Hierarchical Activated Carbon–MnO2 Composite for Wide Potential Window Asymmetric Supercapacitor Devices in Organic Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AC-MnO2 Composite
2.3. Assembly of the Supercapacitor Devices
2.4. Characterization
2.5. Calculation
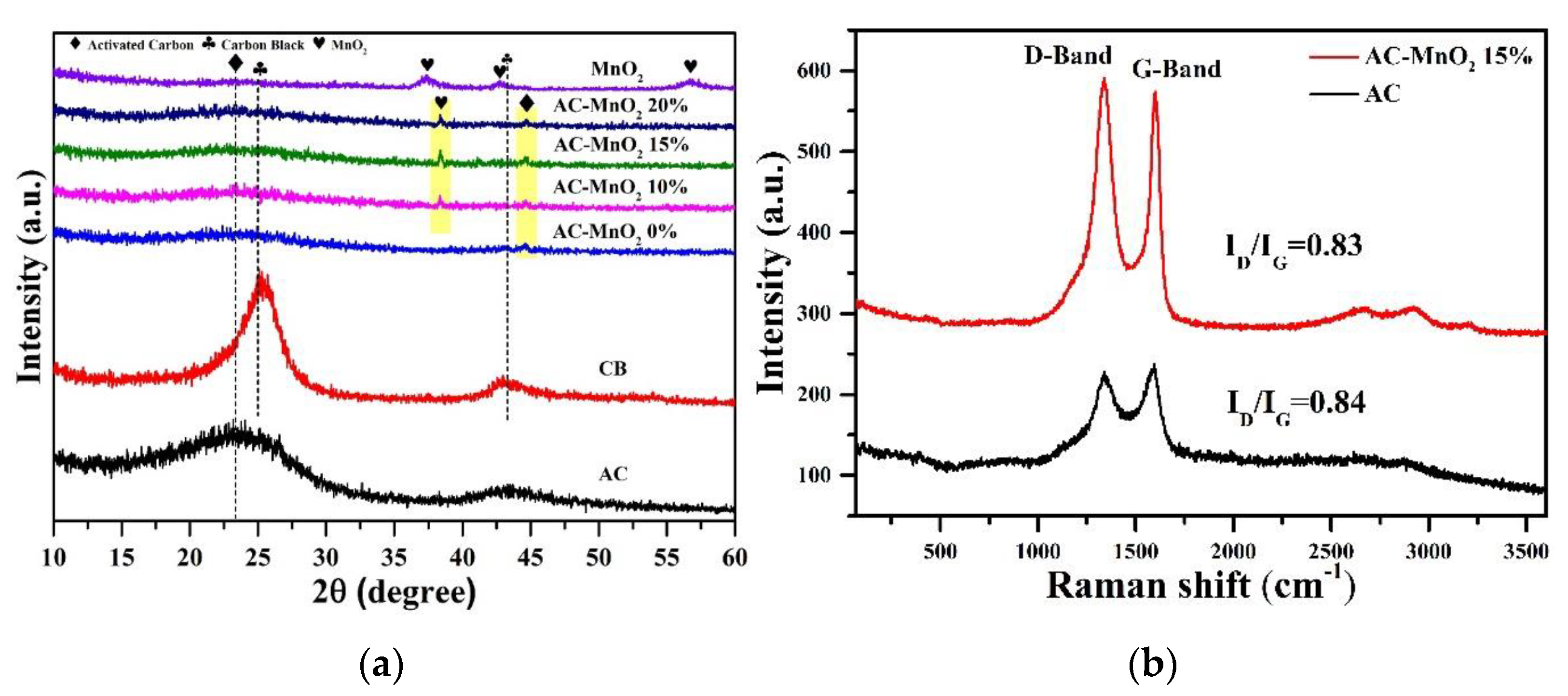
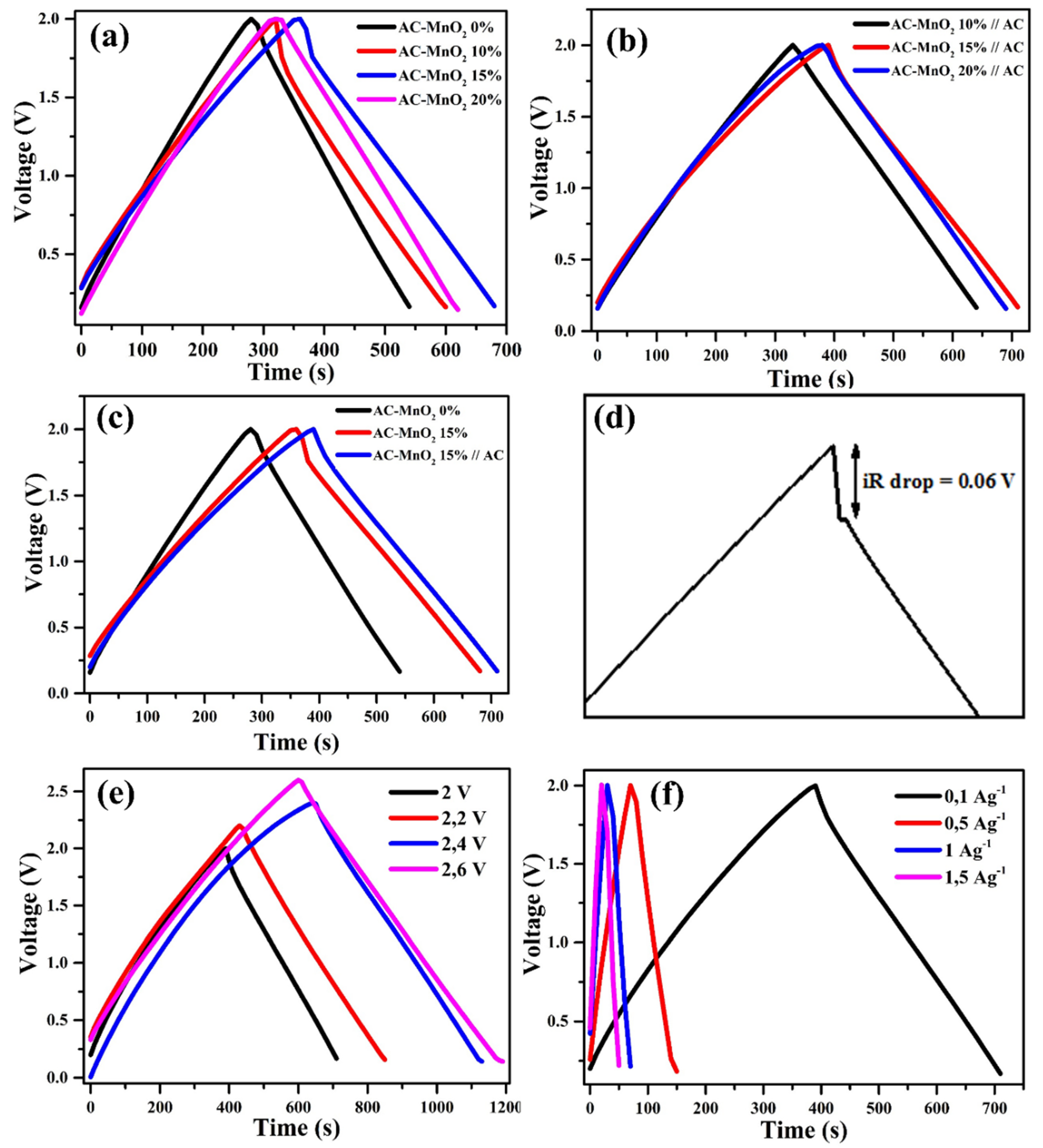
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al-Alami, A.H. Critical Review of Energy Storage Systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A Review of Energy Storage Types, Applications and Recent Developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Jiang, S.H.; Ding, J.; Wang, R.H.; Chen, F.Y.; Sun, J.; Deng, Y.X.; Li, X.L. Solvothermal-Induced Construction of Ultra-Tiny Fe2O3 Nanoparticles/Graphene Hydrogels as Binder-Free High-Capacitance Anode for Supercapacitors. Rare Met. 2021, 40, 3520–3530. [Google Scholar] [CrossRef]
- Diantoro, M.; Luthfiyah, I.; Istiqomah; Wisodo, H.; Utomo, J.; Meevasana, W. Electrochemical Performance of Symmetric Supercapacitor Based on Activated Carbon Biomass TiO2 Nanocomposites. J. Phys. Conf. Ser. 2022, 2243, 012077. [Google Scholar] [CrossRef]
- Zuhri, F.U.; Diantoro, M.; Suryanti, L.; Suprayogi, T.; Nasikhudin; Sunaryono; Meevasana, W. ZnO-FC-NiCo MOF for Prospective Supercapacitor Materials. Mater. Today Proc. 2020, 44, 3385–3389. [Google Scholar] [CrossRef]
- Son, Y.H.; Bui, P.T.M.; Lee, H.R.; Akhtar, M.S.; Shah, D.K.; Yang, O.B. A Rapid Synthesis of Mesoporous Mn2O3 Nanoparticles for Supercapacitor Applications. Coatings 2019, 9, 631. [Google Scholar] [CrossRef]
- Cai, X.; Sun, K.; Qiu, Y.; Jiao, X. Recent Advances in Graphene and Conductive Polymer Composites for Supercapacitor Electrodes: A Review. Crystals 2021, 11, 947. [Google Scholar] [CrossRef]
- Yumak, T. Electrochemical Performance of Fabricated Supercapacitors Using MnO2/Activated Carbon Electrodes. Hacettepe J. Biol. Chem. 2019, 47, 115–122. [Google Scholar] [CrossRef]
- Shanmuga Priya, M.; Divya, P.; Rajalakshmi, R. A Review Status on Characterization and Electrochemical Behaviour of Biomass Derived Carbon Materials for Energy Storage Supercapacitors. Sustain. Chem. Pharm. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Omokafe, S.M.; Adeniyi, A.A.; Igbafen, E.O.; Oke, S.R.; Olubambi, P.A. Fabrication of Activated Carbon from Coconut Shells and Its Electrochemical Properties for Supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 10854–10865. [Google Scholar] [CrossRef]
- Kumar, Y.; Chopra, S.; Gupta, A.; Kumar, Y.; Uke, S.J.; Mardikar, S.P. Low Temperature Synthesis of MnO2 Nanostructures for Supercapacitor Application. Mater. Sci. Energy Technol. 2020, 3, 566–574. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.M.; Jung, S.C.; Chung, D.C.; Kim, B.J. Bamboo-Based Mesoporous Activated Carbon for High-Power-Density Electric Double-Layer Capacitors. Nanomaterials 2021, 11, 2750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zuo, S.; Shen, B.; Wang, Y.; Xia, H. Fabrication of Nanosized Layered-MnO2/Activated Carbon Composites Electrodes for High-Performance Supercapacitor Ya. Int. J. Electrochem. Sci. 2020, 15, 7646–7662. [Google Scholar] [CrossRef]
- Tseng, L.H.; Hsiao, C.H.; Nguyen, D.D.; Hsieh, P.Y.; Lee, C.Y.; Tai, N.H. Activated Carbon Sandwiched Manganese Dioxide/Graphene Ternary Composites for Supercapacitor Electrodes. Electrochim. Acta 2018, 266, 284–292. [Google Scholar] [CrossRef]
- Zhou, B.; Sui, Y.; Qi, J.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Zhang, X. Synthesis of Ultrathin MnO2 Nanosheets/Bagasse Derived Porous Carbon Composite for Supercapacitor with High Performance. J. Electron. Mater. 2019, 48, 3026–3035. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the Use of Transition-Metal-Oxide and Conducting Polymer-Based Fibres for High-Performance Supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Suryanti, L.; Suryani, S.E.I.; Hartatiek; Nasikhudin; Utomo, J.; Taufiq, A.; Suryana, R.; Aspanut, Z.; Diantoro, M. The Effect of Mn2O3 nanoparticles on Its Specific Capacitance of Symmetric Supercapacitors FC-ZnO-x(Mn2O3). Mater. Today Proc. 2020, 44, 3355–3360. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Z.; Deng, Q. The E Ff Ect of an External Magnetic Field on the Electrochemical Capacitance of Nanoporous Nickel for Energy Storage. Nanomaterials 2019, 9, 694. [Google Scholar] [CrossRef]
- Choi, J.R.; Lee, J.W.; Yang, G.; Heo, Y.J.; Park, S.J. Activated Carbon/MnO2 Composites as Electrode for High Performance Supercapacitors. Catalysts 2020, 10, 256. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Ma, X.; Li, J. In Situ Hydrothermal Synthesis of MnO2 Nanowires/Wood Derived Activated Carbon Hollow Fibers Composite and Its Application in Supercapacitor. Wood Res. 2020, 65, 737–746. [Google Scholar] [CrossRef]
- Mothkuri, S.; Gupta, H.; Jain, P.K.; Rao, T.N.; Padmanabham, G.; Chakrabarti, S. Functionalized Carbon Nanotube and MnO2 Nanoflower Hybrid as an Electrode Material for Supercapacitor Application. Micromachines 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, F.; Javanbakht, M.; Shahrokhian, S. Anodic Pulse Electrodeposition of Mesoporous Manganese Dioxide Nanostructures for High Performance Supercapacitors. J. Alloys Compd. 2021, 887, 161376. [Google Scholar] [CrossRef]
- Majumdar, D. Review on Current Progress of MnO2-Based Ternary Nanocomposites for Supercapacitor Applications. ChemElectroChem 2020, 8, 291–336. [Google Scholar] [CrossRef]
- Raheem, Z.H.; Al-Sammarraie Ali, A.M. Hydrothermal Synthesis of Y-MnO2 Nanostructures with Different Morphologies Using Different Mn+2 Precursors. Syst. Rev. Pharm. 2020, 11, 453–458. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 Battery Chemistry with Dissolution-Deposition Mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Bhat, T.S.; Jadhav, S.A.; Beknalkar, S.A.; Patil, S.S.; Patil, P.S. MnO2 Core-Shell Type Materials for High-Performance Supercapacitors: A Short Review. Inorg. Chem. Commun. 2022, 141, 109493. [Google Scholar] [CrossRef]
- Singu, B.S.; Goda, E.S.; Yoon, K.R. Carbon Nanotube–Manganese Oxide Nanorods Hybrid Composites for High-Performance Supercapacitor Materials. J. Ind. Eng. Chem. 2021, 97, 239–249. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the Supercapacitor Performances of Activated Carbon with Carbon Nanomaterials. J. Power Sources 2020, 450, 227678. [Google Scholar] [CrossRef]
- Yang, H.; Kannappan, S.; Pandian, A.S.; Jang, J.H.; Lee, Y.S.; Lu, W. Graphene Supercapacitor with Both High Power and Energy Density. Nanotechnology 2017, 28, 445401. [Google Scholar] [CrossRef]
- Huang, C.L.; Chiang, L.M.; Su, C.A.; Li, Y.Y. MnO2/Carbon Nanotube-Embedded Carbon Nanofibers as Core–Shell Cables for High Performing Asymmetric Flexible Supercapacitors. J. Ind. Eng. Chem. 2021, 103, 142–153. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, Q.; Qian, Y.; He, J.; Dong, X. Alkali Cation Incorporated MnO2 Cathode and Carbon Cloth Anode for Flexible Aqueous Supercapacitor with High Wide-Voltage and Power Density; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 342. [Google Scholar] [CrossRef]
- Roy, C.K.; Shah, S.S.; Reaz, A.H.; Sultana, S.; Chowdhury, A.N.; Firoz, S.H.; Zahir, M.H.; Ahmed Qasem, M.A.; Aziz, M.A. Preparation of Hierarchical Porous Activated Carbon from Banana Leaves for High-Performance Supercapacitor: Effect of Type of Electrolytes on Performance. Chem.—Asian J. 2021, 16, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, M.; Kali, R.; Bello, A.; Padya, B.; Kalu-Uka, G.M.; Wasswa, J.; Jain, P.K.; Onwualu, P.A.; Dzade, N.Y. Modified Activation Process for Supercapacitor Electrode Materials from African Maize Cob. Materials 2020, 13, 5412. [Google Scholar] [CrossRef] [PubMed]
- Tagsin, P.; Suksangrat, P.; Klangtakai, P.; Srepusharawoot, P.; Ruttanapun, C.; Kumnorkaew, P.; Pimanpang, S.; Amornkitbamrung, V. Electrochemical Mechanisms of Activated Carbon, α-MnO2 and Composited Activated Carbon-α-MnO2 Films in Supercapacitor Applications. Appl. Surf. Sci. 2021, 570, 151056. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-Supported Pd Catalyst for Highly Selective Hydrogenation of Resorcinol to 1, 3-Cyclohexanedione through Giant π-Conjugate Interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef]
- Yan, S.; Lin, J.; Liu, P.; Zhao, Z.; Lian, J.; Chang, W.; Yao, L.; Liu, Y.; Lin, H.; Han, S. Preparation of Nitrogen-Doped Porous Carbons for High-Performance Supercapacitor Using Biomass of Waste Lotus Stems. RSC Adv. 2018, 8, 6806–6813. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated Amorphous Carbon With High-Porosity Derived From Camellia Pollen Grains as Anode Materials for Lithium/Sodium Ion Batteries. Front. Chem. 2018, 6, 366. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of Garlic Skin-Derived 3D Hierarchical Porous Carbon for High-Performance Supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef]
- Luo, X.Y.; Chen, Y.; Mo, Y. A Review of Charge Storage in Porous Carbon-Based Supercapacitors. Xinxing Tan Cailiao/New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Çıplak, Z.; Yıldız, N. The Effect of Ag Loading on Supercapacitor Performance of Graphene Based Nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 65–76. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W.-P. Enhancing the Pore Wettability of Coal-Based Porous Carbon as Electrode Materials for High Performance Supercapacitors. Mater. Chem. Phys. 2020, 252, 123381. [Google Scholar] [CrossRef]
- Markoulidis, F.; Trapalis, C.; Todorova, N.; Grilli, R.; Lekakou, C. Composite Electrodes of Activated Carbon and Multiwall Carbon Nanotubes Decorated with Silver Nanoparticles for High Power Energy Storage. J. Compos. Sci. 2019, 3, 97. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Ye, Z.; Jin, Y.; Cui, C.; Xie, Q.; Wang, J.; Zhang, G.; Dong, Z.; Miao, Y.; et al. High Energy and High Power Density Supercapacitor with 3D Al Foam-Based Thick Graphene Electrode: Fabrication and Simulation. Energy Storage Mater. 2020, 33, 18–25. [Google Scholar] [CrossRef]
- Abdul Mageeth, A.M.; Park, S.J.; Jeong, M.; Kim, W.; Yu, C. Planar-Type Thermally Chargeable Supercapacitor without an Effective Heat Sink and Performance Variations with Layer Thickness and Operation Conditions. Appl. Energy 2020, 268, 114975. [Google Scholar] [CrossRef]
- Kumagai, S.; Mukaiyachi, K.; Tashima, D. Rate and Cycle Performances of Supercapacitors with Different Electrode Thickness Using Non-Aqueous Electrolyte. J. Energy Storage 2015, 3, 10–17. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhang, Q.; Yang, J.; Duan, G.; Xu, W.; Yang, F.; Jiang, S. Electrode Thickness Design toward Bulk Energy Storage Devices with High Areal/Volumetric Energy Density. Appl. Energy 2021, 289, 116734. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Deng, T.; Zhang, W. Metal Organic Framework Derived Porous Carbon Materials Excel as an Excellent Platform for High-Performance Packaged Supercapacitors. Nanoscale 2021, 13, 5570–5593. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiang, B.; Liu, Z.; Li, C.; Yan, F.; Liu, X.; Li, H.; Chao, Y.; Dong, D. Effects of Specific Surface Area of Electrode and Different Electrolyte on Capacitance Properties in Nano Porous-Structure CrN Thin Film Electrode for Supercapacitor. Ceram. Int. 2021, 47, 18540–18549. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Zhong, R.; Zhou, A.; Wang, X.; Yang, Z. A Quasi-Solid Asymmetric Supercapacitor Based on MnO2-Coated and N-Doped Pinecone Porous Carbon. J. Mater. Sci. Mater. Electron. 2022, 33, 1899–1909. [Google Scholar] [CrossRef]
- Abbas, A.; Abbas, S.; Bhattarai, A.; Latiff, N.M.; Wai, N.; Phan, A.N.; Lim, T.M. Effect of Electrode Porosity on the Charge Transfer in Vanadium Redox Flow Battery. J. Power Sources 2021, 488, 229411. [Google Scholar] [CrossRef]
- Zhang, L. Storage Properties of the Shale Reservoir of the Lower Keluke Formation in the Shihuigou Area: Implications for the Controlling Factors. Geofluids 2022, 2022, 9007217. [Google Scholar] [CrossRef]
- Majid, S.; Ali, A.S.G.; Cao, W.Q.; Reza, R.; Ge, Q. Biomass-Derived Porous Carbons as Supercapacitor Electrodes-A Review. Xinxing Tan Cailiao/New Carbon Mater. 2021, 36, 546–572. [Google Scholar] [CrossRef]
- Jow, T.R.; Xu, K.; Ding, S.P. Nonaqueous Electrolyte Development for Electrochemical Capacitors; U.S. Army Research Laboratory: Adelphi, MD, USA, 1999; pp. 1–39. [Google Scholar] [CrossRef][Green Version]
- Borghei, S.A.; Zare, M.H.; Ahmadi, M.; Sadeghi, M.H.; Marjani, A.; Shirazian, S.; Ghadiri, M. Synthesis of Multi-Application Activated Carbon from Oak Seeds by KOH Activation for Methylene Blue Adsorption and Electrochemical Supercapacitor Electrode. Arab. J. Chem. 2021, 14, 102958. [Google Scholar] [CrossRef]
- Lee, K.C.; Lim, M.S.W.; Hong, Z.Y.; Chong, S.; Tiong, T.J.; Pan, G.T.; Huang, C.M. Coconut Shell-Derived Activated Carbon for High-Performance Solid-State Supercapacitors. Energies 2021, 14, 4546. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Li, L.; Peng, Z.; Tang, X.; Hu, T.; Yuan, K.; Chen, Y. Molecular Crowding Agents Engineered to Make Bioinspired Electrolytes for High-Voltage Aqueous Supercapacitors. eScience 2021, 1, 83–90. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Ahankari, S.; Lasrado, D.; Subramaniam, R. Advances in Materials and Fabrication of Separators in Supercapacitors. Mater. Adv. 2022, 3, 1472–1496. [Google Scholar] [CrossRef]
- Ashraf, M.; Shah, S.S.; Khan, I.; Aziz, M.A.; Ullah, N.; Khan, M.; Adil, S.F.; Liaqat, Z.; Usman, M.; Tremel, W.; et al. High-Performance Asymmetric Supercapacitor Based on Tungsten Oxide Nanoplates and Highly Reduced Graphene Oxide Electrodes. Chem.—Eur. J. 2021, 27, 6973–6984. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Han, Q.; Zhao, W.; Dai, L.; Ni, Y. Screen Printing Fabricating Patterned and Customized Full Paper-Based Energy Storage Devices with Excellent Photothermal, Self-Healing, High Energy Density and Good Electromagnetic Shielding Performances. J. Mater. Sci. Technol. 2022, 97, 190–200. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass Derived Carbon for Supercapacitor Applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Mohammadi, N.; Pourreza, K.; Bahrami Adeh, N.; Omidvar, M. Defective Mesoporous Carbon/MnO2 Nanocomposite as an Advanced Electrode Material for Supercapacitor Application. J. Alloys Compd. 2021, 883, 160874. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Cevik, E.; Gondal, M.A.; Bozkurt, A.; Akhtar, S.; Hassan, M. Synthesis of Manganese (IV) Oxide at Activated Carbon on Reduced Graphene Oxide Sheets via Laser Irradiation Technique for Organic Binder-Free Electrodes in Flexible Supercapacitors. Ceram. Int. 2021, 47, 7416–7424. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Han, Y.K.; Lee, D.W. Carbon Alternative Pseudocapacitive V2O5 Nanobricks and δ-MnO2 nanoflakes @α-MnO2 nanowires hetero-phase for high-energy pseudocapacitor. J. Power Sources 2020, 453, 227766. [Google Scholar] [CrossRef]
- Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. One-Pot Synthesis of Sulfur and Nitrogen Co-Functionalized Graphene Material using Deep Eutectic Solvents for Supercapacitors. Chem. Sustain. Chem. 2019, 12, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Du, H. Electrochemical Capacitance of a Carbon Quantum Dots-Polypyrrole/Titania Nanotube Hybrid. RSC Adv. 2015, 5, 89689–89697. [Google Scholar] [CrossRef]
- Huang, J.J.; Zhang, Y.X.; Zhang, J.X. Characterization of MnO2 and AgNWs Co-Doped into an Activated Carbon Thin Film Electrode for Supercapacitors. J. Electron. Mater. 2021, 50, 6535–6544. [Google Scholar] [CrossRef]
- Xu, L.; Jia, M.; Li, Y.; Jin, X.; Zhang, F. High-Performance MnO2-Deposited Graphene/Activated Carbon Film Electrodes for Flexible Solid-State Supercapacitor. Sci. Rep. 2017, 7, 12857. [Google Scholar] [CrossRef]
- Yang, K.; Cho, K.; Yoon, D.S.; Kim, S. Bendable Solid-State Supercapacitors with Au Nanoparticle-Embedded Graphene Hydrogel Films. Sci. Rep. 2017, 7, 40163. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Zu, L.; Cai, X.; Liu, Y.; Wang, X.; Lian, H. New Supercapacitors Based on the Synergetic Redox Effect between Electrode and Electrolyte. Materials 2016, 9, 734. [Google Scholar] [CrossRef]
- Doloksaribu, M.; Harsojo; Triyana, K.; Prihandoko, B. The Effect of Concentration Nanoparticles MnO2 DOPED in Activated Carbon as Supercapacitor Electrodes. Int. J. Appl. Eng. Res. 2017, 12, 8625–8631. [Google Scholar]
- Zhang, J.; Sun, J.; Ahmed Shifa, T.; Wang, D.; Wu, X.; Cui, Y. Hierarchical MnO2/Activated Carbon Cloth Electrode Prepared by Synchronized Electrochemical Activation and Oxidation for Flexible Asymmetric Supercapacitors. Chem. Eng. J. 2019, 372, 1047–1055. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Udaya, B.K.; Panemangalore, D.B. Ionic Liquid Electrolytes for Flexible Supercapacitors. Flex. Supercapacitor Nanoarchitectonics 2021, 575–610. [Google Scholar] [CrossRef]
- Kundu, S.; Mogera, U.; George, S.J.; Kulkarni, G.U. A Planar Supercapacitor Made of Supramolecular Nanofibre Based Solid Electrolyte Exhibiting 8 V Window. Nano Energy 2019, 61, 259–266. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Anothumakkool, B.; Torris, A.T.; Nair, S.B.; Badiger, M.V.; Kurungot, S. An All-Solid-State-Supercapacitor Possessing a Non-Aqueous Gel Polymer Electrolyte Prepared Using a UV-Assisted: In Situ Polymerization Strategy. J. Mater. Chem. A 2017, 5, 8461–8476. [Google Scholar] [CrossRef]
- Xiao, T.; Li, J.; Zhuang, X.; Zhang, W.; Wang, S.; Chen, X.; Xiang, P.; Jiang, L.; Tan, X. Wide Potential Window and High Specific Capacitance Triggered via Rough NiCo2S4 Nanorod Arrays with Open Top for Symmetric Supercapacitors. Electrochim. Acta 2018, 269, 397–404. [Google Scholar] [CrossRef]
- Ali, M.S.M.; Zainal, Z.; Hussein, M.Z.; Wahid, M.H.; Bahrudin, N.N.; Muzakir, M.M.; Jalil, R. Porous Carboxymethyl Cellulose Carbon of Lignocellulosic Based Materials Incorporated Manganese Oxide for Supercapacitor Application. Int. J. Biol. Macromol. 2021, 180, 654–666. [Google Scholar] [CrossRef]
- Long, X.; Tian, L.; Wang, J.; Zhang, L.; Chen, Y.; Emin, A.; Wang, X.; Xie, W.; Liu, D.; Fu, Y.; et al. Interconnected δ-MnO2 Nanosheets Anchored on Activated Carbon Cloth as Flexible Electrode for High-Performance Aqueous Asymmetric Supercapacitors. J. Electroanal. Chem. 2020, 877, 114656. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Song, L.; Wu, M.; Gao, Y.H.; Chen, J.; Mao, C.; Song, J.; Niu, H. Co2+ Induced Phase Transformation from δ- to α-MnO2 and Their Hierarchical α-MnO2@δ-MnO2 Nanostructures for Efficient Asymmetric Supercapacitor. J. Mater. Chem. A 2019, 7, 12661–12668. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, C.; Qian, W.; Teng, C. Flower-like MnO2 on Layered Carbon Derived from Sisal Hemp for Asymmetric Supercapacitor with Enhanced Energy Density. J. Alloys Compd. 2020, 826, 154133. [Google Scholar] [CrossRef]
- Wang, W.; Qi, J.; Sui, Y.; He, Y.; Meng, Q.; Wei, F.; Jin, Y. An Asymmetric Supercapacitor Based on Activated Porous Carbon Derived from Walnut Shells and NiCo2O4 Nanoneedle Arrays Electrodes. J. Nanosci. Nanotechnol. 2018, 18, 5600–5608. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Hou, L.; Zhang, X.; Zhou, Y.; Yang, Y.; Hu, Z. Graphene Hydrogels Functionalized Non-Covalently by Fused Heteroaromatic Molecule for Asymmetric Supercapacitor with Ultra-Long Cycle Life. Electrochim. Acta 2019, 317, 437–448. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.; Wu, B.; Hu, N. Vertical Carbon Skeleton Introduced Three-Dimensional MnO2 Nanostructured Composite Electrodes for High-Performance Asymmetric Supercapacitors. J. Power Sources 2020, 476, 228527. [Google Scholar] [CrossRef]
- Miao, J.; Zhou, C.; Yan, X.; Jiang, H.; You, M.; Zhu, Y.; Li, Y.; Zhou, W.; Cheng, X. Electrochemical Performance of an Asymmetric Coin Cell Supercapacitor Based on Marshmallow-like MnO2/Carbon Cloth in Neutral and Alkaline Electrolytes. Energy Fuels 2021, 35, 2766–2774. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Yang, W.D.; Lee, K.C.; Huang, C.M. An Effective Electrodeposition Mode for Porous MnO2/Ni Foam Composite for Asymmetric Supercapacitors. Materials 2016, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Wen, Z.Q.; Guo, X.L.; Zhang, S.M.; Zhang, Y.X. Engineering Firecracker-like Beta-Manganese Dioxides@spinel Nickel Cobaltates Nanostructures for High-Performance Supercapacitors. J. Power Sources 2014, 270, 426–433. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Huang, M.; Li, F.; Wang, X.L.; Wen, Z.Q. One-Pot Synthesis of Hierarchical MnO2-Modified Diatomites for Electrochemical Capacitor Electrodes. J. Power Sources 2014, 246, 449–456. [Google Scholar] [CrossRef]
- He, X.; Chen, Q.; Mao, X.; Liu, W.; Zhou, Y.; Yang, W.; Yang, Y.; Xu, J. Pseudocapacitance Electrode and Asymmetric Supercapacitor Based on Biomass Juglone/Activated Carbon Composites. RSC Adv. 2019, 9, 30809–30814. [Google Scholar] [CrossRef] [PubMed]
- Hatzell, K.B.; Fan, L.; Beidaghi, M.; Boota, M.; Pomerantseva, E.; Kumbur, E.C.; Gogotsi, Y. Composite Manganese Oxide Percolating Networks as a Suspension Electrode for an Asymmetric Flow Capacitor. ACS Appl. Mater. Interfaces 2014, 6, 8886–8893. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J.; Long, X.; Wang, X.; Zou, S.; Zhang, L.; Xu, H.; Fu, Y.; Liu, D.; Li, Y.; et al. One-Step Construction of δ-MnO2 Cathodes with an Interconnected Nanosheet Structure on Graphite Paper for High-Performance Aqueous Asymmetric Supercapacitors. J. Energy Storage 2021, 35, 102308. [Google Scholar] [CrossRef]
- Koseoglou, M.; Tsioumas, E.; Papagiannis, D.; Jabbour, N.; Mademlis, C. A Novel On-Board Electrochemical Impedance Spectroscopy System for Real-Time Battery Impedance Estimation. IEEE Trans. Power Electron. 2021, 36, 10776–10787. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ganesh, V. Electrochemical Characterization of Self-Assembled Monolayers ( SAMs ) of Silanes on Indium Tin Oxide (ITO) Electrodes—Tuning Electron Transfer Behaviour across Electrode—Electrolyte Interface. J. Colloid Interface Sci. 2012, 374, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Sun, Y.; Zhao, X.; Lin, B.; Yang, H.; Zhang, X.; Lingxiang, G. Long-Term-Stable, Solution-Processable, Electrochromic Carbon Nanotubes/Polymer Composite for Smart Supercapacitor with Wide Working Potential Window. J. Mater. Chem. A 2018, 6, 18994–19003. [Google Scholar] [CrossRef]
- Lukács, Z.; Kristóf, T. A Generalized Model of the Equivalent Circuits in the Electrochemical Impedance Spectroscopy. Electrochim. Acta 2020, 363, 137199. [Google Scholar] [CrossRef]
- Vieira, D.S.; Fernandes, P.R.G.; Mukai, H.; Zola, R.S.; Lenzi, G.G.; Lenzi, E.K. Surface Roughness Influence on CPE Parameters in Electrolytic Cells. Int. J. Electrochem. Sci. 2016, 11, 7775–7784. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Yang, F. A High-Performance Symmetric Supercapacitor from Porous Activated Carbon under Compression. Energy Technol. 2021, 9, 2100068. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Yang, F. Tuning Electrochemical Performance of Carbon-Sphere-Based Supercapacitors by Compressive Stress. Electrochim. Acta 2020, 357, 136874. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.M. Improved Performances of Hybrid Supercapacitors Using Granule Li4Ti5O12/Activated Carbon Composite Anode. Mater. Lett. 2018, 228, 220–223. [Google Scholar] [CrossRef]






| Sample | SBET (m2g−1) | Vtotal (cm3g−1) | Pore Size (nm) |
|---|---|---|---|
| Activated Carbon (AC) | 1227.96 | 0.73 | 3.83 |
| MnO2 | 99.91 | 0.23 | 7.72 |
| AC–MnO2 0% | 808.22 | 0.46 | 3.9 |
| AC–MnO2 10% | 784.02 | 0.44 | 3.9 |
| AC–MnO2 15% | 727.13 | 0.43 | 3.9 |
| AC–MnO2 20% | 666.28 | 0.42 | 3.9 |
| Sample | Gravimetric Capacitance (Fg−1) | Gravimetric Energy Density (Wh.kg−1) | Gravimetric Power Density (W.kg−1) |
|---|---|---|---|
| AC–MnO2 0% | 70.71 | 8.53 | 69.59 |
| AC–MnO2 10% | 73.91 | 8.83 | 74.61 |
| AC–MnO2 15% | 79.43 | 9.07 | 85.44 |
| AC–MnO2 20% | 74.32 | 8.85 | 72.01 |
| AC–MnO2 10%//AC | 74.50 | 8.94 | 78.59 |
| AC–MnO2 15%//AC | 81.63 | 9.21 | 70.83 |
| AC–MnO2 20%//AC | 77.31 | 9.64 | 86.75 |
| Cathode | Anode | Electrolyte | Gravimetric Capacitance (F/g) | Gravimetric Energy Density (Wh/kg) | Gravimetric Power Density (W/kg) | Retention | Ref. |
|---|---|---|---|---|---|---|---|
| α-MnO2@-δ-MnO2 | AC | 1 M Na2SO4 | 28.9 | 12.9 | 230 | 73% (10,000) | [81] |
| MnO2/SHAC | SHAC | 1 M Na2SO4 | 49.2 | 46.2 | 3679 | 80.4% (5000) | [82] |
| NiCo2O4 | AC | 2 M KOH | 52.3 | 21 | 424.5 | 99.3% (5000) | [83] |
| Graphene hydrogel | BDTD-rGO | 1 M H2SO4 | 54 | 9.52 | 450 | 81.3% (5000) | [84] |
| rGO/C/MnO2 | AC | 3 M KOH | 59.5 | 21.2 | 190 | 72% (2500) | [85] |
| MnO2/Carbon Cloth | AC | 1 M Na2SO4 | 67.8 | 18.46 | 699.54 | 97.3% (2000) | [86] |
| AC-MnO2 15%//AC | AC | 1 M Et4NBF4 | 98.45 | 21.07 | 103.98 | 91.97% (1000) | This work |
| Sample | CPE | Rs | α | CPE 2 | Rct | α | W |
|---|---|---|---|---|---|---|---|
| AC–MnO2 15% | 9.0534 × 10−6 | 4.9748 | 0.8021 | 5.1498 × 10−6 | 22.014 | 0.32438 | 6.2736 |
| AC–MnO2 15%//AC | 27.741 × 10−6 | 3.1401 | 0.4643 | 3.954 × 10−7 | 19.976 | 1 | 6.9894 |
| Cell | Gravimetric Capacitance (F/g) | Gravimetric Energy Density (Wh/kg) | Gravimetric Power Density(W/kg) | Energy Discharge (mWh) |
|---|---|---|---|---|
| Coin Cell | 89.43 | 16.34 | 100.04 | 0.244 |
| Cylindrical Cell | 84.28 | 14.88 | 96.68 | 18.062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diantoro, M.; Istiqomah, I.; Fath, Y.A.; Mufti, N.; Nasikhudin, N.; Meevasana, W.; Alias, Y.B. Hierarchical Activated Carbon–MnO2 Composite for Wide Potential Window Asymmetric Supercapacitor Devices in Organic Electrolyte. Micromachines 2022, 13, 1989. https://doi.org/10.3390/mi13111989
Diantoro M, Istiqomah I, Fath YA, Mufti N, Nasikhudin N, Meevasana W, Alias YB. Hierarchical Activated Carbon–MnO2 Composite for Wide Potential Window Asymmetric Supercapacitor Devices in Organic Electrolyte. Micromachines. 2022; 13(11):1989. https://doi.org/10.3390/mi13111989
Chicago/Turabian StyleDiantoro, Markus, Istiqomah Istiqomah, Yusril Al Fath, Nandang Mufti, Nasikhudin Nasikhudin, Worawat Meevasana, and Yatimah Binti Alias. 2022. "Hierarchical Activated Carbon–MnO2 Composite for Wide Potential Window Asymmetric Supercapacitor Devices in Organic Electrolyte" Micromachines 13, no. 11: 1989. https://doi.org/10.3390/mi13111989
APA StyleDiantoro, M., Istiqomah, I., Fath, Y. A., Mufti, N., Nasikhudin, N., Meevasana, W., & Alias, Y. B. (2022). Hierarchical Activated Carbon–MnO2 Composite for Wide Potential Window Asymmetric Supercapacitor Devices in Organic Electrolyte. Micromachines, 13(11), 1989. https://doi.org/10.3390/mi13111989








