Belessiotis-Richards, A.; Backlund, C.; Wang, J.; Hidaka, K.; Milling, L.E.; Bhagchandani, S.; Melo, M.B.; Wu, S.; Li, N.; et al. STING agonist delivery by tumor-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 2022, 21, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamada, K.; Sato, Y.; Harashima, H. Lipid nanoparticles fuse with cell membranes of immune cells at low temperatures leading to the loss of transfection activity. Int. J. Pharm. 2020, 587, 119652. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.M.; Nakamura, T.; Sato, Y.; Sato, T.; Hyodo, M.; Hayakawa, Y.; Harashima, H. Interval- and cycle-dependent combined effect of STING agonist loaded lipid nanoparticles and a PD-1 antibody. Int. J. Pharm. 2022, 624, 122034. [Google Scholar] [CrossRef] [PubMed]
- Messenheimer, D.J.; Jensen, S.M.; Afentoulis, M.E.; Wegmann, K.W.; Feng, Z.; Friedman, D.J.; Gough, M.J.; Urba, W.J.; Fox, B.A. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin. Cancer Res. 2017, 23, 6165–6177. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Z.; Chen, J.; Guo, S.; You, W.; Wang, S.; Ma, J.; Wang, H.; Wang, X.; Wang, H.; et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer 2022, 10, e004381. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Chen, B.Q.; Zhao, Y.; Zhang, Y.; Pan, Y.J.; Xia, H.Y.; Kankala, R.K.; Wang, S.B.; Liu, G.; Chen, A.Z. Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy. Bioact. Mater. 2023, 21, 1–19. [Google Scholar] [CrossRef]
- Hassannia, H.; Chaleshtari, M.G.; Atyabi, F.; Nosouhian, M.; Masjedi, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Ghalamfarsa, G.; et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology 2020, 159, 75–87. [Google Scholar] [CrossRef]
- Barshidi, A.; Karpisheh, V.; Noukabadi, F.K.; Kiani, F.K.; Mohammadi, M.; Afsharimanesh, N.; Ebrahimi, F.; Kiaie, S.H.; Navashenaq, J.G.; Hojjat-Farsangi, M.; et al. Dual Blockade of PD-1 and LAG3 Immune Checkpoints Increases Dendritic Cell Vaccine Mediated T Cell Responses in Breast Cancer Model. Pharm. Res. 2022, 39, 1851–1866. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Shofolawe-Bakare, O.T.; Stokes, L.D.; Hossain, M.; Smith, A.E.; Werfel, T.A. Immunostimulatory biomaterials to boost tumor immunogenicity. Biomater. Sci. 2020, 8, 5516–5537. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ma, S.; Peng, S.; Zhou, G.; Xie, R.; Jiang, Q.; Guo, S.; He, Q.; Yang, W. Zwitterionic Polymer Coating of Sulfur Dioxide-Releasing Nanosystem Augments Tumor Accumulation and Treatment Efficacy. Adv. Healthc. Mater. 2020, 9, e1901582. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.M.; Hossainy, S.; Rowan, S.J.; Hubbell, J.A.; Esser-Kahn, A.P. Immunostimulatory Polymers as Adjuvants, Immunotherapies, and Delivery Systems. Macromolecules 2022, 55, 6913–6937. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185. [Google Scholar] [CrossRef]
- Hagan, C.T.t.; Mi, Y.; Knape, N.M.; Wang, A.Z. Enhancing Combined Immunotherapy and Radiotherapy through Nanomedicine. Bioconjug. Chem. 2020, 31, 2668–2678. [Google Scholar] [CrossRef]
- Lerner, E.C.; Edwards, R.M.; Wilkinson, D.S.; Fecci, P.E. Laser ablation: Heating up the anti-tumor response in the intracranial compartment. Adv. Drug Deliv. Rev. 2022, 185, 114311. [Google Scholar] [CrossRef]
- Deng, B.; Ma, B.; Ma, Y.; Cao, P.; Leng, X.; Huang, P.; Zhao, Y.; Ji, T.; Lu, X.; Liu, L. Doxorubicin and CpG loaded liposomal spherical nucleic acid for enhanced Cancer treatment. J. Nanobiotechnol. 2022, 20, 140. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Q.; Wang, M.; Hoover, A.; Wang, X.; Zhou, F.; Towner, R.A.; Smith, N.; Saunders, D.; Song, J.; et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem. Eng. J. 2020, 396, 125239. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Song, Y.; Lv, M.; Mao, Y.; Song, H.; Wang, Y.; Nie, G.; Liu, X.; Cui, J.; et al. IR792-MCN@ZIF-8-PD-L1 siRNA drug delivery system enhances photothermal immunotherapy for triple-negative breast cancer under near-infrared laser irradiation. J. Nanobiotechnol. 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gao, A.; Lin, Z. Fluorescence imaging of tumor immune contexture in immune checkpoint blockade therapy. Int. Immunopharmacol. 2022, 106, 108617. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Patel, C.B.; Ramakrishnan, S.; Panesar, P.S.; Long, S.R.; Gambhir, S.S. A Novel Engineered Small Protein for Positron Emission Tomography Imaging of Human Programmed Death Ligand-1: Validation in Mouse Models and Human Cancer Tissues. Clin. Cancer Res. 2019, 25, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.R.B.; Smith, C.C.; Bixby, L.M.; Glatt, D.M.; Dunn, S.S.; Saito, R.; Kim, W.Y.; Serody, J.S.; Vincent, B.G.; Parrott, M.C. Immuno-PET imaging of tumor-infiltrating lymphocytes using the zirconium-89 radiolabeled anti-CD3 antibody in immune-competent mice bearing syngeneic tumors. PLoS ONE 2018, 13, e0193832. [Google Scholar]
- Xie, N.; Hou, Y.; Wang, S.; Ai, X.; Bai, J.; Lai, X.; Zhang, Y.; Meng, X.; Wang, X. Second near-infrared (NIR-II) imaging: A novel diagnostic technique for brain diseases. Rev. Neurosci. 2022, 33, 467–490. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; Yetisen, A.K.; et al. Near-Infrared-II Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. [Google Scholar] [CrossRef]
- Luo, X.; Peng, X.; Hou, J.; Wu, S.; Shen, J.; Wang, L. Folic acid-functionalized polyethyleneimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int. J. Nanomed. 2017, 12, 5331–5343. [Google Scholar] [CrossRef]
- Li, X.; Ji, Y.; Chen, M.; Zhang, S.; Wang, Z.; Su, D.; Luo, N. A Dual-Mode Imaging Nanoparticle Probe Targeting PD-L1 for Triple-Negative Breast Cancer. Contrast Media Mol. Imaging 2022, 2022, 2431026. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Shaegh, S.A.; Kashaninejad, N.; Phan, D.T. Design, fabrication, and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Z.; Shamsi, M.H. Desktop Fabrication of Lab-On-Chip Devices on Flexible Substrates: A Brief Review. Micromachines 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Parlato, S.; Grisanti, G.; Sinibaldi, G.; Peruzzi, G.; Casciola, C.M.; Gabriele, L. Tumor-on-a-chip platforms to study cancer-immune system crosstalk in the era of immunotherapy. Lab Chip 2021, 21, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xiao, Y.; Liu, H.; Fan, Y.; Dao, M. Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation. Adv. Biol. 2021, 5, e2000024. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Segal, E. Lab-on-a-Chip Devices for Point-of-Care Medical Diagnostics. Adv. Biochem. Eng. Biotechnol. 2022, 179, 247–265. [Google Scholar]
- Lee, J.; Kim, S.E.; Moon, D.; Doh, J. A multilayered blood vessel/tumor tissue chip to investigate T cell infiltration into solid tumor tissues. Lab Chip 2021, 21, 2142–2152. [Google Scholar] [CrossRef]
- Paterson, K.; Paterson, S.; Mulholland, T.; Coffelt, S.B.; Zagnoni, M. Assessment of CAR-T Cell-Mediated Cytotoxicity in 3D Microfluidic Cancer Co-Culture Models for Combination Therapy. IEEE Open J. Eng. Med. Biol. 2022, 3, 86–95. [Google Scholar] [CrossRef]
- Adriani, G.; Pavesi, A.; Tan, A.T.; Bertoletti, A.; Thiery, J.P.; Kamm, R.D. Microfluidic models for adoptive cell-mediated cancer immunotherapies. Drug Discov. Today 2016, 21, 1472–1478. [Google Scholar] [CrossRef]
- Aref, A.R.; Campisi, M.; Ivanova, E.; Portell, A.; Larios, D.; Piel, B.P.; Mathur, N.; Zhou, C.; Coakley, R.V.; Bartels, A.; et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip 2018, 18, 3129–3143. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Zeng, S.; Ren, X.; Yan, Y.; Gong, Z. Applying artificial intelligence for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3393–3405. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, C.; Jain, K.; Masanam, H.B.; Narasimhan, A.K.; Natarajan, A. Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications. Micromachines 2022, 13, 2217. https://doi.org/10.3390/mi13122217
John C, Jain K, Masanam HB, Narasimhan AK, Natarajan A. Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications. Micromachines. 2022; 13(12):2217. https://doi.org/10.3390/mi13122217
Chicago/Turabian StyleJohn, Clyde, Kaahini Jain, Hema Brindha Masanam, Ashwin Kumar Narasimhan, and Arutselvan Natarajan. 2022. "Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications" Micromachines 13, no. 12: 2217. https://doi.org/10.3390/mi13122217
APA StyleJohn, C., Jain, K., Masanam, H. B., Narasimhan, A. K., & Natarajan, A. (2022). Recent Trends and Opportunities for the Targeted Immuno-Nanomaterials for Cancer Theranostics Applications. Micromachines, 13(12), 2217. https://doi.org/10.3390/mi13122217






