Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens
Abstract
1. Introduction
2. Nano/Micromotors in the Gastrointestinal Tract
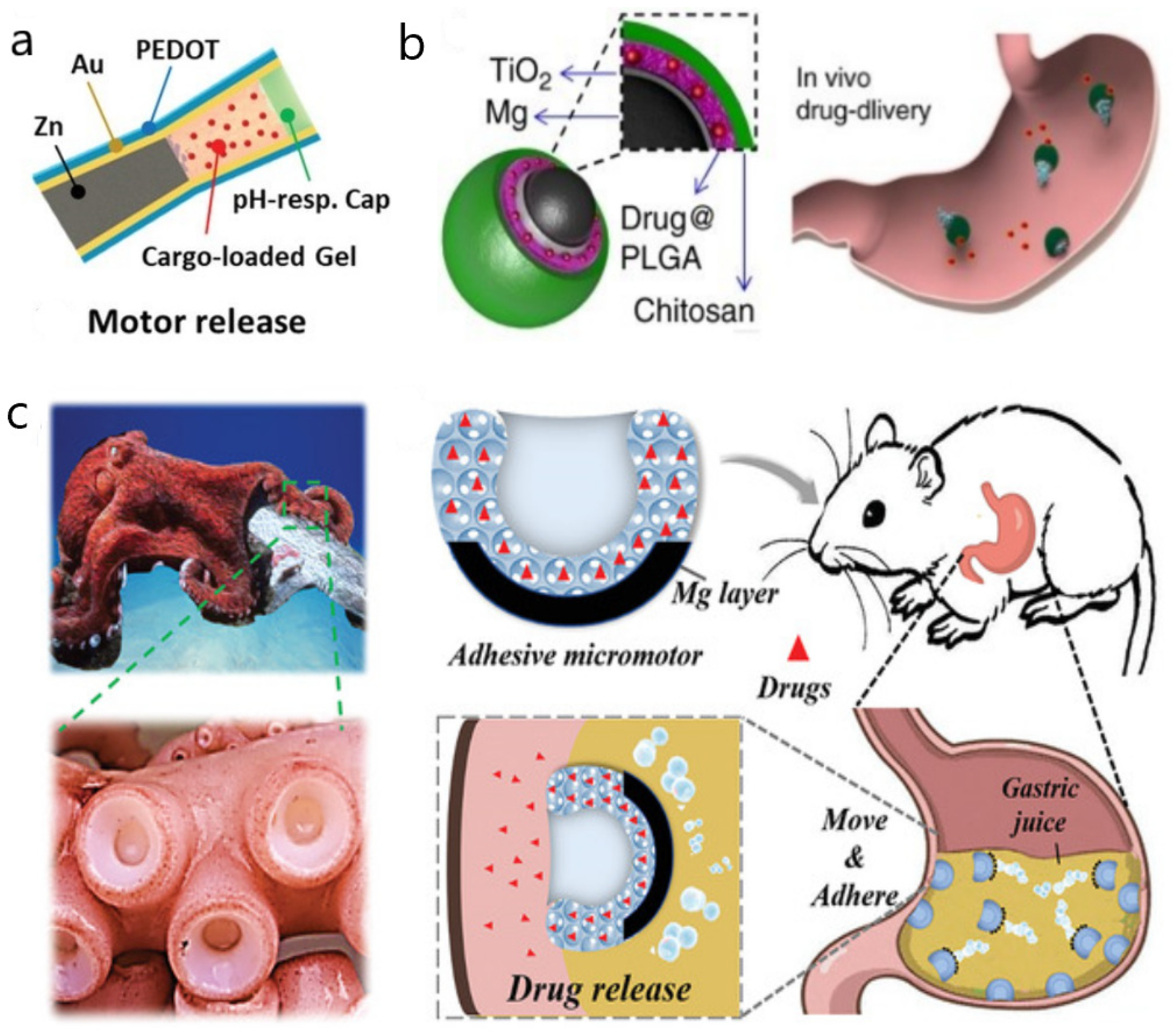
3. Nano/Micromotors in the Urinary Tract
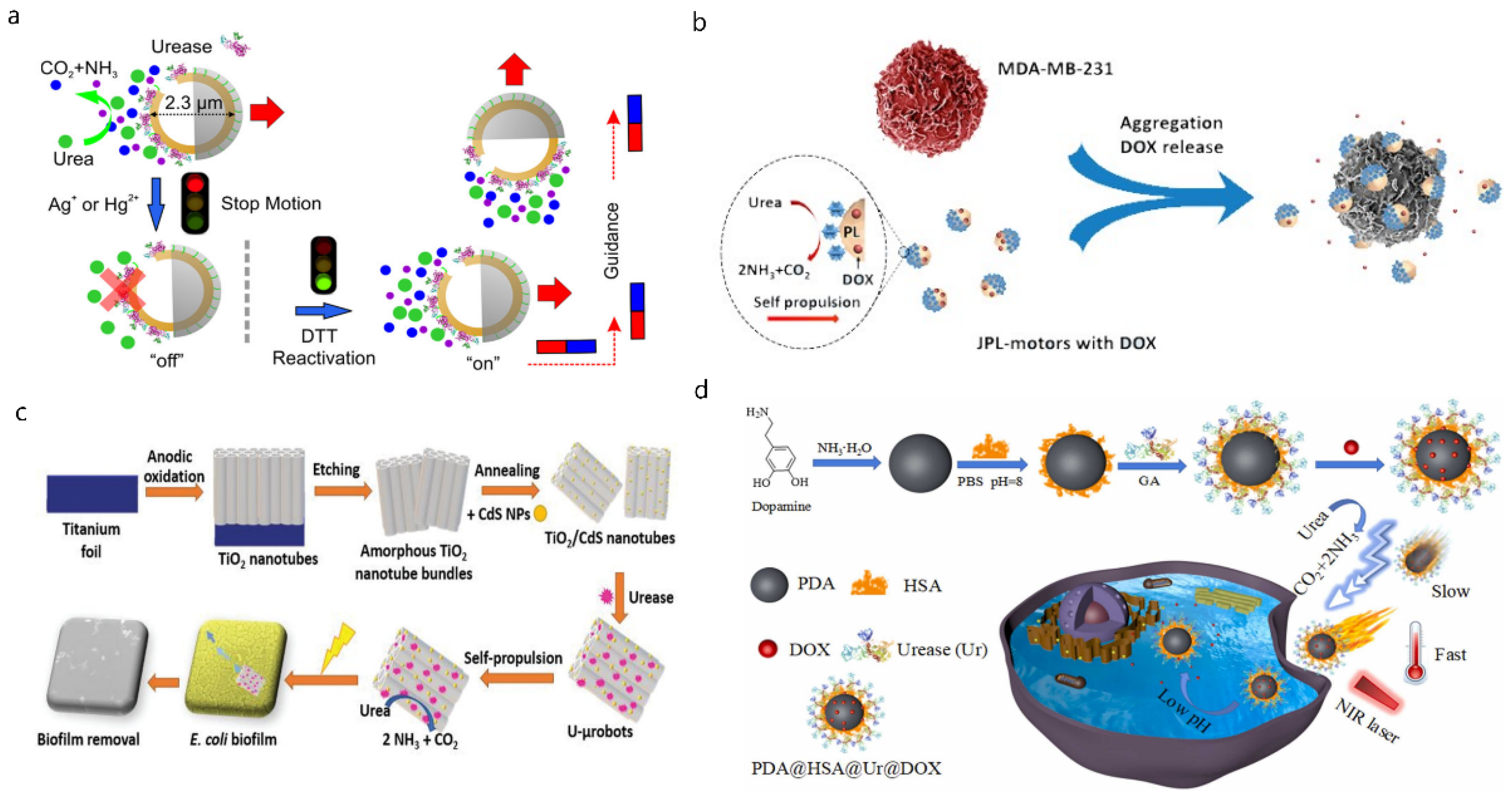
4. Nano/Micromotors in Blood Vessels
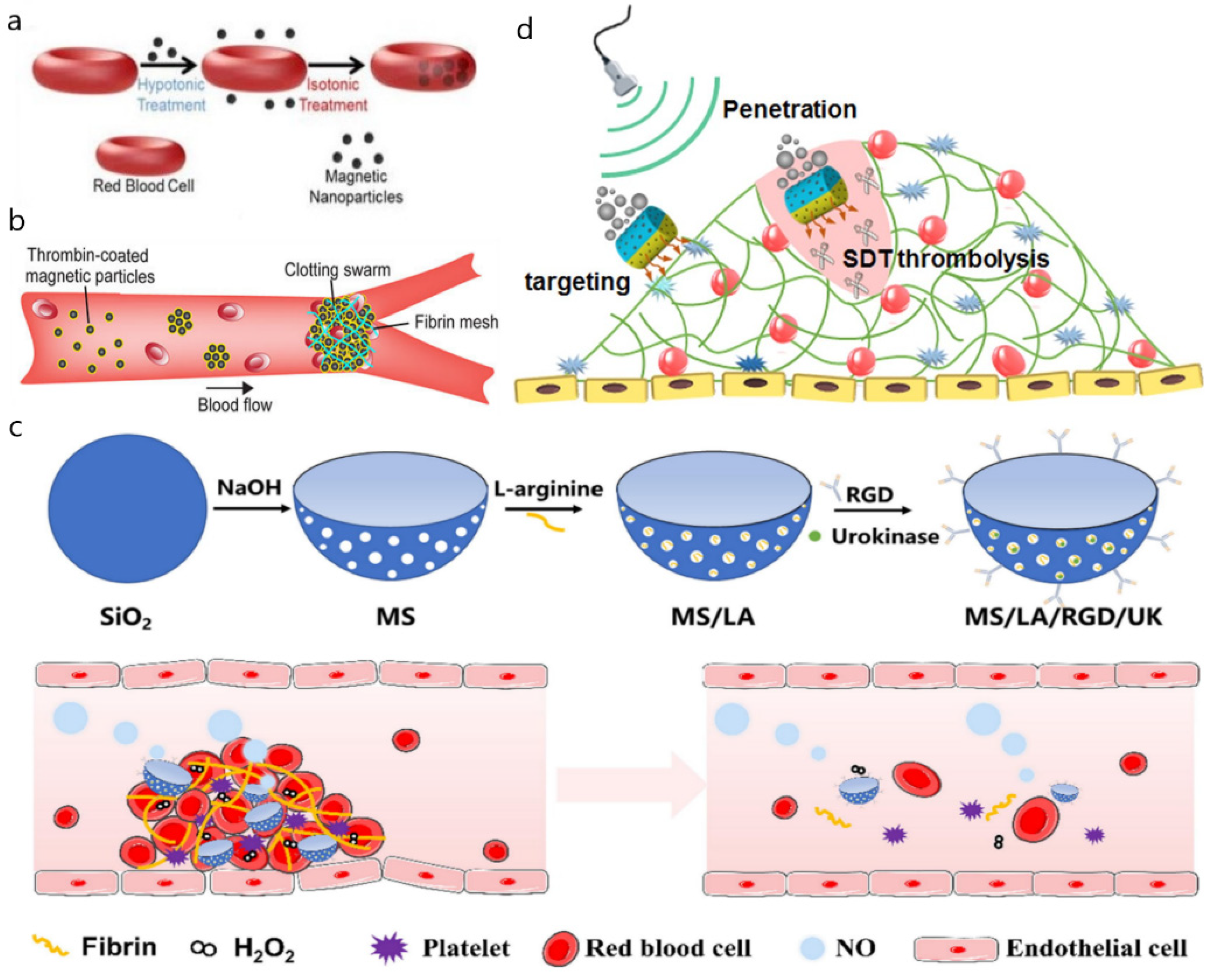
5. Nano/Micromotors in Other Lumens
6. Summary and Expectation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Rowland, M.; Benet, L.Z.; Graham, G.G. Clearance concepts in pharmacokinetics. J. Pharm. Biopharm. 1973, 1, 123–136. [Google Scholar] [CrossRef]
- Yamashita, F.; Hashida, M. Pharmacokinetic considerations for targeted drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 139–147. [Google Scholar] [CrossRef]
- Tezel, G.; Timur, S.S.; Kuralay, F.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Current status of micro/nanomotors in drug delivery. J. Drug Target. 2021, 29, 29–45. [Google Scholar] [CrossRef]
- Xu, T.; Gao, W.; Xu, L.P.; Zhang, X.; Wang, S. Fuel-Free Synthetic Micro-/Nanomachines. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Paxton, W.F.; Baker, P.T.; Kline, T.R.; Wang, Y.; Mallouk, T.E.; Sen, A. Catalytically induced electrokinetics for motors and micropumps. J. Am. Chem. Soc. 2006, 128, 14881–14888. [Google Scholar] [CrossRef]
- Pavlick, R.A.; Sengupta, S.; McFadden, T.; Zhang, H.; Sen, A. A polymerization-powered motor. Angew. Chem. Int. Ed. Engl. 2011, 50, 9374–9377. [Google Scholar] [CrossRef]
- Howse, J.R.; Jones, R.A.; Ryan, A.J.; Gough, T.; Vafabakhsh, R.; Golestanian, R. Self-motile colloidal particles: From directed propulsion to random walk. Phys. Rev. Lett. 2007, 99, 048102. [Google Scholar] [CrossRef]
- Brown, A.; Poon, W. Ionic effects in self-propelled Pt-coated Janus swimmers. Soft Matter 2014, 10, 4016–4027. [Google Scholar] [CrossRef]
- Safdar, M.; Khan, S.U.; Jänis, J. Progress toward Catalytic Micro- and Nanomotors for Biomedical and Environmental Applications. Adv. Mater. 2018, 30, e1703660. [Google Scholar] [CrossRef]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. Engl. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, C.; Shen, B.; Zhu, X.; Li, Y.; Shi, L.; Zhang, Y.; Liu, J.; Wang, Y.; Sun, L. Upconversion-Magnetic Carbon Sphere for Near Infrared Light-Triggered Bioimaging and Photothermal Therapy. Theranostics 2019, 9, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, T.; Chen, H.; Wang, Q.; Liu, Z.; Fang, L.; Wan, M.; Mao, C.; Shen, J. Engineered Platelet-Based Micro/Nanomotors for Cancer Therapy. Small 2021, 17, e2104912. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chang, X.; Liu, H.; Hu, Y.; Li, T.; Li, L. Multi-response biocompatible Janus micromotor for ultrasonic imaging contrast enhancement. Appl. Mater. Today 2021, 23, 101026. [Google Scholar] [CrossRef]
- Das, S.S.; Erez, S.; Karshalev, E.; Wu, Y.; Wang, J.; Yossifon, G. Switching from Chemical to Electrical Micromotor Propulsion across a Gradient of Gastric Fluid via Magnetic Rolling. ACS Appl. Mater. Interfaces 2022, 14, 30290–30298. [Google Scholar] [CrossRef]
- Cao, H.X.; Jung, D.; Lee, H.S.; Go, G.; Nan, M.; Choi, E.; Kim, C.S.; Park, J.O.; Kang, B. Micromotor Manipulation Using Ultrasonic Active Traveling Waves. Micromachines 2021, 12, 192. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xiu, W.; Liu, Y.; Ren, L.; Xiao, H.; Yang, F.; Gao, Y.; Xu, C.; Wang, L. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 2020, 6, eaaz8204. [Google Scholar] [CrossRef]
- Alapan, Y.; Bozuyuk, U.; Erkoc, P.; Karacakol, A.C.; Sitti, M. Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow. Sci. Robot 2020, 5, eaba5726. [Google Scholar] [CrossRef]
- Bernad, S.I.; Susan-Resiga, D.; Bernad, E.S. Hemodynamic Effects on Particle Targeting in the Arterial Bifurcation for Different Magnet Positions. Molecules 2019, 24, 2509. [Google Scholar] [CrossRef]
- Mena-Giraldo, P.; Orozco, J. Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers 2021, 13, 3920. [Google Scholar] [CrossRef]
- Ji, F.; Li, T.; Yu, S.; Wu, Z.; Zhang, L. Propulsion Gait Analysis and Fluidic Trapping of Swinging Flexible Nanomotors. ACS Nano 2021, 15, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Liu, C.; Zhang, Q.; Meng, H.; Yu, S.; Zeng, K.; Han, J.; Jin, X.; Shi, S.; Yu, P.; et al. Magnetic-Driven Hydrogel Microrobots Selectively Enhance Synthetic Lethality in MTAP-Deleted Osteosarcoma. Front. Bioeng. Biotechnol. 2022, 10, 911455. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, S.; Liao, J.; Qing, X.; Sun, D.; Ji, F.; Song, W.; Wang, L.; Li, T. A Robot Platform for Highly Efficient Pollutant Purification. Front. Bioeng. Biotechnol. 2022, 10, 903219. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, A.; Shao, G.; Wei, M.; Guo, B.; Zhang, G.; Li, L.; Wang, W. Janus Microdimer Surface Walkers Propelled by Oscillating Magnetic Fields. Adv. Funct. Mater. 2018, 28, 1706066. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; Sui, X.; Men, Y.; White, P.B.; van Hest, J.C.M.; Wilson, D.A. Self-propelled supramolecular nanomotors with temperature-responsive speed regulation. Nat. Chem. 2017, 9, 480–486. [Google Scholar] [CrossRef]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef]
- Carlsen, R.W.; Sitti, M. Bio-hybrid cell-based actuators for microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef]
- Servant, A.; Qiu, F.; Mazza, M.; Kostarelos, K.; Nelson, B.J. Controlled in vivo swimming of a swarm of bacteria-like microrobotic flagella. Adv. Mater. 2015, 27, 2981–2988. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Zhang, H.; Chang, X.; Song, W.; Hu, Y.; Shao, G.; Sandraz, E.; Zhang, G.; Li, L.; et al. Magnetically Propelled Fish-Like Nanoswimmers. Small 2016, 12, 6098–6105. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef]
- Sattayasamitsathit, S.; Kou, H.; Gao, W.; Thavarajah, W.; Kaufmann, K.; Zhang, L.; Wang, J. Fully loaded micromotors for combinatorial delivery and autonomous release of cargoes. Small 2014, 10, 2830–2833. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial micromotors in the mouse’s stomach: A step toward in vivo use of synthetic motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Mundaca-Uribe, R.; Wei, X.; Ramírez-Herrera, D.E.; Karshalev, E.; Nguyen, B.; Fang, R.H.; Zhang, L.; Wang, J. Multicompartment Tubular Micromotors Toward Enhanced Localized Active Delivery. Adv. Mater. 2020, 32, e2000091. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.; Angsantikul, P.; Li, J.; Angel Lopez-Ramirez, M.; Ramírez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, C.; Wang, D.; He, Q. Bubble-Propelled Janus Gallium/Zinc Micromotors for the Active Treatment of Bacterial Infections. Angew. Chem. Int. Ed. Engl. 2021, 60, 8750–8754. [Google Scholar] [CrossRef]
- Wei, X.; Beltrán-Gastélum, M.; Karshalev, E.; Esteban-Fernández de Ávila, B.; Zhou, J.; Ran, D.; Angsantikul, P.; Fang, R.H.; Wang, J.; Zhang, L. Biomimetic Micromotor Enables Active Delivery of Antigens for Oral Vaccination. Nano Lett. 2019, 19, 1914–1921. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, C.; Chen, H.; Fan, L.; Zhao, Y.; Qian, X.; Chai, R. Suction-Cup-Inspired Adhesive Micromotors for Drug Delivery. Adv. Sci. 2022, 9, e2103384. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chu, M.; Yin, N.; Huang, W.; Liu, W.; Zhang, Z.; Liu, J.; Shi, J. Biological chemotaxis-guided self-thermophoretic nanoplatform augments colorectal cancer therapy through autonomous mucus penetration. Sci. Adv. 2022, 8, eabn3917. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Pacheco, M.; Maria-Hormigos, R.; Escarpa, A. Perspectives on Janus micromotors: Materials and applications. Appl. Mater. Today 2017, 9, 407–418. [Google Scholar] [CrossRef]
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. Design and synthesis of Janus micro- and nanoparticles. J. Mater. Chem. 2005, 15, 3745–3760. [Google Scholar] [CrossRef]
- Walker, D.; Käsdorf, B.T.; Jeong, H.H.; Lieleg, O.; Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 2015, 1, e1500501. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J. The controls of gastrointestinal movements: Some old and new views. N. Engl. J. Med. 1971, 285, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Li, C.Y.; Wang, D.; Lv, H.; Lv, S.W.; Zhao, N.; Ma, H.; Wang, S. Bacterial Biofilm Bioinspired Persistent Luminescence Nanoparticles with Gut-Oriented Drug Delivery for Colorectal Cancer Imaging and Chemotherapy. ACS Appl. Mater. Interfaces. 2019, 11, 36409–36419. [Google Scholar] [CrossRef]
- Hickling, D.R.; Sun, T.T.; Wu, X.R. Anatomy and Physiology of the Urinary Tract: Relation to Host Defense and Microbial Infection. Microbiol. Spectr. 2015, 3, 1–25. [Google Scholar] [CrossRef]
- Chou, R.; Selph, S.; Buckley, D.I.; Fu, R.; Griffin, J.C.; Grusing, S.; Gore, J.L. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 1189–1199. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. Jama 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Tyagi, P.; Kashyap, M.; Majima, T.; Kawamorita, N.; Yoshizawa, T.; Yoshimura, N. Intravesical liposome therapy for interstitial cystitis. Int. J. Urol. 2017, 24, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Mozolowski, W. Chemical composition of normal urine. Lancet 1948, 1, 423. [Google Scholar] [CrossRef]
- Dey, K.K.; Zhao, X.; Tansi, B.M.; Méndez-Ortiz, W.J.; Córdova-Figueroa, U.M.; Golestanian, R.; Sen, A. Micromotors Powered by Enzyme Catalysis. Nano Lett. 2015, 15, 8311–8315. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X.; Hahn, K.; Sánchez, S. Motion Control of Urea-Powered Biocompatible Hollow Microcapsules. ACS Nano 2016, 10, 3597–3605. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Cho, S.H.; Hahn, S.K. Urease-Powered Polydopamine Nanomotors for Intravesical Therapy of Bladder Diseases. ACS Nano 2020, 14, 6683–6692. [Google Scholar] [CrossRef]
- Poinard, B.; Kamaluddin, S.; Tan, A.Q.Q.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 4777–4789. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Gong, H.; Wei, F.; Zhuang, J.; Karshalev, E.; Esteban-Fernández de Ávila, B.; Huang, C.; Zhou, Z.; Li, Z.; et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot 2020, 5, eaba6137. [Google Scholar] [CrossRef]
- Suchanski, J.; Grzegrzolka, J.; Owczarek, T.; Pasikowski, P.; Piotrowska, A.; Kocbach, B.; Nowak, A.; Dziegiel, P.; Wojnar, A.; Ugorski, M. Sulfatide decreases the resistance to stress-induced apoptosis and increases P-selectin-mediated adhesion: A two-edged sword in breast cancer progression. Breast Cancer Res. 2018, 20, 133. [Google Scholar] [CrossRef]
- Kerrigan, S.W.; Cox, D. Platelet-bacterial interactions. Cell Mol. Life Sci. 2010, 67, 513–523. [Google Scholar] [CrossRef]
- Villa, K.; Sopha, H.; Zelenka, J.; Motola, M.; Dekanovsky, L.; Beketova, D.C.; Macak, J.M.; Ruml, T.; Pumera, M. Enzyme-Photocatalyst Tandem Microrobot Powered by Urea for Escherichia coli Biofilm Eradication. Small 2022, 18, e2106612. [Google Scholar] [CrossRef]
- Wu, M.; Liu, S.; Liu, Z.; Huang, F.; Xu, X.; Shuai, Q. Photothermal interference urease-powered polydopamine nanomotor for enhanced propulsion and synergistic therapy. Colloids Surf. B Biointerfaces 2022, 212, 112353. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wu, S.; Sun, Y.; Ren, J.; Qu, X. Self-Propelled Active Photothermal Nanoswimmer for Deep-Layered Elimination of Biofilm In Vivo. Nano Lett. 2020, 20, 7350–7358. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Jurado-Sánchez, B.; Escarpa, A. Dual-Propelled Lanbiotic Based Janus Micromotors for Selective Inactivation of Bacterial Biofilms. Angew. Chem. Int. Ed. Engl. 2021, 60, 4915–4924. [Google Scholar] [CrossRef]
- Kong, L.; Mayorga-Martinez, C.C.; Guan, J.; Pumera, M. Photocatalytic Micromotors Activated by UV to Visible Light for Environmental Remediation, Micropumps, Reversible Assembly, Transportation, and Biomimicry. Small 2020, 16, e1903179. [Google Scholar] [CrossRef]
- Lee, D.N.; Kim, Y.R.; Yang, S.; Tran, N.M.; Park, B.J.; Lee, S.J.; Kim, Y.; Yoo, H.; Kim, S.J.; Shin, J.H. Controllable Nitric Oxide Storage and Release in Cu-BTC: Crystallographic Insights and Bioactivity. Int. J. Mol. Sci. 2022, 23, 9098. [Google Scholar] [CrossRef]
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharm. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Li, J.; Gao, W.; Xu, T.; Christianson, C.; Gao, W.; Galarnyk, M.; He, Q.; Zhang, L.; et al. Turning erythrocytes into functional micromotors. ACS Nano 2014, 8, 12041–12048. [Google Scholar] [CrossRef]
- Gao, C.; Lin, Z.; Wang, D.; Wu, Z.; Xie, H.; He, Q. Red Blood Cell-Mimicking Micromotor for Active Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23392–23400. [Google Scholar] [CrossRef]
- Munerati, M.; Cortesi, R.; Ferrari, D.; Di Virgilio, F.; Nastruzzi, C. Macrophages loaded with doxorubicin by ATP-mediated permeabilization: Potential carriers for antitumor therapy. Biochim. Biophys Acta 1994, 1224, 269–276. [Google Scholar] [CrossRef]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-Guided Hybrid Neutrophil Micromotors for Targeted Drug Transport. Angew. Chem. Int. Ed. Engl. 2017, 56, 12935–12939. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chang, X.; Wu, Z.; Li, J.; Shao, G.; Deng, X.; Qiu, J.; Guo, B.; Zhang, G.; He, Q.; et al. Autonomous Collision-Free Navigation of Microvehicles in Complex and Dynamically Changing Environments. ACS Nano 2017, 11, 9268–9275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, R.; Chen, H.; Li, T.; Jiang, H.; Xu, X.; Tang, X.; Wan, M.; Mao, C.; Shi, D. Near-Infrared Light-Driven Multifunctional Tubular Micromotors for Treatment of Atherosclerosis. ACS Appl. Mater Interfaces 2021, 13, 30930–30940. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Wang, Q.; Wang, R.; Wu, R.; Li, T.; Fang, D.; Huang, Y.; Yu, Y.; Fang, L.; Wang, X.; et al. Platelet-derived porous nanomotor for thrombus therapy. Sci. Adv. 2020, 6, eaaz9014. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, T.; Gao, W.; Wang, Q.; Li, X.; Mao, C.; Zhou, M.; Wan, M.; Shen, J. Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J. Mater. Chem. B 2020, 8, 5765–5775. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Functional coatings enable navigation of light-propelled micromotors in blood for effective biodetoxification. Nanoscale 2021, 13, 17106–17115. [Google Scholar] [CrossRef]
- Tao, Y.; Li, X.; Wu, Z.; Chen, C.; Tan, K.; Wan, M.; Zhou, M.; Mao, C. Nitric oxide-driven nanomotors with bowl-shaped mesoporous silica for targeted thrombolysis. J. Colloid Interface Sci. 2022, 611, 61–70. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, R.; Li, X.; Wang, X.; Tang, X.; Tan, K.; Wan, M.; Mao, C.; Xu, X.; Jiang, H.; et al. Multi-Pathway Microenvironment Regulation for Atherosclerosis Therapy Based on Beta-Cyclodextrin/L-Arginine/Au Nanomotors with Dual-Mode Propulsion. Small 2022, 18, e2104120. [Google Scholar] [CrossRef]
- Fang, D.; Li, T.; Wu, Z.; Wang, Q.; Wan, M.; Zhou, M.; Mao, C. Dual drive mode polydopamine nanomotors for continuous treatment of an inferior vena cava thrombus. J. Mater. Chem B 2021, 9, 8659–8666. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Zhu, L.; Huang, W.; Zhao, Y.; Jin, K.; ZhuGe, Q. Tissue Plasminogen Activator-Porous Magnetic Microrods for Targeted Thrombolytic Therapy after Ischemic Stroke. ACS Appl. Mater. Interfaces 2018, 10, 32988–32997. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, Y.; Ran, P.; He, J.; Xie, S.; Weng, J.; Li, X. Ultrasound-Propelled Janus Rod-Shaped Micromotors for Site-Specific Sonodynamic Thrombolysis. ACS Appl. Mater. Interfaces 2021, 13, 58411–58421. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Albadawi, H.; Chong, B.W.; Deipolyi, A.R.; Sheth, R.A.; Khademhosseini, A.; Oklu, R. Advances in Biomaterials and Technologies for Vascular Embolization. Adv. Mater. 2019, 31, e1901071. [Google Scholar] [CrossRef]
- Bilbao, J.I.; Martínez-Cuesta, A.; Urtasun, F.; Cosín, O. Complications of embolization. Semin. Interv. Radiol. 2006, 23, 126–142. [Google Scholar] [CrossRef]
- Law, J.; Wang, X.; Luo, M.; Xin, L.; Du, X.; Dou, W.; Wang, T.; Shan, G.; Wang, Y.; Song, P.; et al. Microrobotic swarms for selective embolization. Sci. Adv. 2022, 8, eabm5752. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xie, H.; Liang, X.; Li, X.; Duan, J.; Chen, Y.; Yang, Z.; Liu, C.; Wang, C.; Zhang, H.; et al. Bilayered Nanoparticles with Sequential Release of VEGF Gene and Paclitaxel for Restenosis Inhibition in Atherosclerosis. ACS Appl. Mater. Interfaces 2017, 9, 27522–27532. [Google Scholar] [CrossRef]
- Zhang, J.; Zu, Y.; Dhanasekara, C.S.; Li, J.; Wu, D.; Fan, Z.; Wang, S. Detection and treatment of atherosclerosis using nanoparticles. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2017, 9, e1412. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Feng, G.; Shen, J.; Kong, D.; Zhao, Q. Nitric Oxide-Releasing Biomaterials for Biomedical Applications. Curr. Med. Chem. 2016, 23, 2579–2601. [Google Scholar] [CrossRef]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Stricker, R.B.; Wong, D.; Shiu, D.T.; Reyes, P.T.; Shuman, M.A. Activation of plasminogen by tissue plasminogen activator on normal and thrombasthenic platelets: Effects on surface proteins and platelet aggregation. Blood 1986, 68, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, T.; Ji, F.; Zhao, S.; Liu, K.; Zhang, Z.; Zhang, W.; Mei, Y. Trimer-like microrobots with multimodal locomotion and reconfigurable capabilities. Mater. Today Adv. 2022, 14, 100231. [Google Scholar] [CrossRef]
- Xie, S.; Li, S.; Cao, W.; Mo, C.; Zhang, Z.; Huang, K.; Li, X. Bacteria-Templated and Dual Enzyme-Powered Microcapsule Motors To Promote Thrombus Penetration and Thrombolytic Efficacy. ACS Appl. Mater. Interfaces 2022, 14, 37553–37565. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Mo, C.; Cao, W.; Xie, S.; Li, S.; Zhang, Z.; Li, X. Bacteria-propelled microtubular motors for efficient penetration and targeting delivery of thrombolytic agents. Acta Biomater. 2022, 142, 49–59. [Google Scholar] [CrossRef]
- Liu, J.; Yu, S.; Xu, B.; Tian, Z.; Zhang, H.; Liu, K.; Shi, X.; Zhao, Z.; Liu, C.; Lin, X.; et al. Magnetically propelled soft microrobot navigating through constricted microchannels. Appl. Mater. Today 2021, 25, 101237. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Schmidt, O.G. Medical microbots need better imaging and control. Nature 2017, 545, 406–408. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yang, H.; Song, W.; Dai, L.; Yu, S.; Liu, X.; Li, T. Magnetic microswarm for MRI contrast enhancer. Chem. Asian J. 2022, 17, e202200561. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Garg, S.; Goldman, D.; Krumme, M.; Rohan, L.C.; Smoot, S.; Friend, D.R. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antivir. Res. 2010, 88 (Suppl. 1), S19–S29. [Google Scholar] [CrossRef]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Nanomedicines for the topical treatment of vulvovaginal infections: Addressing the challenges of antimicrobial resistance. Adv. Drug Deliv. Rev. 2021, 178, 113855. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular Cargo Delivery: Toward Assisted Fertilization by Sperm-Carrying Micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Medina-Sánchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Brazdova, A.; Senechal, H.; Peltre, G.; Poncet, P. Immune Aspects of Female Infertility. Int. J. Fertil. Steril. 2016, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Shende, P. Biotherapy using Sperm Cell-oriented Transportation of Therapeutics in Female Reproductive Tract Cancer. Curr. Pharm. Biotechnol. 2022, 23, 1359–1366. [Google Scholar] [CrossRef]
- Magdanz, V.; Guix, M.; Hebenstreit, F.; Schmidt, O.G. Dynamic Polymeric Microtubes for the Remote-Controlled Capture, Guidance, and Release of Sperm Cells. Adv. Mater. 2016, 28, 4084–4089. [Google Scholar] [CrossRef]
- Striggow, F.; Medina-Sánchez, M.; Auernhammer, G.K.; Magdanz, V.; Friedrich, B.M.; Schmidt, O.G. Sperm-Driven Micromotors Moving in Oviduct Fluid and Viscoelastic Media. Small 2020, 16, e2000213. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Maitz, M.F.; Werner, C.; Schmidt, O.G. Sperm Micromotors for Cargo Delivery through Flowing Blood. ACS Nano 2020, 14, 2982–2993. [Google Scholar] [CrossRef]
- Striggow, F.; Nadporozhskaia, L.; Friedrich, B.M.; Schmidt, O.G.; Medina-Sánchez, M. Micromotor-mediated sperm constrictions for improved swimming performance. Eur. Phys. J. E Soft Matter 2021, 44, 67. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, J.; Li, Z.; Gong, H.; de Ávila, B.E.; Duan, Y.; Zhang, Q.; Zhou, J.; Yin, L.; Karshalev, E.; et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 2022. [Google Scholar] [CrossRef]
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef]
- Kim, D.I.; Lee, H.; Kwon, S.H.; Sung, Y.J.; Song, W.K.; Park, S. Bilayer Hydrogel Sheet-Type Intraocular Microrobot for Drug Delivery and Magnetic Nanoparticles Retrieval. Adv. Healthc. Mater. 2020, 9, e2000118. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, B.; Zhao, Z.; Yang, X.; Xing, Y.; You, C.; Kong, Y.; Cui, J.; Zhu, L.; Lin, S.; et al. Flying Squirrel-Inspired Motion Control of a Light-Deformed Pt-PAzoMA Micromotor through Drag Force Manipulation. ACS Appl. Mater. Interfaces 2021, 13, 30106–30117. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, C.; Ulbricht, C.; Truglas, T.; Wielend, D.; Bednorz, M.; Groiss, H.; Brüggemann, O.; Teasdale, I.; Salinas, Y. Reversible Speed Regulation of Self-Propelled Janus Micromotors via Thermoresponsive Bottle-Brush Polymers. Chemistry 2021, 27, 3262–3267. [Google Scholar] [CrossRef] [PubMed]

| Name | Size | Power Source | Locomotion Speed | Lifespan | Biocompatibility | Applicable Environment |
|---|---|---|---|---|---|---|
| Double-conical Zinc Micromotors [31] | 20 μm | Chemical power: Zn | 110 μm/s | 15 s | / | Stomach |
| PEDOT/Zn Bilayer Micromotors [32] | 20 μm | Chemical power: Zn | ~60 μm/s | ~10 min | showed no apparent toxicity | Stomach |
| Multicompartment Tubular Micromotors [33] | ~15 μm | Chemical power: Zn | ~70 μm/s | 15 s–60 s (the length of the Zn segment) | showed no apparent toxicity | Stomach and Duodenum |
| Mg-based Micromotors [34] | ~20 μm | Chemical power: Mg | ~120 μm/s | ~6 min | showed no apparent toxicity | Stomach |
| Bubble-propelled Janus Ga/Zn Micromotors [35] | ~8.9 μm | Chemical power: Zn | 383–161.2 μm/s | 0.55–5.2 min | showed no apparent toxicity | Stomach |
| Motor Toxoids [36] | ~25 μm | Chemical power: Mg | 160–200 μm/s | / | showed no apparent toxicity | Stomach and Small intestine |
| Suction-cup-inspired Micromotors [37] | ~300 μm | Chemical power: Mg | 200 μm/s | ~16 s | / | Stomach |
| Biological Chemotaxis-guided Self-thermophoretic Micromotors [38] | ~80 μm | Photocatalytic activity | 2 μm/s | / | / | Colorectum |
| Name | Size | Power Source | Locomotion Speed | Lifespan | Biocompatibility | Applicable Environment |
|---|---|---|---|---|---|---|
| Active Hybrid Microcapsule Motors [54] | ~2.32 μm | Powered by the biocatalytic decomposition of urea | ~11 μm/s | over 10 min | / | Bladder |
| Enzyme-powered Polymer Micromotors [55] | ~1 μm | Powered by the biocatalytic decomposition of urea | 10.67 μm/s | / | showed no apparent toxicity | Bladder |
| Enzyme-powered Janus Platelet Micromotors [57] | ~2 μm | Powered by the biocatalytic decomposition of urea | ~6 μm/s | over 30 min | / | Bladder |
| Enzyme-photocatalyst Tandem Micromotors [60] | ~15 μm | Enzyme and the photocatalytic activity | ~3.3 μm/s | over 2 h | / | Bladder/Urinary catheters |
| Photothermal Interference (PTI) Urease-modified Polydopamine (PDA) Micromotors [61] | ~330 nm | Enzyme and the photocatalytic activity | increased mean square displacement | / | showed no apparent toxicity | Bladder |
| Name | Size | Power Source | Locomotion Speed | Lifespan | Biocompatibility | Applicable Environment |
|---|---|---|---|---|---|---|
| RBC Micromotors [69] | ~7 μm | Ultrasound propulsion and magnetic guidance | 5 μm/s | / | anti-phagocytosis capability against macrophages | Blood vessel |
| Red Blood Cell-mimicking (RBCM) Micromotors [70] | ~2.1 μm | Ultrasound propulsion and magnetic guidance | ~7 μm/s | / | showed no apparent toxicity | Blood vessel |
| Engineered PLT Micromotors [13] | ~2 μm | Photocatalytic activity | 2.2~12.2 μm/s | / | showed no apparent toxicity | Blood vessel |
| NIR Light-driven Multifunctional Mesoporous/Macroporous Tubular Micromotors [74] | ~8 μm | Photocatalytic activity | ~5.4 μm/s | / | showed no apparent toxicity | Blood vessel |
| Platelet-derived Porous Micromotors [75] | 410 nm | Photocatalytic activity | increased mean square displacement | / | showed no apparent toxicity | Blood vessel |
| Platelet-derived Nanomotor Coated Balloon [76] | ~500 nm | Photocatalytic activity | increased mean square displacement | / | showed no apparent toxicity | Blood vessel |
| Functional Coatings Enable Navigation of Light-propelled Micromotors [77] | ~20 μm | Photocatalytic activity | ~8.2 μm/s | / | showed no apparent toxicity | Blood vessel |
| Nitric Oxide-driven Nanomotors [78] | ~255 nm | Chemical power: NO | ~3.5 μm/s | / | showed no apparent toxicity | Blood vessel |
| β-cyclodextrin/L-arginine/Au Nanomotors with Dual-mode Propulsion [79] | ~250 nm | Chemical power: NO and Photocatalytic activity | 5~8 μm/s | / | showed no apparent toxicity | Blood vessel |
| Dual Drive Mode Polydopamine Nanomotors [80] | ~200 μm | Chemical power: NO and Photocatalytic activity | ~2.32 μm/s | / | showed no apparent toxicity | Blood vessel |
| tPA-porous Magnetic Microrods [81] | ~1.3 μm | Magnetic guidance | / | / | showed no apparent toxicity | Blood vessel |
| Ultrasound-propelled Janus Rod-shaped Micromotors [82] | ~1.3 μm | Ultrasound propulsion | penetration in agarose gels | / | showed no apparent toxicity | Blood vessel |
| Name | Size | Power Source | Locomotion Speed | Lifespan | Biocompatibility | Applicable Environment |
|---|---|---|---|---|---|---|
| Sperm-hybrid Micromotors [102] | >50 μm | Sperm propulsion and Magnetic guidance | ~50 μm/s | / | / | Female reproductive tract |
| Sperm-driven Micromotors [107] | >50 μm | Sperm propulsion and Magnetic guidance | ~76 μm/s | / | showed no apparent toxicity | Blood vessel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Gao, Y.; Lv, Y.; Wang, Y.; Cao, Y.; Zhao, W.; Zuo, D.; Mu, H.; Hua, Y. Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens. Micromachines 2022, 13, 1780. https://doi.org/10.3390/mi13101780
Huang S, Gao Y, Lv Y, Wang Y, Cao Y, Zhao W, Zuo D, Mu H, Hua Y. Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens. Micromachines. 2022; 13(10):1780. https://doi.org/10.3390/mi13101780
Chicago/Turabian StyleHuang, Shandeng, Yinghua Gao, Yu Lv, Yun Wang, Yinghao Cao, Weisong Zhao, Dongqing Zuo, Haoran Mu, and Yingqi Hua. 2022. "Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens" Micromachines 13, no. 10: 1780. https://doi.org/10.3390/mi13101780
APA StyleHuang, S., Gao, Y., Lv, Y., Wang, Y., Cao, Y., Zhao, W., Zuo, D., Mu, H., & Hua, Y. (2022). Applications of Nano/Micromotors for Treatment and Diagnosis in Biological Lumens. Micromachines, 13(10), 1780. https://doi.org/10.3390/mi13101780







