Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring
Abstract
1. Introduction
- Originality: Retrofitting existing safety belts, first extending of the point-of-care system on the aircraft to passengers besides pilots.
- Feasibility: The cost of the equipment is lower than nearly 90% of commercial products since our design is a cost-saving approach by retrofitting of existing safety belts.
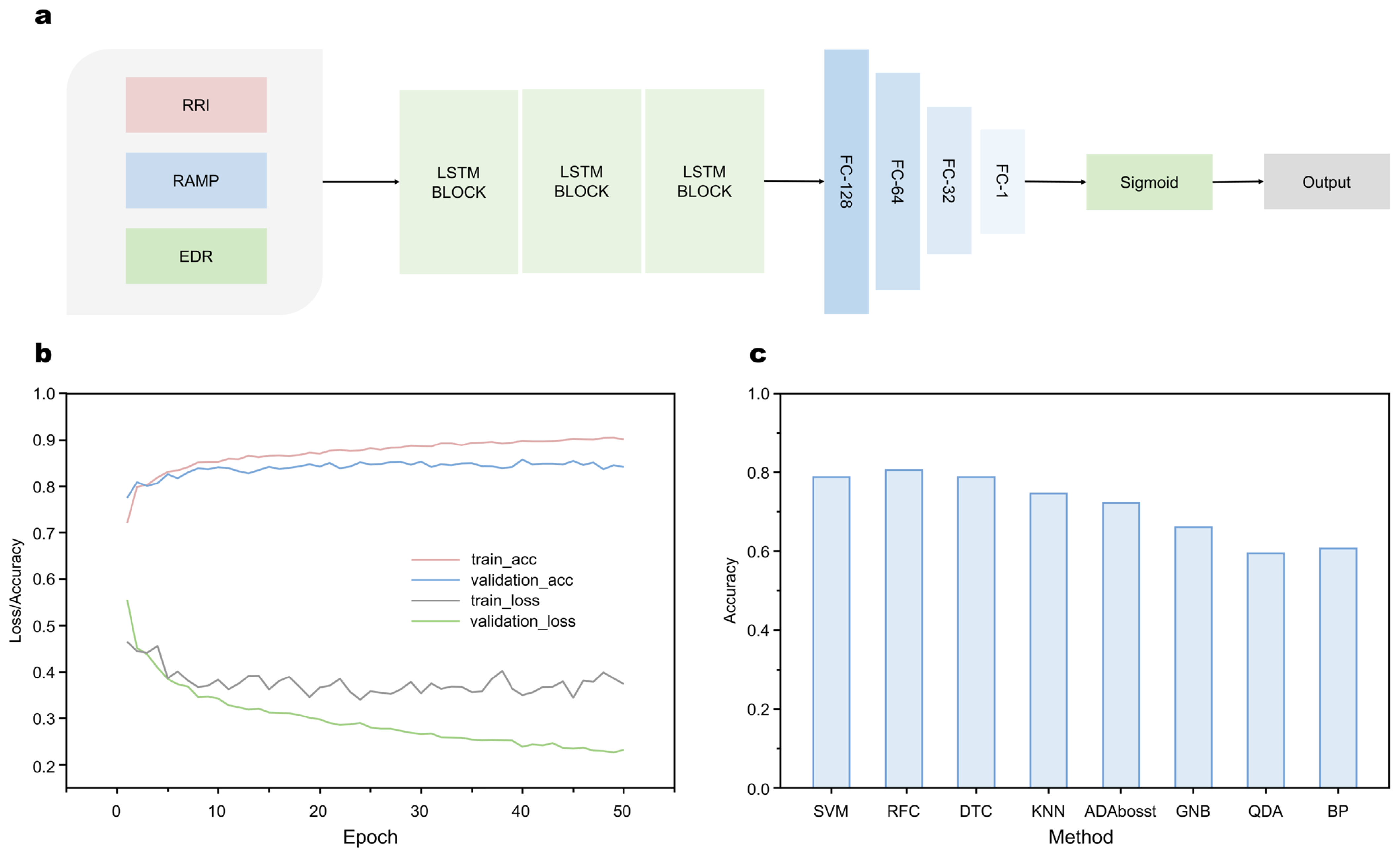
- Accuracy: With the LSTM-RNN based algorithm, the average recognition accuracy of the model is up to 84–85%.
- Extensibility: Data of hybrid ECG, breathing, and motion signals can also be used for monitoring other diseases.
2. Design and Methods
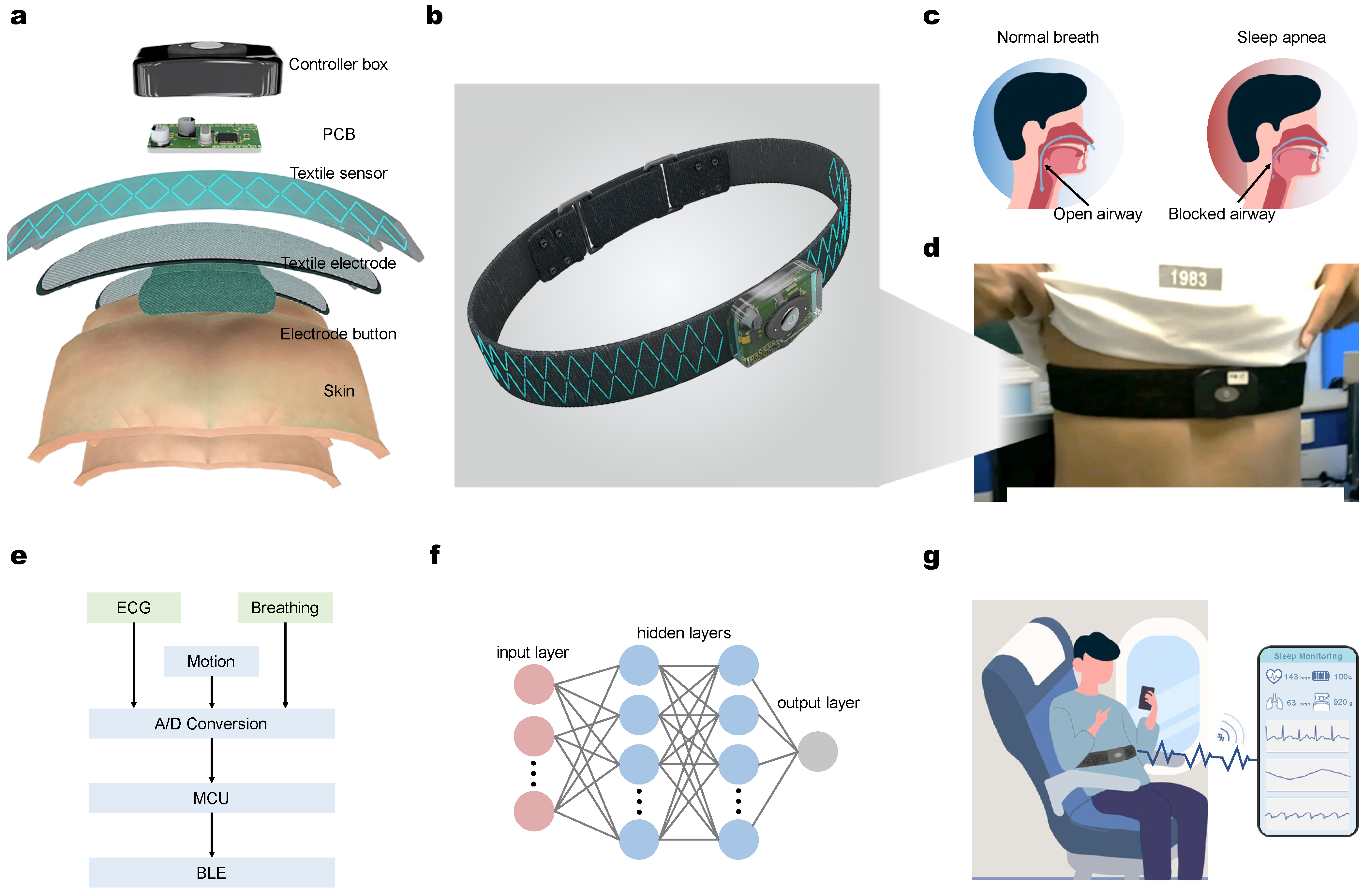
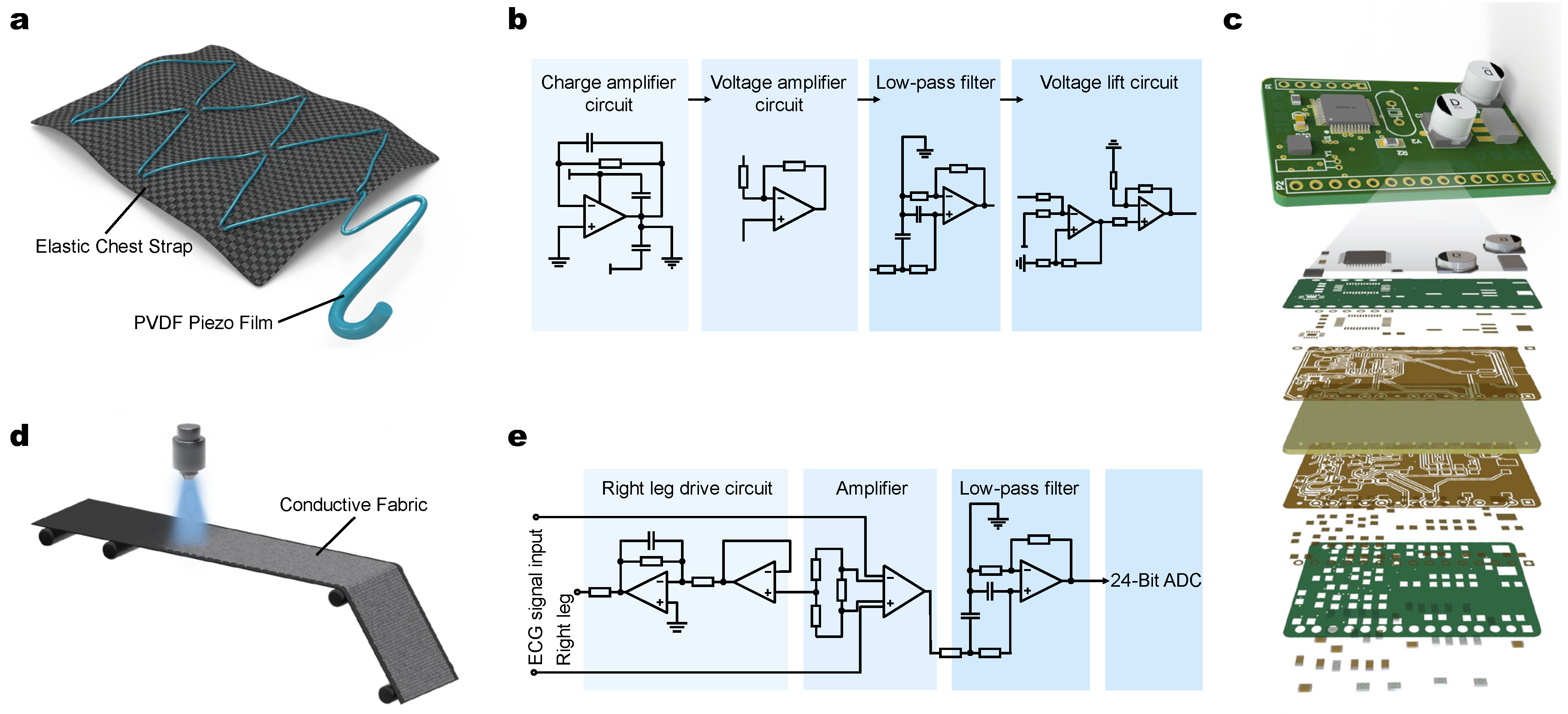
2.1. Hardware Design and Signals Acquisition
2.2. ECG and Breathing Signal Collecting and Processing
2.3. Machine Learning-Enabled SAHS Recognition
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of Care Diagnostics: Status and Future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Trung, T.Q.; Lee, N.-E. Recent Progress, Challenges, and Prospects of Fully Integrated Mobile and Wearable Point-of-Care Testing Systems for Self-Testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.K.; Zhou, X.X.; Byrnes, J.R.; Martinko, A.J.; Lui, I.; Pance, K.; Lim, S.A.; Glasgow, J.E.; Glasgow, A.A.; Turcios, K. Engineering Luminescent Biosensors for Point-of-Care SARS-CoV-2 Antibody Detection. Nat. Biotechnol. 2021, 39, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W. From Prenatal Genomic Diagnosis to Fetal Personalized Medicine: Progress and Challenges. Nat. Med. 2012, 18, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.-H.; Liao, J.C. New and Developing Diagnostic Technologies for Urinary Tract Infections. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef]
- Chen, G.; Xiao, X.; Zhao, X.; Tat, T.; Bick, M.; Chen, J. Electronic Textiles for Wearable Point-of-Care Systems. Chem. Rev. 2022, 122, 3259–3291. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Pearson, S.D. Novel Therapies for an Aging Population: Grappling with Price, Value, and Affordability. JAMA 2019, 321, 1567–1568. [Google Scholar] [CrossRef]
- Osier, F.; Ting, J.P.Y.; Fraser, J.; Lambrecht, B.N.; Romano, M.; Gazzinelli, R.T.; Bortoluci, K.R.; Zamboni, D.S.; Akbar, A.N.; Evans, J. The Global Response to the COVID-19 Pandemic: How Have Immunology Societies Contributed? Nat. Rev. Immunol. 2020, 20, 594–602. [Google Scholar] [CrossRef]
- Yip, W.; Fu, H.; Chen, A.T.; Zhai, T.; Jian, W.; Xu, R.; Pan, J.; Hu, M.; Zhou, Z.; Chen, Q. 10 Years of Health-Care Reform in China: Progress and Gaps in Universal Health Coverage. Lancet 2019, 394, 1192–1204. [Google Scholar] [CrossRef]
- Hamburg, M.A.; Collins, F.S. The Path to Personalized Medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef]
- Schork, N.J. Personalized Medicine: Time for One-Person Trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Hood, L.; Friend, S.H. Predictive, Personalized, Preventive, Participatory (P4) Cancer Medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-Based Devices for Point-of-Care Diagnostic Applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Y.; Jiang, X. Barcoded Point-of-Care Bioassays. Chem. Soc. Rev. 2019, 48, 850–884. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Tahanisaz, S.; Shokuhyar, S. Evaluation of Passenger Satisfaction with Service Quality: A Consecutive Method Applied to the Airline Industry. J. Air Transp. Manag. 2020, 83, 101764. [Google Scholar] [CrossRef]
- Merkert, R.; Swidan, H. Flying with(out) a Safety Net: Financial Hedging in the Airline Industry. Transp. Res. E Logist. Transp. Rev. 2019, 127, 206–219. [Google Scholar] [CrossRef]
- Gerstle, C.R. Parallels in Safety between Aviation and Healthcare. J. Pediatr. Surg. 2018, 53, 875–878. [Google Scholar] [CrossRef]
- Wang, H. Big Data Visualization and Analysis of Various Factors Contributing to Airline Delay in the United States. In Proceedings of the 2022 International Conference on Big Data, Information and Computer Network (BDICN), Sanya, China, 20–22 January 2022; pp. 177–181. [Google Scholar]
- Boyd, D.D.; Scharf, M.; Cross, D. A Comparison of General Aviation Accidents Involving Airline Pilots and Instrument-Rated Private Pilots. J. Saf. Res. 2021, 76, 127–134. [Google Scholar] [CrossRef]
- Borges do Nascimento, I.J.; Jerončić, A.; Arantes, A.J.R.; Brady, W.J.; Guimarães, N.S.; Antunes, N.S.; Carim Junior, G.; Marcolino, M.S. The Global Incidence of In-Flight Medical Emergencies: A Systematic Review and Meta-Analysis of Approximately 1.5 Billion Airline Passengers. Am. J. Emerg. Med. 2021, 48, 156–164. [Google Scholar] [CrossRef]
- Jagoda, A.; Pietrzak, M. MEDICAL EMERGENCIES IN COMMERCIAL AIR TRAVEL. Emerg. Med. Clin. N. Am. 1997, 15, 251–260. [Google Scholar] [CrossRef]
- DeHart, R.L. Health Issues of Air Travel. Annu. Rev. Public Health 2003, 24, 133–151. [Google Scholar] [CrossRef]
- Jou, R.-C.; Kuo, C.-W.; Chiu, Y.-C. Bidding Behaviors for International Airline Seats in Short/Long Distance Flights. Transp. Res. Part A Policy Pr. 2022, 163, 55–79. [Google Scholar] [CrossRef]
- Pedroso Fabrin, B.H.; Ferrari, D. Investigation of Airborne Exposure Risk to Infectious Diseases during Aircraft Boarding Process Using Agent-Based Modeling. In Proceedings of the AIAA AVIATION 2022 Forum, Chicago, IL, USA, 27 June–1 July 2022; p. 3616. [Google Scholar]
- Shao, S.; Zhou, Q.; Liu, Z. A New Assessment Method of the Pilot Stress Using ECG Signals during Complex Special Flight Operation. IEEE Access 2019, 7, 185360–185368. [Google Scholar] [CrossRef]
- Shen, S.; Xiao, X.; Xiao, X.; Chen, J. Wearable Triboelectric Nanogenerators for Heart Rate Monitoring. Chem. Commun. 2021, 57, 5871–5879. [Google Scholar] [CrossRef]
- Smith, D.F.; Amin, R.S. OSA and Cardiovascular Risk in Pediatrics. Chest 2019, 156, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.J.; Gaisl, T.; Wons, A.M.; Kohler, M. CPAP vs. Mandibular Advancement Devices and Blood Pressure in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. JAMA 2015, 314, 2280–2293. [Google Scholar] [CrossRef]
- Jean, R.E.; Duttuluri, M.; Gibson, C.D.; Mir, S.; Fuhrmann, K.; Eden, E.; Supariwala, A. Improvement in Physical Activity in Persons with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. J. Phys. Act. Health 2017, 14, 176–182. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, Y.J.; Jeong, D.-U.; Park, K.S. Apnea–Hypopnea Index Prediction Using Electrocardiogram Acquired during the Sleep-Onset Period. IEEE Trans. Biomed. Eng. 2016, 64, 295–301. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Miyajima, M.; Yamakawa, T.; Abe, E.; Suzuki, Y.; Sawada, Y.; Kano, M.; Maehara, T.; Ohta, K.; Sasai-Sakuma, T. Epileptic Seizure Prediction Based on Multivariate Statistical Process Control of Heart Rate Variability Features. IEEE Trans. Biomed. Eng. 2015, 63, 1321–1332. [Google Scholar] [PubMed]
- Yao, G.; Xu, L.; Cheng, X.; Li, Y.; Huang, X.; Guo, W.; Liu, S.; Wang, Z.L.; Wu, H. Bioinspired Triboelectric Nanogenerators as Self-Powered Electronic Skin for Robotic Tactile Sensing. Adv. Funct. Mater. 2020, 30, 1907312. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, X.; Chen, J.; Li, Q.; Fu, H. Machine-Learning-Assisted Recognition on Bioinspired Soft Sensor Arrays. ACS Nano 2022, 16, 6734–6743. [Google Scholar] [CrossRef]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic Biohybrid Microrobot Multimers Based on Chlorella Cells for Enhanced Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, B.; Peng, G.; Shan, Y.; Hu, H.; Yang, Z. Skin-Inspired Piezoelectric Tactile Sensor Array with Crosstalk-Free Row+Column Electrodes for Spatiotemporally Distinguishing Diverse Stimuli. Adv. Sci. 2021, 8, 2002817. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, F.; Wei, X.; Wang, D.; Wang, Y. A Piezoelectric Tactile Sensor for Tissue Stiffness Detection with Arbitrary Contact Angle. Sensors 2020, 20, 6607. [Google Scholar] [CrossRef]
- Qu, H.; Xiao, X.; Han, Z.; Hu, M.; Shen, S.; Yang, L.; Jia, F.; Wang, T.; Ye, Z.; Sun, W. Graphene Oxide Nanofiltration Membrane Based on Three-Dimensional Size-Controllable Metal–Organic Frameworks for Water Treatment. ACS Appl. Nano Mater. 2022, 5, 5196–5207. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, G.; Libanori, A.; Chen, J. Wearable Triboelectric Nanogenerators for Therapeutics. Trends Chem. 2021, 3, 279–290. [Google Scholar] [CrossRef]
- Xiao, X.; Xiao, X.; Zhou, Y.; Zhao, X.; Chen, G.; Liu, Z.; Wang, Z.; Lu, C.; Hu, M.; Nashalian, A.; et al. An Ultrathin Rechargeable Solid-State Zinc Ion Fiber Battery for Electronic Textiles. Sci. Adv. 2021, 7, eabl3742. [Google Scholar] [CrossRef]
- Lv, J.; Yin, J.; Qin, Y.; Dai, Y.; Cheng, Z.; Luo, L.; Liu, X. Post-Construction of Weaving Structure in Aramid Fiber towards Improvements of Its Transverse Properties. Compos. Sci. Technol. 2021, 208, 108780. [Google Scholar] [CrossRef]
- Yokus, M.A.; Jur, J.S. Fabric-Based Wearable Dry Electrodes for Body Surface Biopotential Recording. IEEE Trans. Biomed. Eng. 2015, 63, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Pani, D.; Dessì, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. Fully Textile, PEDOT: PSS Based Electrodes for Wearable ECG Monitoring Systems. IEEE Trans. Biomed. Eng. 2015, 63, 540–549. [Google Scholar] [CrossRef]
- Claude, A.; Robin, O.; Gehin, C.; Massot, B. Design and Evaluation of a Novel Technology for Ambulatory Monitoring of Bruxism Events. Sens. Actuators A Phys. 2019, 295, 532–540. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Cheng, S. Adaptive Deep Brain Stimulation System Based on ADS1292. In Proceedings of the 2019 IEEE 7th International Conference on Bioinformatics and Computational Biology (ICBCB), Hangzhou, China, 21–23 March 2019; pp. 83–87. [Google Scholar]
- Ding, S.; Zhu, X.; Chen, W.; Wei, D. Derivation of Respiratory Signal from Single- Channel ECGs Based on Source Statistics. Int. J. Bioelectromagn. 2004, 6, 41–48. [Google Scholar]
- Murray, L.P.; Mace, C.R. Usability as a Guiding Principle for the Design of Paper-Based, Point-of-Care Devices—A Review. Anal. Chim. Acta 2020, 1140, 236–249. [Google Scholar] [CrossRef]
- Elola, A.; Aramendi, E.; Irusta, U.; del Ser, J.; Alonso, E.; Daya, M. ECG-Based Pulse Detection during Cardiac Arrest Using Random Forest Classifier. Med. Biol. Eng. Comput. 2019, 57, 453–462. [Google Scholar] [CrossRef]
- Venkatesan, C.; Karthigaikumar, P.; Varatharajan, R. A Novel LMS Algorithm for ECG Signal Preprocessing and KNN Classifier Based Abnormality Detection. Multimed. Tools Appl. 2018, 77, 10365–10374. [Google Scholar] [CrossRef]
- Penzel, T.; Moody, G.B.; Mark, R.G.; Goldberger, A.L.; Peter, J.H. The Apnea-ECG Database. In Proceedings of the Computers in Cardiology 2000, Cambridge, MA, USA, 24–27 September 2000; (Cat. 00CH37163). Volume 27, pp. 255–258. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Rao, Z.; Zhang, W.; Liu, C.; Wang, Z.; Zhang, S.; Zhang, B.; Hu, M.; Servati, P.; Xiao, X. Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring. Micromachines 2022, 13, 1880. https://doi.org/10.3390/mi13111880
Ji X, Rao Z, Zhang W, Liu C, Wang Z, Zhang S, Zhang B, Hu M, Servati P, Xiao X. Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring. Micromachines. 2022; 13(11):1880. https://doi.org/10.3390/mi13111880
Chicago/Turabian StyleJi, Xiaoqiang, Zhi Rao, Wei Zhang, Chang Liu, Zimo Wang, Shuo Zhang, Butian Zhang, Menglei Hu, Peyman Servati, and Xiao Xiao. 2022. "Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring" Micromachines 13, no. 11: 1880. https://doi.org/10.3390/mi13111880
APA StyleJi, X., Rao, Z., Zhang, W., Liu, C., Wang, Z., Zhang, S., Zhang, B., Hu, M., Servati, P., & Xiao, X. (2022). Airline Point-of-Care System on Seat Belt for Hybrid Physiological Signal Monitoring. Micromachines, 13(11), 1880. https://doi.org/10.3390/mi13111880








