Zinc Carboxylate Surface Passivation for Enhanced Optical Properties of In(Zn)P Colloidal Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zinc Oleate (Zn(OA)2) Stock Solution
2.3. Synthesis of In(Zn)P QDs
2.4. ZnS Shell Passivaiton to In(Zn)P QDs
2.5. Characterization Methods
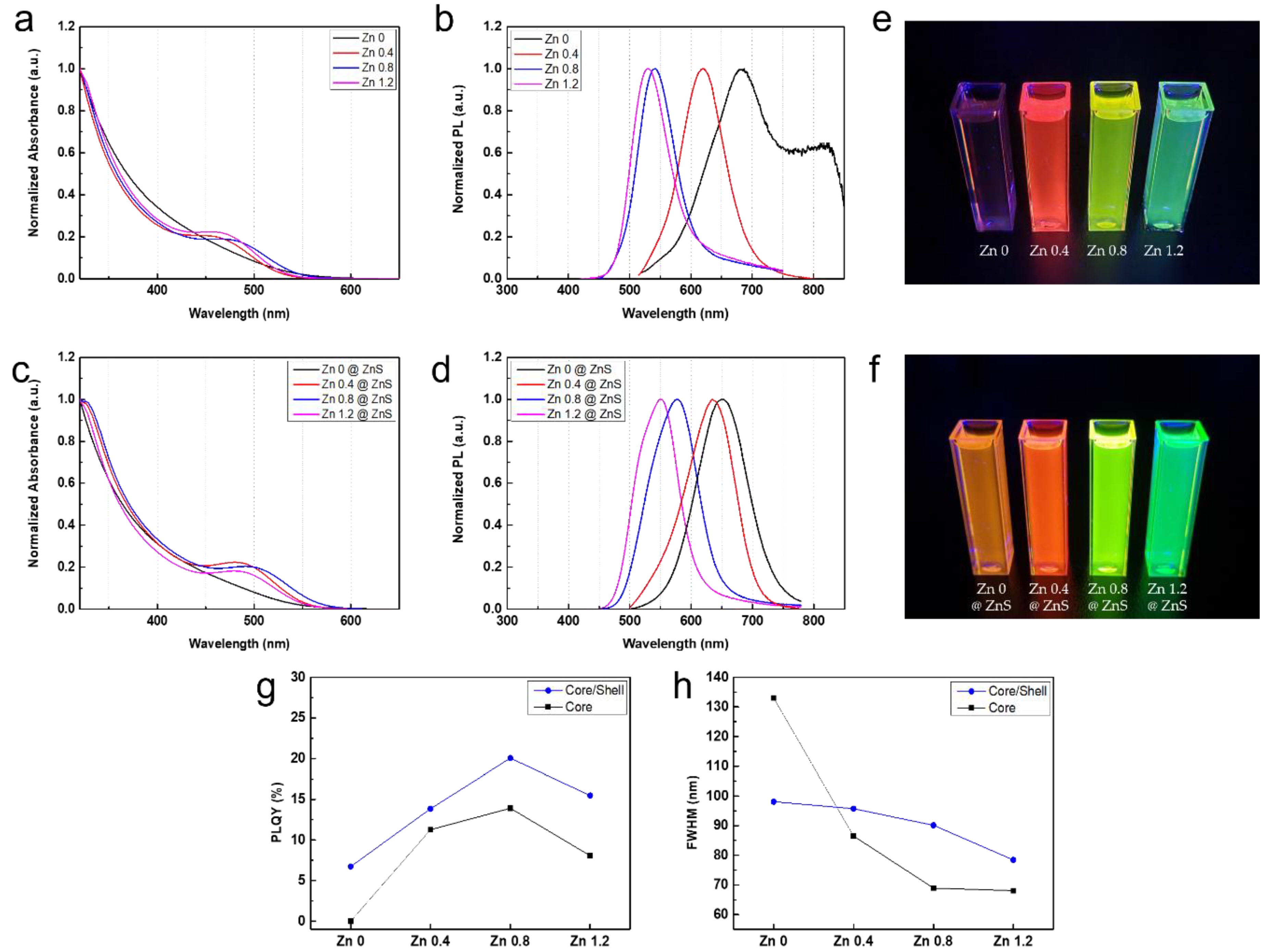
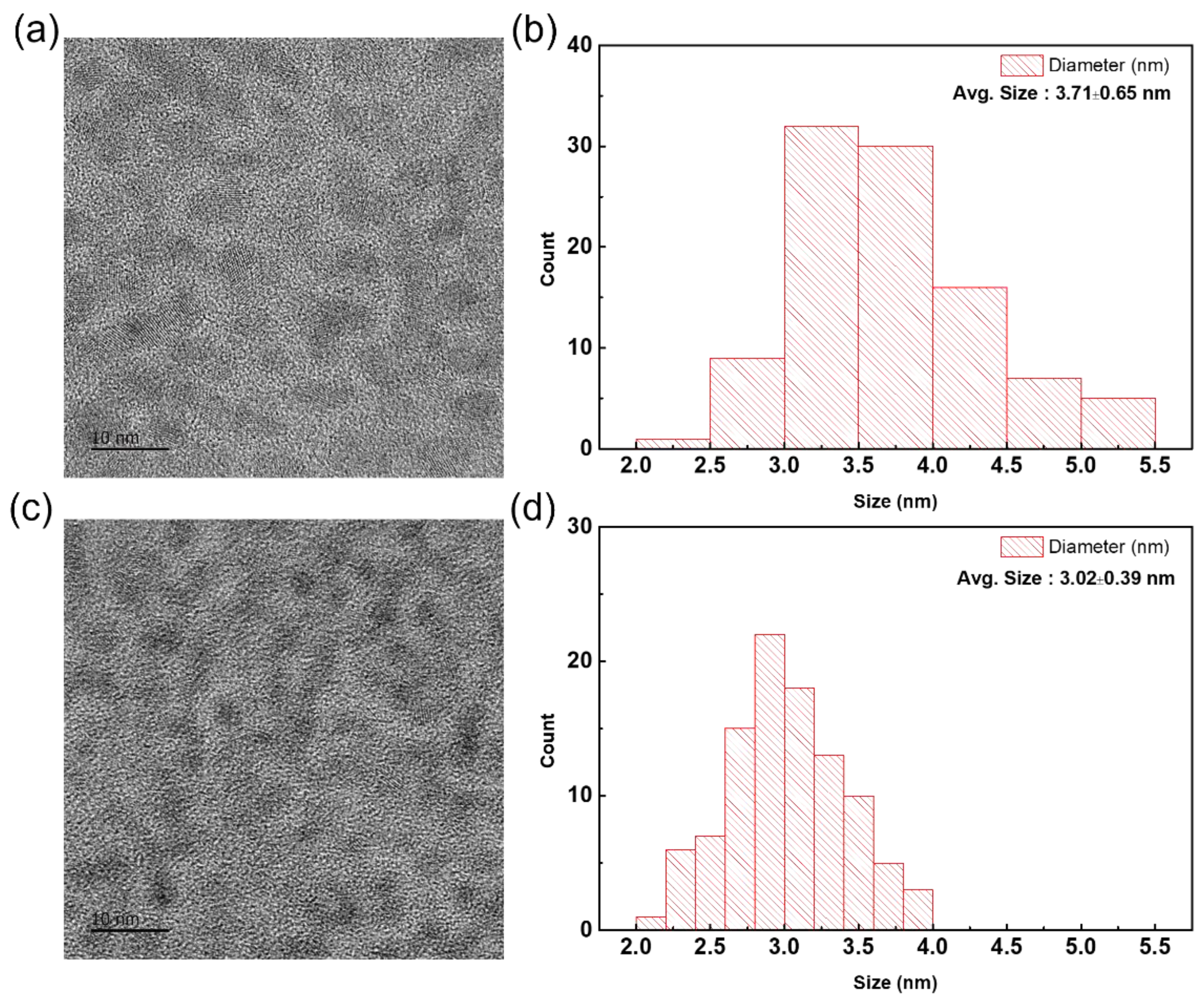
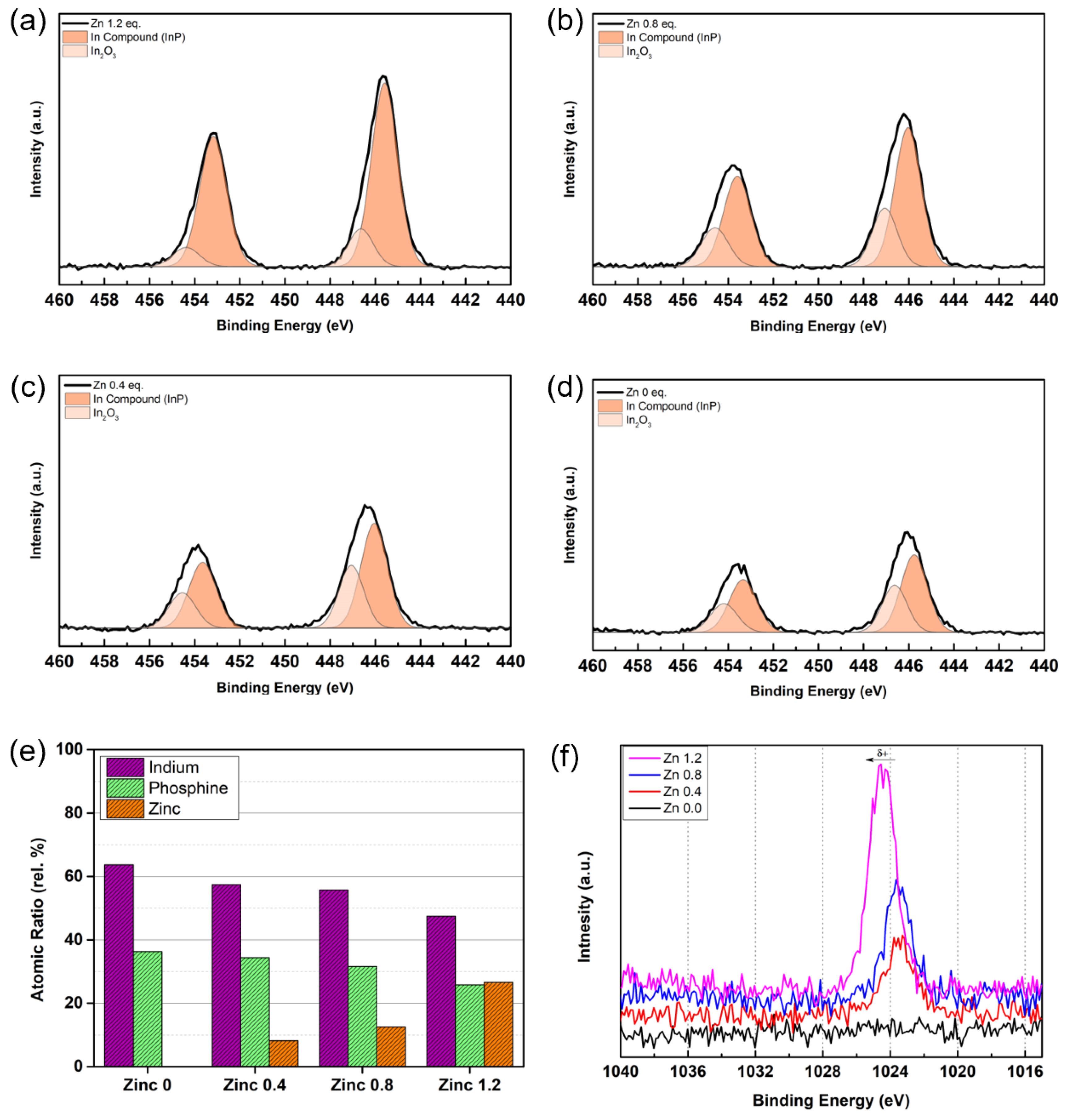
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kovalenko, M.V.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.V.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J.; et al. Prospects of Nanoscience with Nanocrystals. ACS Nano 2015, 9, 1012–1057. [Google Scholar] [CrossRef] [PubMed]
- Moreels, I.; Justo, Y.; De Geyter, B.; Haustraete, K.; Martins, J.C.; Hens, Z. Size-Tunable, Bright, and Stable PbS Quantum Dots: A Surface Chemistry Study. ACS Nano 2011, 5, 2004–2012. [Google Scholar] [CrossRef]
- Tessier, M.D.; Dupont, D.; De Nolf, K.; De Roo, J.; Hens, Z. Economic and Size-Tunable Synthesis of InP/ZnE (E = S, Se) Colloidal Quantum Dots. Chem. Mater. 2015, 27, 4893–4898. [Google Scholar] [CrossRef]
- Reiss, P.; Bleuse, J.; Pron, A. Highly Luminescent CdSe/ZnSe Core/Shell Nanocrystals of Low Size Dispersion. Nano Lett. 2002, 2, 781–784. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, L.; Pang, Y.; Xu, L.; Chen, J.; Ren, Z.; Luo, J.; Wang, L.; Chen, L.; Han, K.; et al. Piezo-phototronic Effect Enhanced Photodetector Based on CH3NH3PbI3 Single Crystals. ACS Nano 2018, 12, 10501–10508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Nanogenerators, self-powered systems, blue energy, piezotronics and piezo-phototronics—A recall on the original thoughts for coining these fields. Nano Energy 2018, 54, 477–483. [Google Scholar] [CrossRef]
- Choi, M.-J.; García de Arquer, F.P.; Proppe, A.H.; Seifitokaldani, A.; Choi, J.; Kim, J.; Baek, S.-W.; Liu, M.; Sun, B.; Biondi, M.; et al. Cascade surface modification of colloidal quantum dot inks enables efficient bulk homojunction photovoltaics. Nat. Commun. 2020, 11, 103. [Google Scholar] [CrossRef]
- Xia, C.; Meeldijk, J.D.; Gerritsen, H.C.; de Mello Donega, C. Highly Luminescent Water-Dispersible NIR-Emitting Wurtzite CuInS2/ZnS Core/Shell Colloidal Quantum Dots. Chem. Mater. 2017, 29, 4940–4951. [Google Scholar] [CrossRef]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. Near-Infrared Photoluminescent Ag2S Quantum Dots from a Single Source Precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef]
- Perez, K.A.; Rogers, C.R.; Weiss, E.A. Quantum Dot-Catalyzed Photoreductive Removal of Sulfonyl-Based Protecting Groups. Angew. Chem. Int. Ed. 2020, 59, 14091–14095. [Google Scholar] [CrossRef]
- Jiang, Y.; Weiss, E.A. Colloidal Quantum Dots as Photocatalysts for Triplet Excited State Reactions of Organic Molecules. J. Am. Chem. Soc. 2020, 142, 15219–15229. [Google Scholar] [CrossRef]
- Wang, R.; Shang, Y.; Kanjanaboos, P.; Zhou, W.; Ning, Z.; Sargent, E.H. Colloidal quantum dot ligand engineering for high performance solar cells. Energy Environ. Sci. 2016, 9, 1130–1143. [Google Scholar] [CrossRef]
- Sliz, R.; Lejay, M.; Fan, J.Z.; Choi, M.-J.; Kinge, S.; Hoogland, S.; Fabritius, T.; García de Arquer, F.P.; Sargent, E.H. Stable Colloidal Quantum Dot Inks Enable Inkjet-Printed High-Sensitivity Infrared Photodetectors. ACS Nano 2019, 13, 11988–11995. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wu, L.; Lu, Z.; Li, M.; Wen, Y.; Yang, Y.; Liu, W.; Zhang, T.; Cao, W.; Tsang, S.-W.; et al. High efficiency and stability of ink-jet printed quantum dot light emitting diodes. Nat. Commun. 2020, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-Light-Emitting Diodes with Quantum Dot Color Converters for Display Backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.Y.; Suh, Y.-H.; Fan, X.-B.; Shin, D.-W.; Lee, S.; Choi, H.W.; Lee, T.H.; Yang, J.; Zhan, S.; Harden-Chaters, W.; et al. Technology progress on quantum dot light-emitting diodes for next-generation displays. Nanoscale Horiz. 2021, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Ginterseder, M.; Franke, D.; Perkinson, C.F.; Wang, L.; Hansen, E.C.; Bawendi, M.G. Scalable Synthesis of InAs Quantum Dots Mediated through Indium Redox Chemistry. J. Am. Chem. Soc. 2020, 142, 4088–4092. [Google Scholar] [CrossRef]
- Song, J.H.; Choi, H.; Pham, H.T.; Jeong, S. Energy level tuned indium arsenide colloidal quantum dot films for efficient photovoltaics. Nat. Commun. 2018, 9, 4267. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8, 5209–5215. [Google Scholar] [CrossRef]
- Vikram, A.; Zahid, A.; Bhargava, S.S.; Jang, H.; Sutrisno, A.; Khare, A.; Trefonas, P.; Shim, M.; Kenis, P.J.A. Unraveling the Origin of Interfacial Oxidation of InP-Based Quantum Dots: Implications for Bioimaging and Optoelectronics. ACS Appl. Nano Mater. 2020, 3, 12325–12333. [Google Scholar] [CrossRef]
- Jang, E.; Kim, Y.; Won, Y.-H.; Jang, H.; Choi, S.-M. Environmentally Friendly InP-Based Quantum Dots for Efficient Wide Color Gamut Displays. ACS Energy Lett. 2020, 5, 1316–1327. [Google Scholar] [CrossRef]
- Tessier, M.D.; Baquero, E.A.; Dupont, D.; Grigel, V.; Bladt, E.; Bals, S.; Coppel, Y.; Hens, Z.; Nayral, C.; Delpech, F. Interfacial Oxidation and Photoluminescence of InP-Based Core/Shell Quantum Dots. Chem. Mater. 2018, 30, 6877–6883. [Google Scholar] [CrossRef]
- Wei, X.; Mei, S.; Zhang, G.; Su, D.; Xie, F.; Zhang, W.; Guo, R. Enhanced tunable dual emission of Cu:InP/ZnS quantum dots enabled by introducing Ag ions. Appl. Surf. Sci. 2019, 493, 605–612. [Google Scholar] [CrossRef]
- Mei, S.; Wei, X.; Yang, D.; Su, D.; Yang, W.; Zhang, G.; Hu, Z.; Yang, B.; Dai, H.; Xie, F.; et al. Color-tunable optical properties of cadmium-free transition metal ions doped InP/ZnS quantum dots. J. Lumin. 2019, 212, 264–270. [Google Scholar] [CrossRef]
- Cui, Z.; Mei, S.; Wen, Z.; Yang, D.; Qin, S.; Xiong, Z.; Yang, B.; He, H.; Bao, R.; Qiu, Y.; et al. Synergistic Effect of Halogen Ions and Shelling Temperature on Anion Exchange Induced Interfacial Restructuring for Highly Efficient Blue Emissive InP/ZnS Quantum Dots. Small 2022, 18, 2108120. [Google Scholar] [CrossRef]
- Kirkwood, N.; De Backer, A.; Altantzis, T.; Winckelmans, N.; Longo, A.; Antolinez, F.V.; Rabouw, F.T.; De Trizio, L.; Geuchies, J.J.; Mulder, J.T.; et al. Locating and Controlling the Zn Content in In(Zn)P Quantum Dots. Chem. Mater. 2020, 32, 557–565. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, D.; Shin, Y.S.; Lee, W.; Orr, S.; Kim, J.Y.; Park, J. Highly luminescent red-emitting In(Zn)P quantum dots using zinc oxo cluster: Synthesis and application to light-emitting diodes. Nanoscale 2022, 14, 2771–2779. [Google Scholar] [CrossRef]
- Hahm, D.; Chang, J.H.; Jeong, B.G.; Park, P.; Kim, J.; Lee, S.; Choi, J.; Kim, W.D.; Rhee, S.; Lim, J.; et al. Design Principle for Bright, Robust, and Color-Pure InP/ZnSexS1–x/ZnS Heterostructures. Chem. Mater. 2019, 31, 3476–3484. [Google Scholar] [CrossRef]
- Lim, J.; Bae, W.K.; Lee, D.; Nam, M.K.; Jung, J.; Lee, C.; Char, K.; Lee, S. InP@ZnSeS, Core@Composition Gradient Shell Quantum Dots with Enhanced Stability. Chem. Mater. 2011, 23, 4459–4463. [Google Scholar] [CrossRef]
- Mulder, J.T.; Kirkwood, N.; De Trizio, L.; Li, C.; Bals, S.; Manna, L.; Houtepen, A.J. Developing Lattice Matched ZnMgSe Shells on InZnP Quantum Dots for Phosphor Applications. ACS Appl. Nano Mater. 2020, 3, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- Pietra, F.; De Trizio, L.; Hoekstra, A.W.; Renaud, N.; Prato, M.; Grozema, F.C.; Baesjou, P.J.; Koole, R.; Manna, L.; Houtepen, A.J. Tuning the Lattice Parameter of InxZnyP for Highly Luminescent Lattice-Matched Core/Shell Quantum Dots. ACS Nano 2016, 10, 4754–4762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, J.; Miller, E.M.; Luther, J.M.; Beard, M.C. Diffusion-Controlled Synthesis of PbS and PbSe Quantum Dots with in Situ Halide Passivation for Quantum Dot Solar Cells. ACS Nano 2014, 8, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Dussert, F.; Truffier-Boutry, D.; Benayad, A.; Beal, D.; Mattera, L.; Ling, W.L.; Carrière, M.; Reiss, P. Influence of the Core/Shell Structure of Indium Phosphide Based Quantum Dots on Their Photostability and Cytotoxicity. Front. Chem. 2019, 7, 466. [Google Scholar] [CrossRef]
- Jeong, B.G.; Chang, J.H.; Hahm, D.; Rhee, S.; Park, M.; Lee, S.; Kim, Y.; Shin, D.; Park, J.W.; Lee, C.; et al. Interface polarization in heterovalent core–shell nanocrystals. Nat. Mater. 2022, 21, 246–252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Bak, E.; Ju, H.M.; Shin, Y.M.; Choi, M.-J. Zinc Carboxylate Surface Passivation for Enhanced Optical Properties of In(Zn)P Colloidal Quantum Dots. Micromachines 2022, 13, 1775. https://doi.org/10.3390/mi13101775
Yoo D, Bak E, Ju HM, Shin YM, Choi M-J. Zinc Carboxylate Surface Passivation for Enhanced Optical Properties of In(Zn)P Colloidal Quantum Dots. Micromachines. 2022; 13(10):1775. https://doi.org/10.3390/mi13101775
Chicago/Turabian StyleYoo, Doheon, Eunyoung Bak, Hae Mee Ju, Yoo Min Shin, and Min-Jae Choi. 2022. "Zinc Carboxylate Surface Passivation for Enhanced Optical Properties of In(Zn)P Colloidal Quantum Dots" Micromachines 13, no. 10: 1775. https://doi.org/10.3390/mi13101775
APA StyleYoo, D., Bak, E., Ju, H. M., Shin, Y. M., & Choi, M.-J. (2022). Zinc Carboxylate Surface Passivation for Enhanced Optical Properties of In(Zn)P Colloidal Quantum Dots. Micromachines, 13(10), 1775. https://doi.org/10.3390/mi13101775






