Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer
Abstract
1. Introduction
2. Materials and Methods
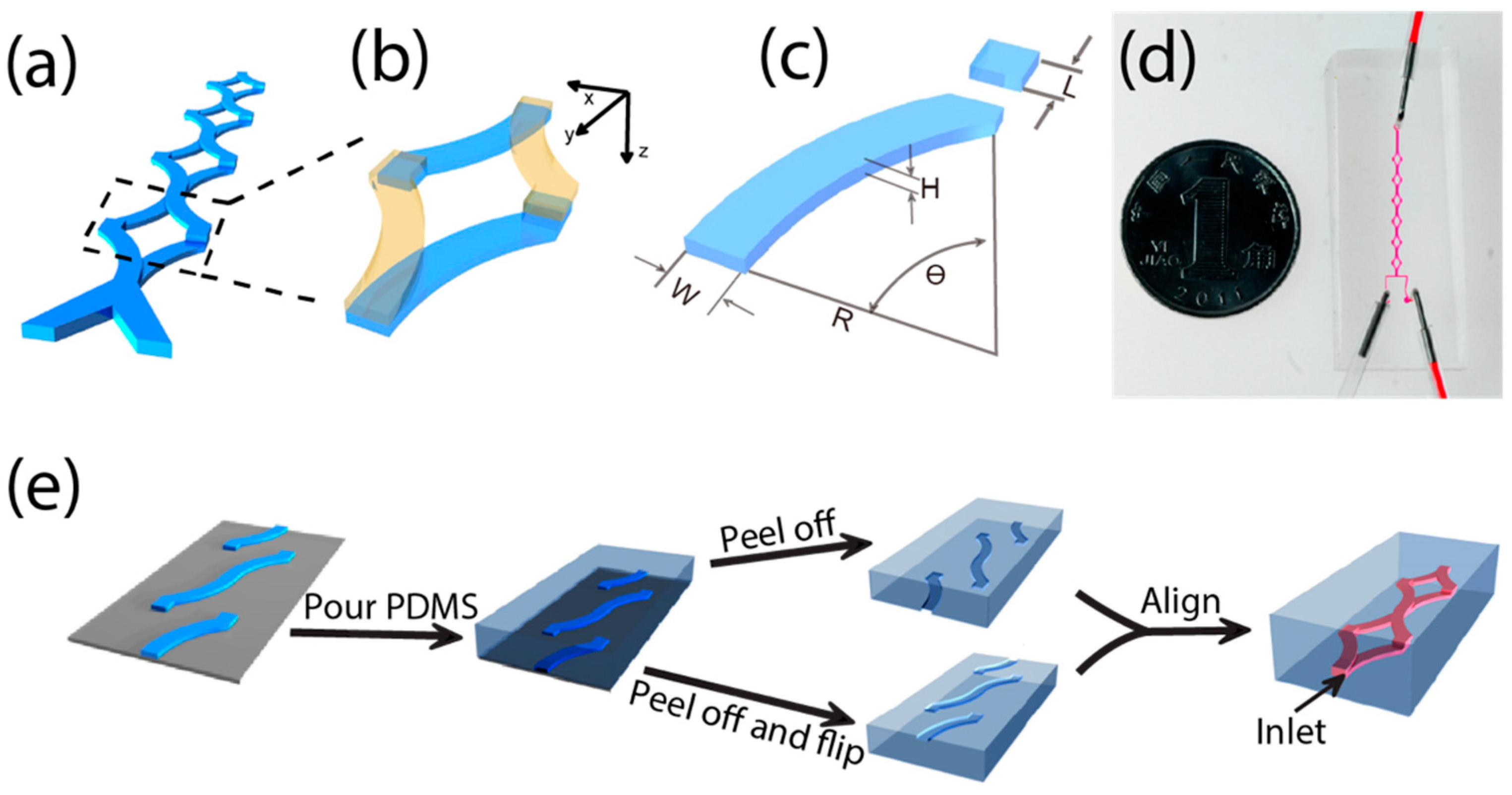
2.1. The Design of Micromixer
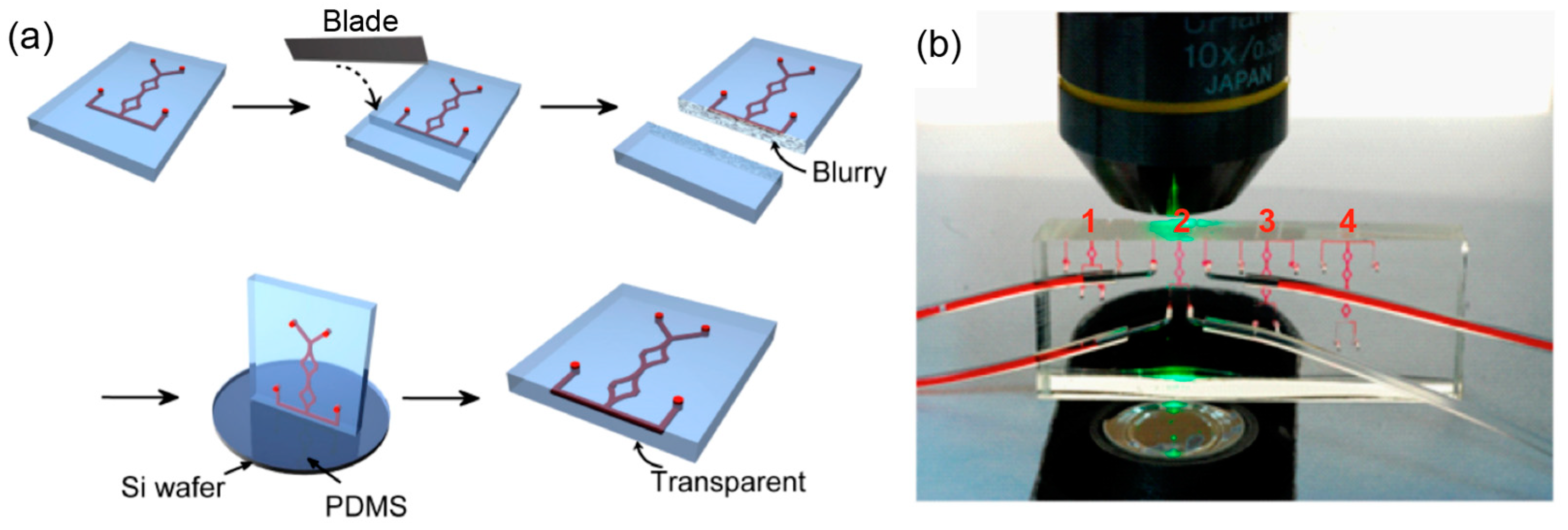
2.2. The Fabrication of PDMS SAR Micromixer
2.3. Numerical Simulation
2.4. Experimental Setup
3. Results
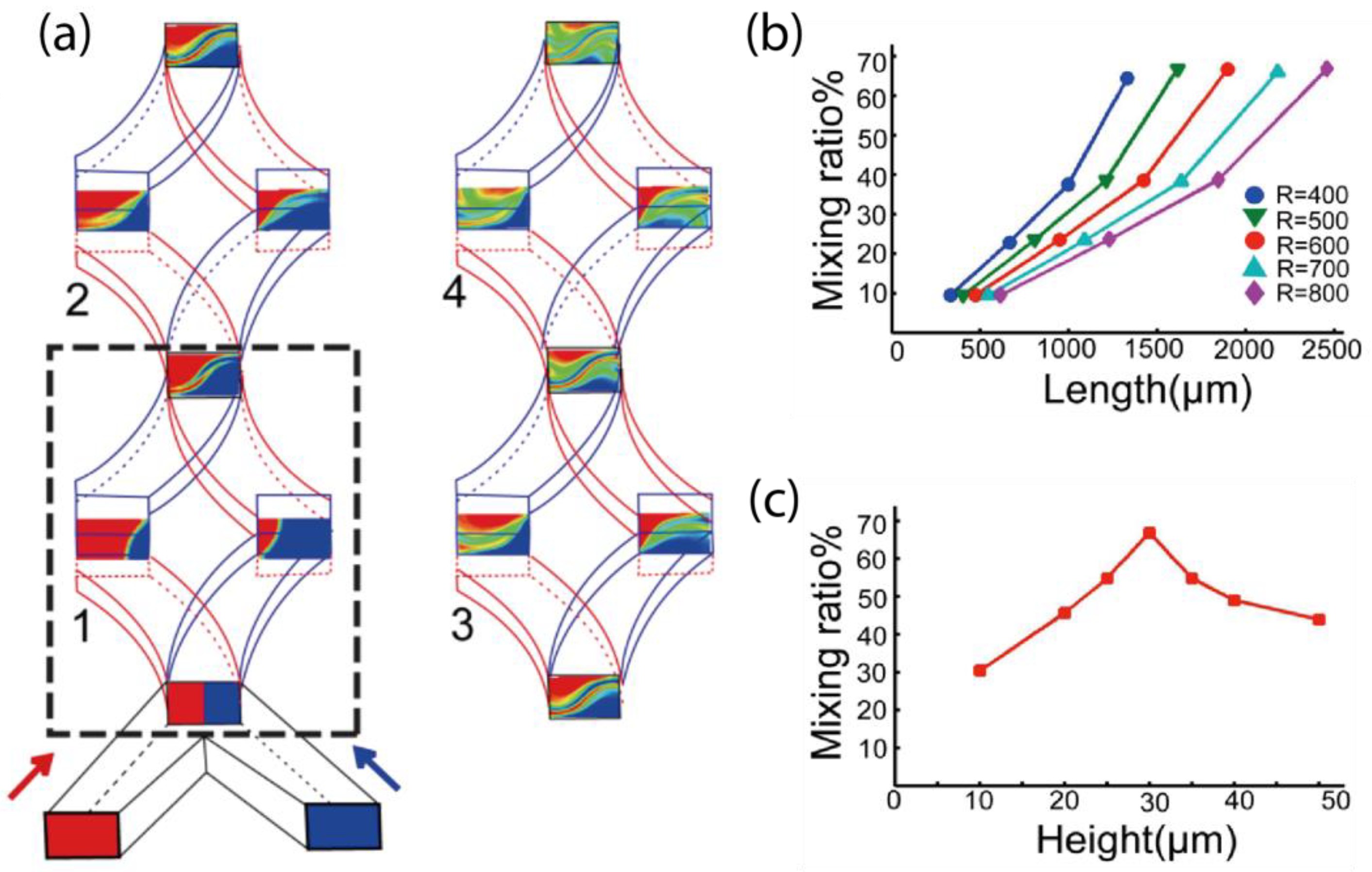
3.1. The Design and Optimization of the SAR Micromixer Structure
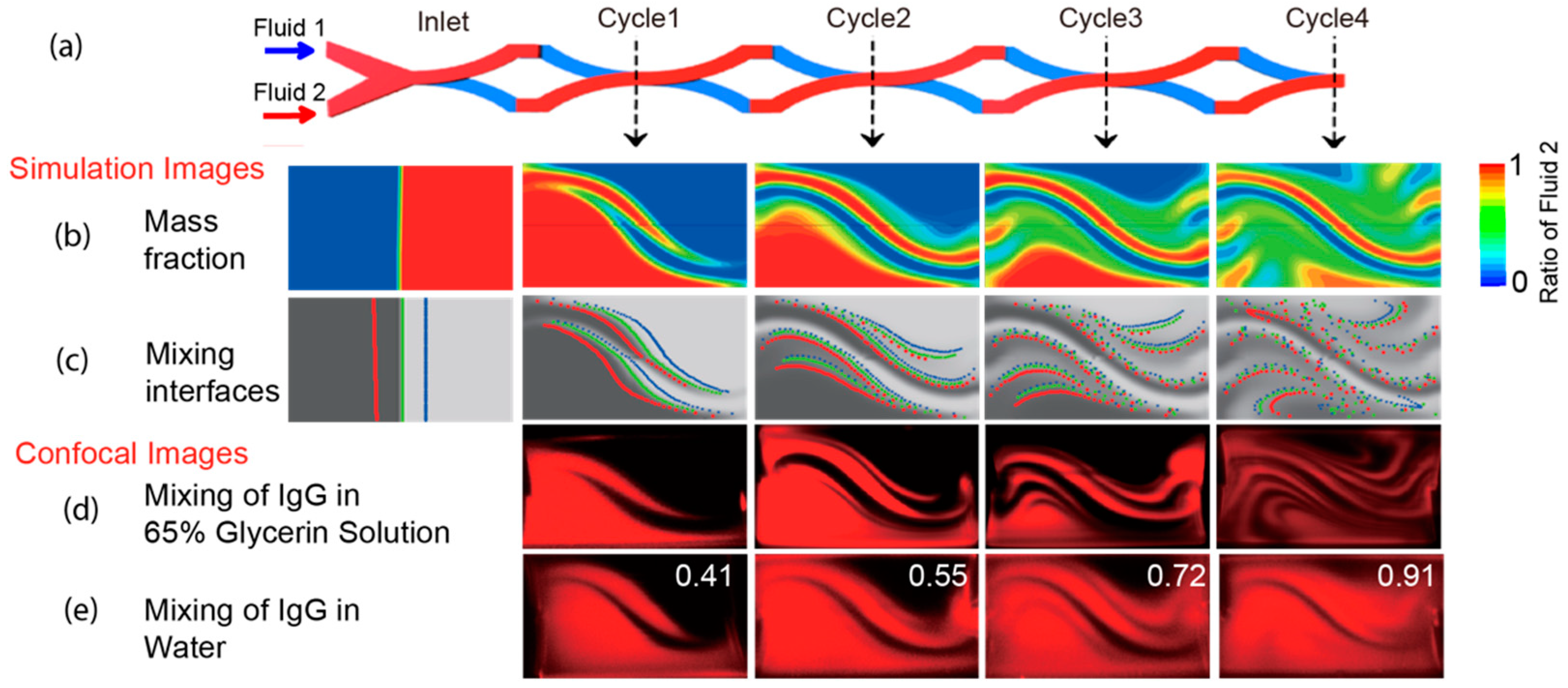
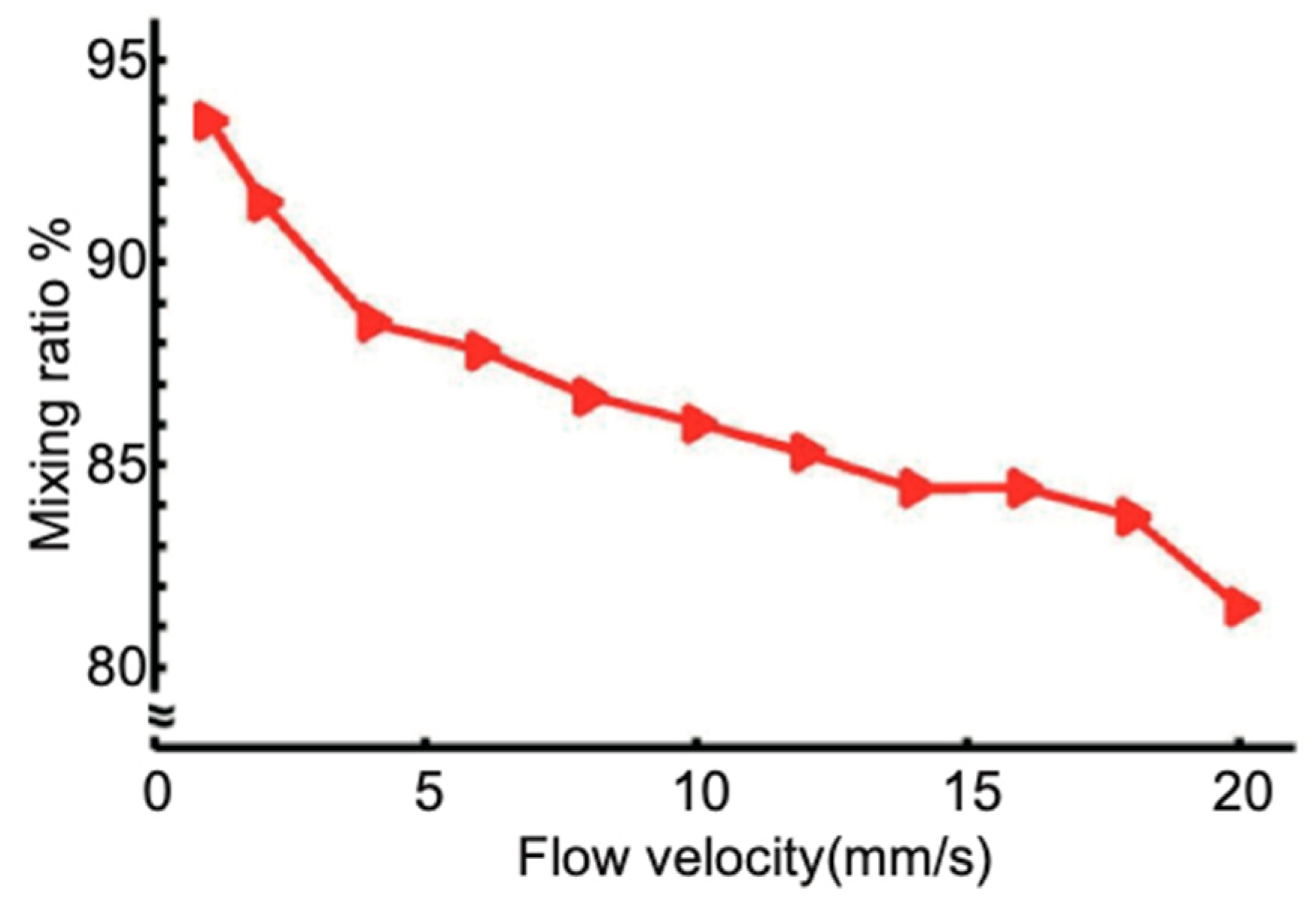
3.2. Numerical Simulation and Experimental Results
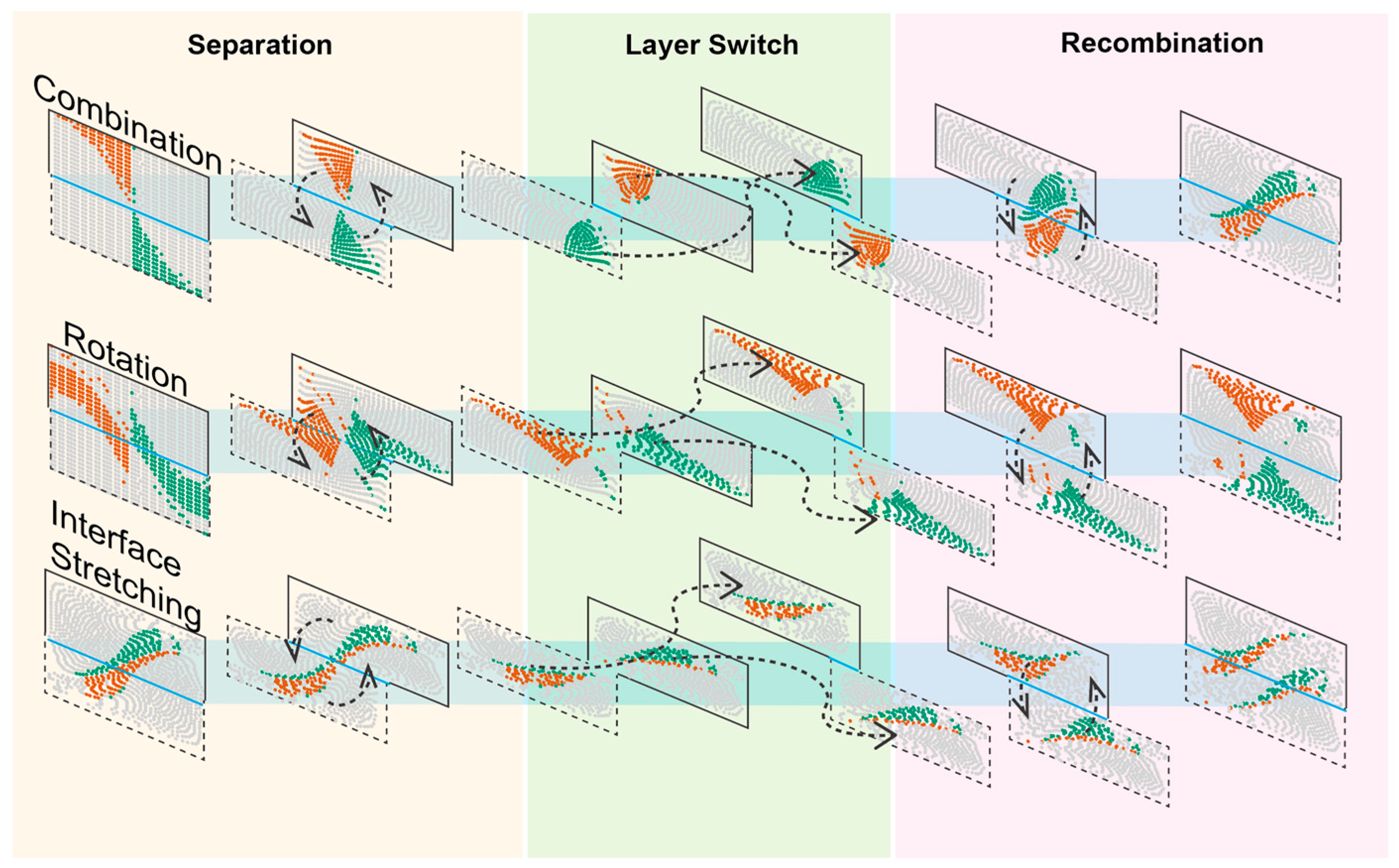
3.3. Mixing Mechanism Dissected Using Streamline Particle Trajectory Tracing
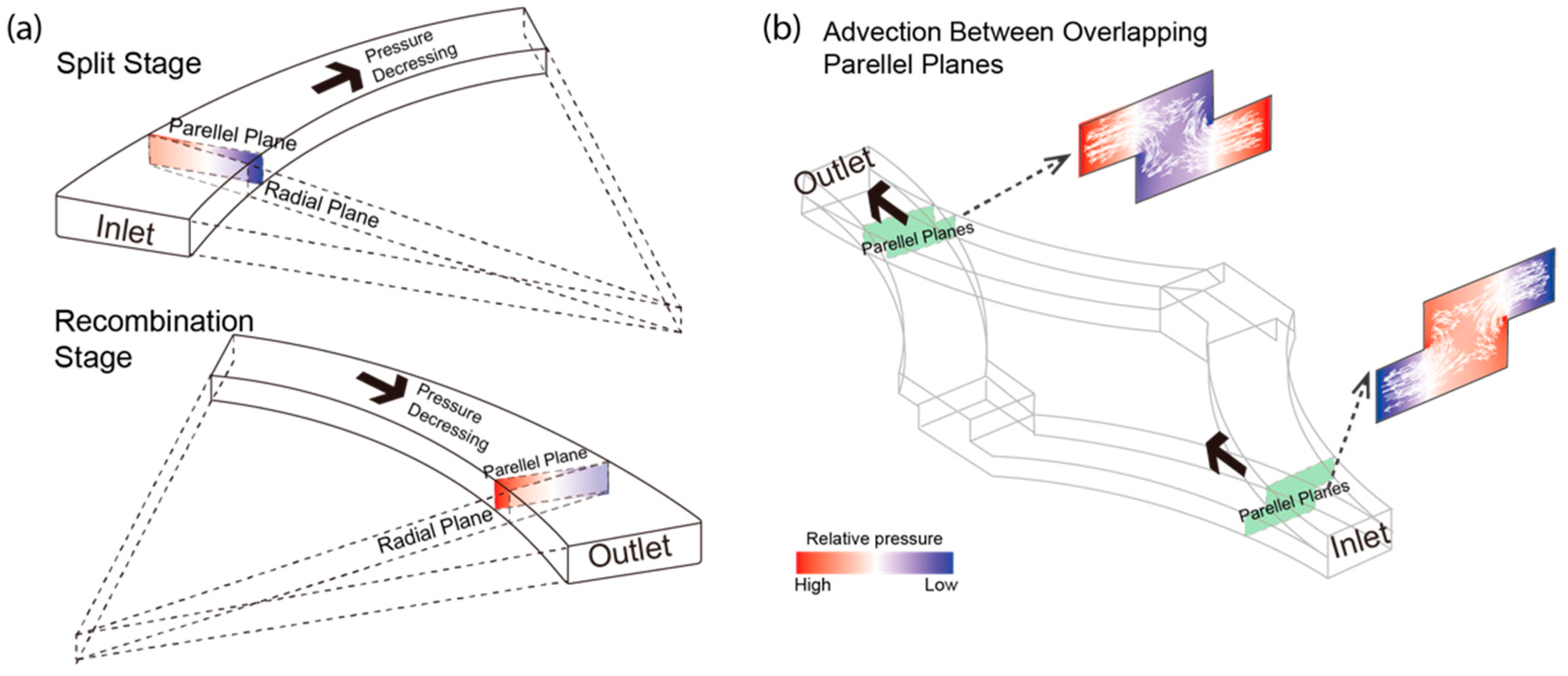
3.4. Radial Pressure Gradients in Curvature Channels Induced Advection in Overlapping Channels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, X.; Zhang, Y.S.; Santiago, G.T.-d.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A.; et al. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 17016. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Streets, A.M.; Huang, Y.Y. Chip in a lab: Microfluidics for next generation life science research. Biomicrofluidics 2013, 7, 11302. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimania, N.; Rabieec, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, D.H.; Park, J.-K. Towards practical sample preparation in point-of-care testing: User-friendly microfluidic devices. Lab Chip 2020, 20, 1191–1203. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhao, W. There can be turbulence in microfluidics at low Reynolds number. Lab Chip 2014, 14, 1452–1458. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A review on mixing in microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Meijer, H.E.; Singh, M.K.; Kang, T.G.; den Toonder, J.M.; Anderson, P.D. Passive and active mixing in microfluidic devices. Macromol. Symp. 2009, 279, 201–209. [Google Scholar] [CrossRef]
- Niu, X.; Liu, L.; Wen, W.; Sheng, P. Active microfluidic mixer chip. Appl. Phys. Lett. 2006, 88, 153508. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Multivortex micromixing. Proc. Natl. Acad. Sci. USA 2006, 103, 7228–7233. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.S. Rotation effect in split and recombination micromixing. Sens. Actuators B Chem. 2008, 129, 364–371. [Google Scholar] [CrossRef]
- Schonfeld, F.; Hessel, V.; Hofmann, C. An optimised split-and-recombine micro-mixer with uniform chaotic mixing. Lab Chip 2004, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Stremler, M.A.; Haselton, F.R.; Aref, H. Designing for chaos: Applications of chaotic advection at the microscale. Phil. Trans. R. Soc. Lond. A 2004, 362, 1019–1036. [Google Scholar] [CrossRef]
- Sivashankar, S.; Agambayev, S.; Mashraei, Y.; Li, E.Q.; Thoroddsen, S.T.; Salama, K.N. A “twisted” microfluidic mixer suitable for a wide range of flow rate applications. Biomicrofluidics 2016, 10, 034120. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.H.; Kwon, T.H.; Ahn, C.H. A serpentine laminating micromixer combining splitting/recombination and advection. Lab Chip 2005, 5, 739–747. [Google Scholar] [CrossRef]
- Raza, W.; Hossain, S.; Kim, K.-Y. Effective mixing in a short serpentine split-and-recombination micromixer. Sens. Actuators B Chem. 2018, 258, 381–392. [Google Scholar]
- Frommelt, T.; Kostur, M.; Wenzel-Schäfer, M.; Talkner, P.; Hänggi, P.; Wixforth, A. Microfluidic mixing via acoustically driven chaotic advection. Phys. Rev. Lett. 2008, 100, 034502. [Google Scholar]
- Nguyen, T.; Kim, M.-C.; Park, J.-S.; Lee, N.-E. An effective passive microfluidic mixer utilizing chaotic advection. Sens. Actuators B Chem. 2008, 132, 172–181. [Google Scholar] [CrossRef]
- Jeon, W.; Shin, C.B. Design and simulation of passive mixing in microfluidic systems with geometric variations. Chem. Eng. J. 2009, 152, 575–582. [Google Scholar] [CrossRef]
- Yasui, T.; Omoto, Y.; Osato, K.; Kaji, N.; Suzuki, N.; Naito, T.; Baba, Y. Microfluidic baker’s transformation device for three-dimensional rapid mixing. Lab Chip 2011, 11, 3356–3360. [Google Scholar] [CrossRef]
- Chen, J.J.; Lai, Y.R.; Tsai, R.T.; der Lin, J.; Wu, C.Y. Crosswiseridge micromixers with split and recombination helical flows. Chem. Eng. Sci. 2011, 66, 2164–2176. [Google Scholar] [CrossRef]
- Feng, X.; Ren, Y.; Jiang, H. An effective splitting-and-recombination micromixer with self-rotated contact surface for wide Reynolds number range applications. Biomicrofluidics 2013, 7, 54121. [Google Scholar] [CrossRef]
- Raza, W.; Hossain, S.; Kim, K.Y. A Review of Passive Micromixers with a Comparative Analysis. Micromachines 2020, 11, 455. [Google Scholar] [CrossRef]
- Duong, L.H.; Chen, P.-C. Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 2019, 13, 024108. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J.; Beebe, D.J. Passive Mixing in a Three-Dimensional Serpentine Microchannel. J. Microelectromech. Syst. 2000, 9, 190. [Google Scholar] [CrossRef]
- Beebe, D.J.; Adrian, R.J.; Olsen, M.G.; Stremler, M.A.; Aref, H.; Jo, B.H. Passive mixing in microchannels: Fabrication and flow experiments. Mec. Ind. 2001, 2, 343–348. [Google Scholar]
- Fei, P.; Chen, Z.; Men, Y.; Li, A.; Shen, Y.; Huang, Y. A compact optofluidic cytometer with integrated liquid-core/PDMS-cladding waveguides. Lab Chip 2012, 12, 3700–3706. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab Chip 2006, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qian, Z.; Jiang, M.; Li, W.; Huang, Y.; Men, Y. Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer. Micromachines 2022, 13, 1720. https://doi.org/10.3390/mi13101720
Zhang X, Qian Z, Jiang M, Li W, Huang Y, Men Y. Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer. Micromachines. 2022; 13(10):1720. https://doi.org/10.3390/mi13101720
Chicago/Turabian StyleZhang, Xiannian, Zhenwei Qian, Mengcheng Jiang, Wentao Li, Yanyi Huang, and Yongfan Men. 2022. "Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer" Micromachines 13, no. 10: 1720. https://doi.org/10.3390/mi13101720
APA StyleZhang, X., Qian, Z., Jiang, M., Li, W., Huang, Y., & Men, Y. (2022). Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer. Micromachines, 13(10), 1720. https://doi.org/10.3390/mi13101720






