A Stretching Force Control-Based Cyclic Loading Method for the Evaluation of Mechanical Properties of Gelation Methacrylate (GelMA) Microfibers
Abstract
1. Introduction
2. Materials and Methods
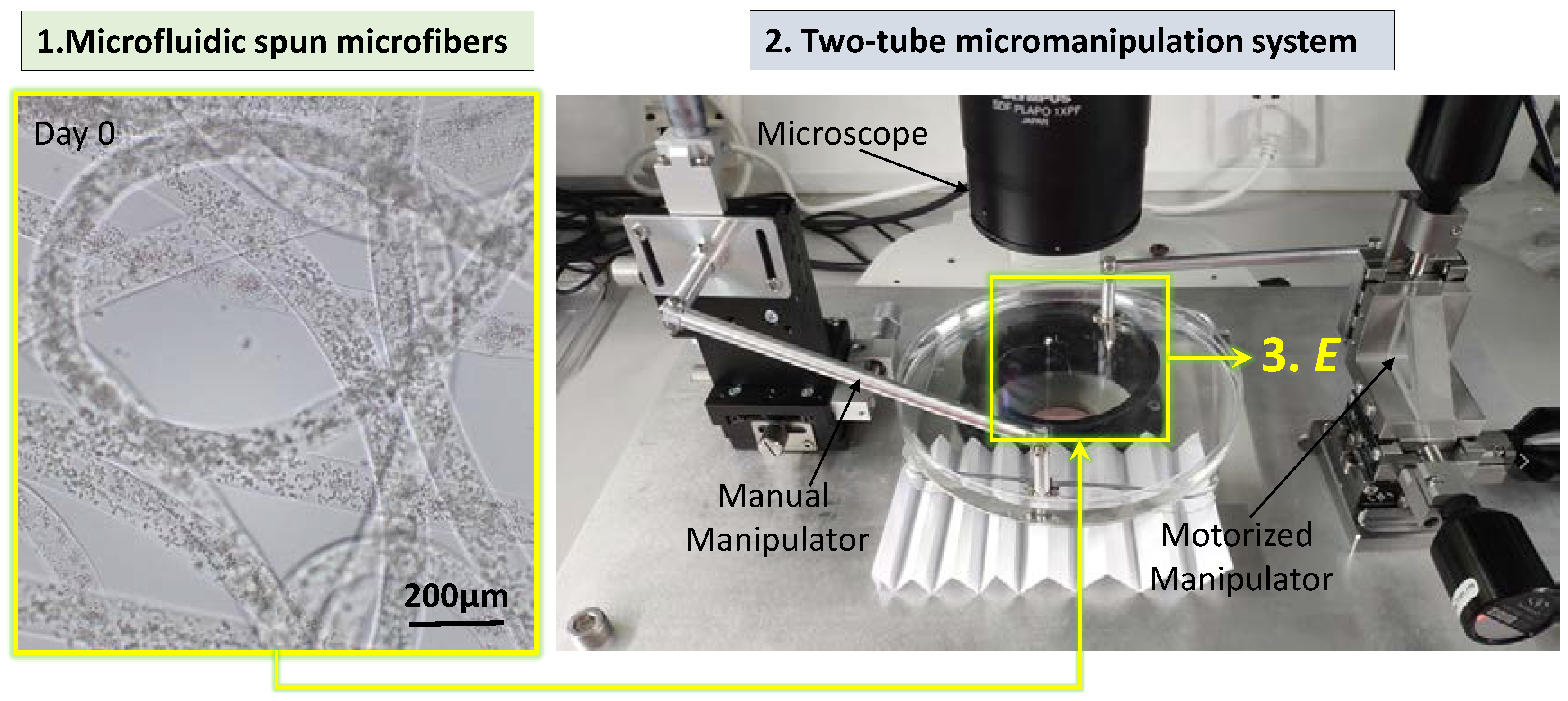
2.1. Microfluidic Spinning of the GelMA Microfibers
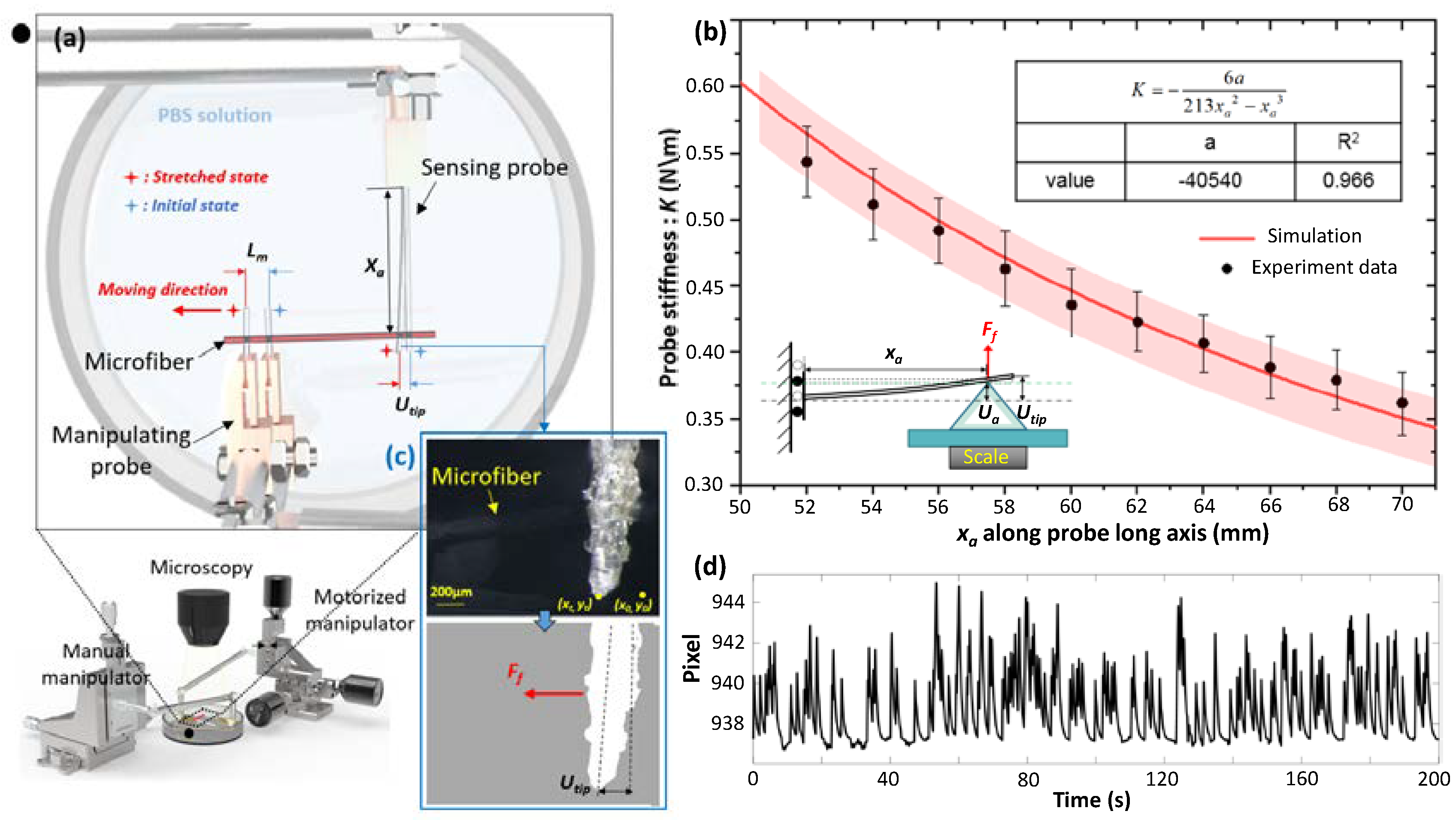
2.2. Two-Tube Micromanipulation System Setup for Stretching the Microfiber
2.3. Calculation of the Stretching Force Applied on the GelMA Microfiber
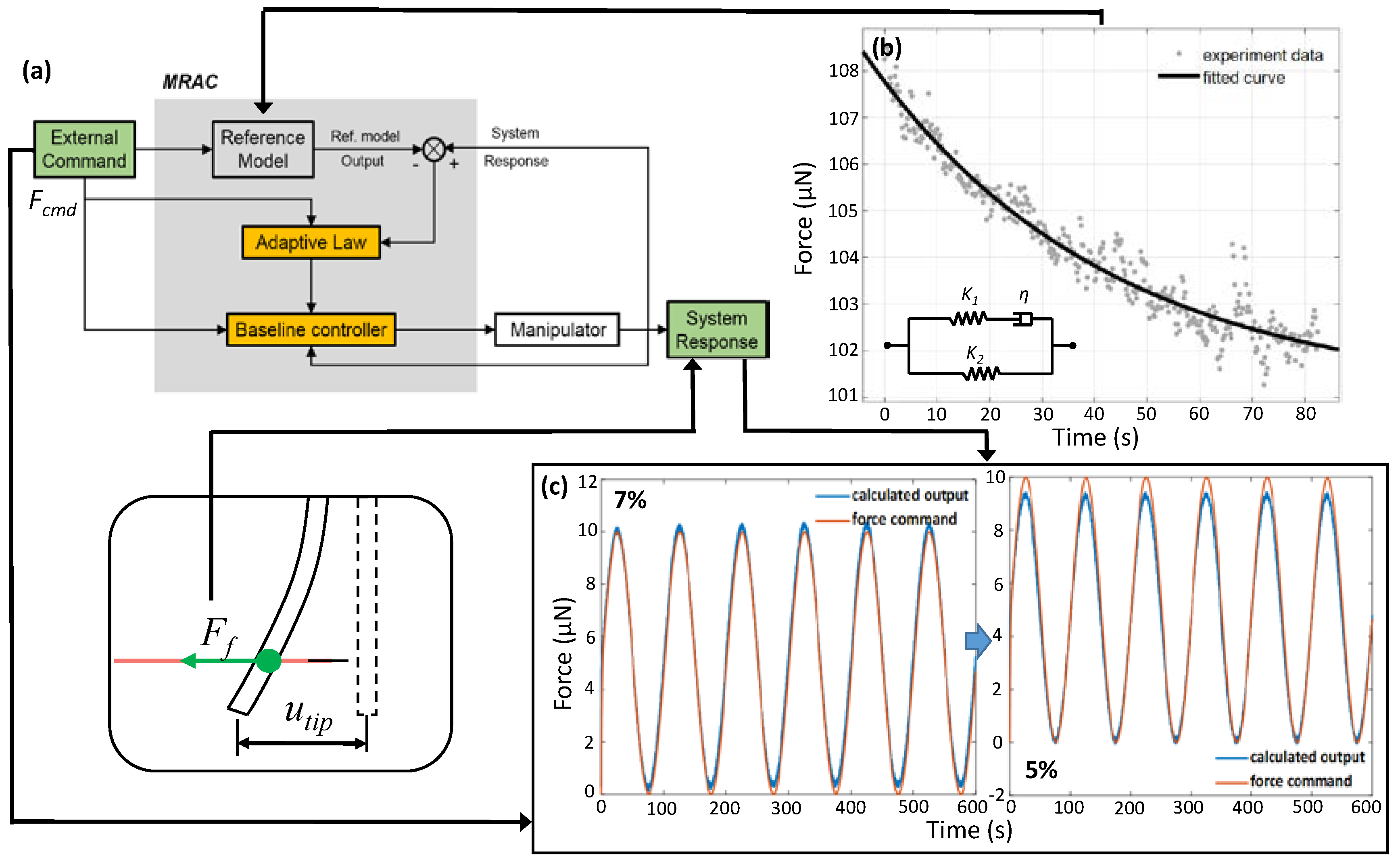
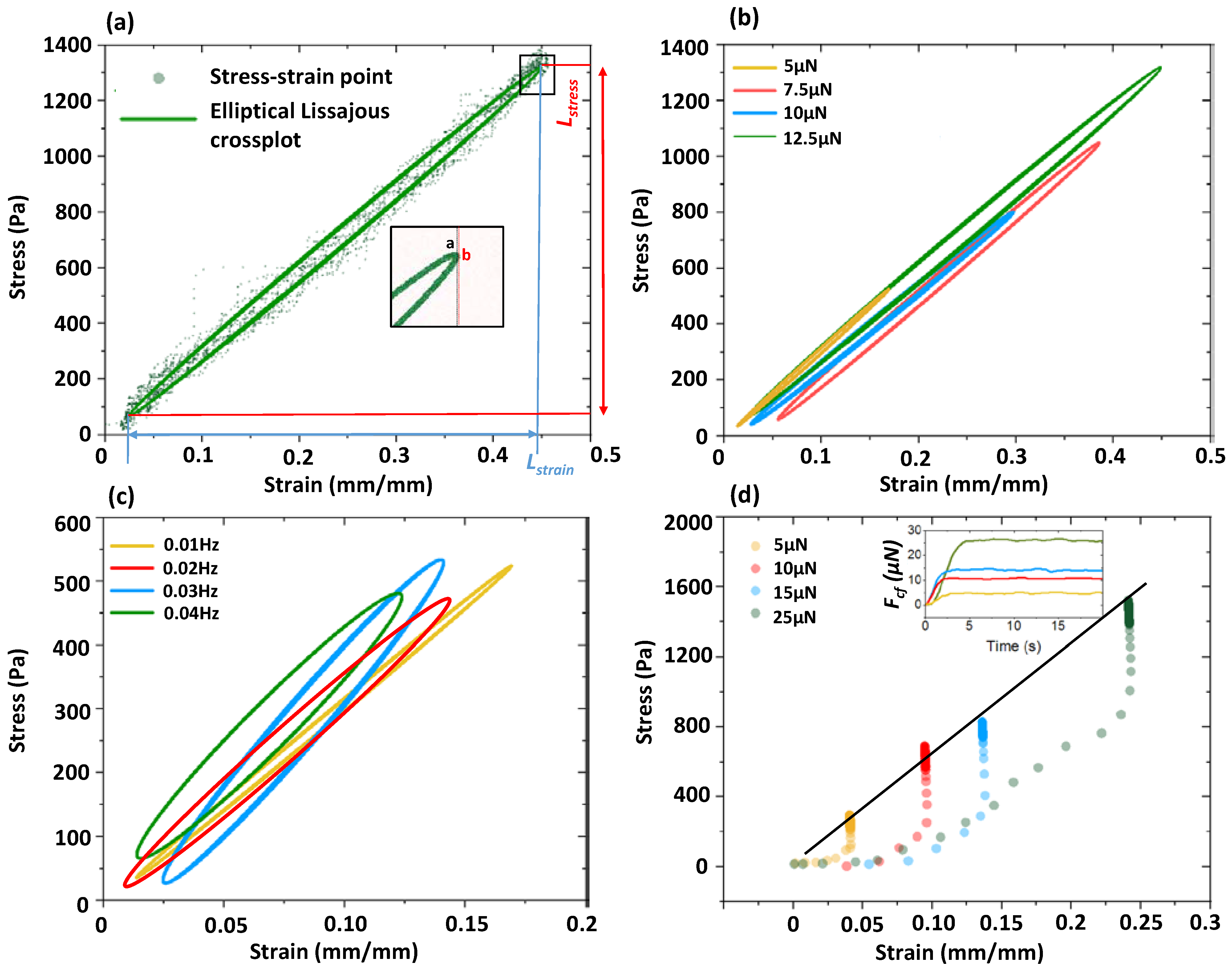
2.4. Measurement of the Microfiber’s Elastic Modulus Based on Cyclic Loading
2.5. 3D Cell Culture in the GelMA Microfiber and cell Viability Staining
3. Results and Discussion
3.1. Accuracy Analysis for Visual-Based Evaluation of the Stretching Force
3.2. Design and Validation of MRAC Strategy for the Stretching Force Control
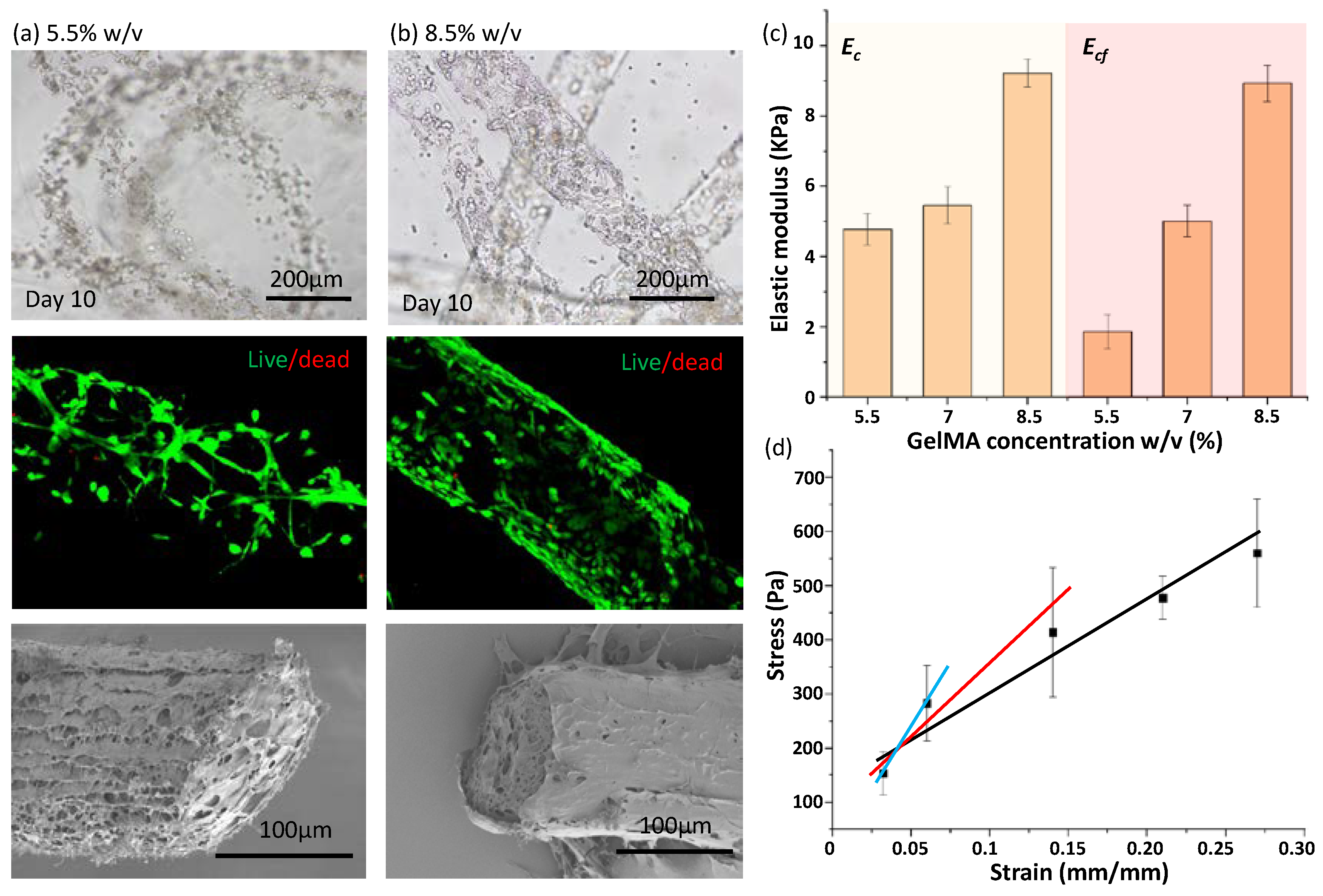
3.3. Measurement of Elastic Modulus: Ec of GelMA Microfibers
3.4. Evaluation of Measurement Reasonableness Based on Cell Response in 3D
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, T.; Shimoda, S.; Chen, Z.; Chen, X.; Wang, H.; Huang, Q.; Fukuda, T.; Shi, Q. Bio-inspired engineering of a perfusion culture platform for guided three-dimensional nerve cell growth and differentiation. Lab Chip 2022, 22, 1006–1017. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Q.; Zhao, H.; Xie, C.; Fu, J.; Liu, Z.; Xiang, M.; He, Y. Fiber-Based Mini Tissue with Morphology-Controllable GelMA Microfibers. Small 2018, 14, 1802187. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, Z.; Hu, N.; Zhou, Y.; Maggio, L.; Miri, A.K.; Fragasso, A.; Jin, X.; Khademhosseini, A.; Zhan, Y.S. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell favorable gelatin methacryloyl microenvironments. Biofabrication 2018, 10, 024102. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Colosi, C.; Święszkowski, W.; Barbetta, A. Co-axial wet-spinning in 3D bioprinting: State of the art and future perspective of microfluidic integration. Biofabrication 2019, 11, 012001. [Google Scholar] [CrossRef]
- Zuo, Y.; He, X.; Yang, Y.; Wei, D.; Sun, J.; Zhong, M.; Xie, R.; Fan, H.; Zhang, X. Microfluidic-based generation of functional microfibers for biomimetic complex tissue construction. Acta Biomater. 2016, 38, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef]
- Costantini, M.; Testa, S.; Fornetti, E.; Barbetta, A.; Trombetta, M.; Cannata, S.M.; Gargioli, C.; Rainer, A. Engineering Muscle networks in 3D gelatin Methacryloyl hydrogels: Influence of Mechanical stiffness and geometrical confinement. Front. Bioeng. Biotechnol. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shi, Q.; Liang, Q.; Yao, Y.; Wang, H.; Sun, J.; Huang, Q.; Fukuda, T. Fabrication of vascular smooth muscle-like tissues based on self-organization of circumferentially aligned cells in microengineered hydrogels. Lab A Chip 2020, 20, 3120–3131. [Google Scholar] [CrossRef]
- Chansoria, P.; Asif, S.; Polkoff, K.; Chung, J.; Piedrahita, J.A.; Shirwaiker, R.A. Characterizing the Effects of Synergistic Thermal and Photo-Cross-Linking during Biofabrication on the Structural and Functional Properties of Gelatin Methacryloyl (GelMA) Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 5175–5188. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Gao, F.; Zeng, Z.; Yang, J.; Wu, M.; Gao, C.; Cheng, D.; Pan, H.; Liu, W.; Ruan, C. Coaxial Scale-Up Printing of Diameter-Tunable Biohybrid Hydrogel Microtubes with High Strength, Perfusability, and Endothelialization. Adv. Funct. Mater. 2020, 30, 2001485. [Google Scholar] [CrossRef]
- Samandari, M.; Alipanah, F.; Majidzadeh-A, K.; Alvarez, M.M.; Trujillo-de Santiago, G.; Tamayol, A. Controlling cellular organization in bioprinting through designed 3D microcompartmentalization. Appl. Phys. Rev. 2021, 8, 021404. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Cuadros, T.R.; Skurtys, O.; Aguilera, J.M. Mechanical properties of calcium alginate fibers produced with a microfluidic device. Carbohydr. Polym. 2012, 89, 1198–1206. [Google Scholar] [CrossRef]
- Wei, D.; Charlton, L.; Glidle, A.; Qi, N.; Dobson, P.S.; Dalby, M.J.; Fan, H.; Yin, H. Dynamically Modulated Core−Shell Microfibers to Study the Effect of Depth Sensing of Matrix Stiffness on Stem Cell Fate. ACS Appl. Mater. Interfaces 2021, 13, 37997–38006. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Schexnailder, P.J.; Dundigalla, A.; White, J.D.; Matos-Pe´rez, C.R.; Cloud, J.L.; Seifert, S.; Wilker, J.J.; Schmidt, G. Highly Extensible Bio-Nanocomposite Fibers. Macromol. Rapid Commun. 2011, 32, 50–57. [Google Scholar] [CrossRef]
- Akiyama, Y. Design of Temperature-Responsive Cell Culture Surfaces for Cell Sheet Engineering. Cyborg Bionic Syst. 2021, 2021, 5738457. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.-C.; Asada, H.H. Extracellular matrix remodeling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues. Sci. Rep. 2019, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Nakayama, A.; Nakano, S.; Amiya, R.; Hirose, J. An Electrical Stimulation Culture System for Daily Maintenance-Free Muscle Tissue Production. Cyborg Bionic Syst. 2021, 2021, 9820505. [Google Scholar] [CrossRef]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef]
- Chen, X.; Sun, T.; Wei, Z.; Chen, Z.; Wang, H.; Huang, Q.; Fukuda, T.; Shi, Q. A clamp-free micro-stretching system for evaluating the viscoelastic response of cell-laden microfibers. Biosens. Bioelectron. 2022, 214, 114517. [Google Scholar] [CrossRef]
- Nguyen, N.T. Model-Reference Adaptive Control. In Model-Reference Adaptive Control: A Primer; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–123. [Google Scholar]
- Marques, S.P.C.; Creus, G.J. Rheological Models: Integral and Differential Representations. In Computational Viscoelasticity; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–21. [Google Scholar]
- Menner, M.; Annaswamy, A.M.; Holzapfel, F. Robust Design of Adaptive Output Feedback Controllers for Direct Feedthrough Systems. J. Guid. Control. Dyn. 2018, 41, 1919–1928. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Wang, W.; Xi, N.; Liu, L. A Manta Ray-Inspired Biosyncretic Robot with Stable Controllability by Dynamic Electric Stimulation. Cyborg Bionic Syst. 2022, 2022, 9891380. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic Cell Microenvironment: Hydrogel-Based Strategy for Recapitulating Dynamic ECM Mechanics. Adv. Funct. Mater. 2021, 14, 2100848. [Google Scholar] [CrossRef]

| Parameter | Goodness of Fit: R2 | ||||
|---|---|---|---|---|---|
| value | 11.23 | 0.7629 | 44.94 | 9 | 0.948 |
| Parameter | |||
|---|---|---|---|
| 7% w/v GelMA | 11.23 | 0.7629 | 44.94 |
| 5.5% w/v GelMA | 30.72 | 3.584 | 56.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Yu, X.; Chen, X.; Huang, Q.; Sun, T. A Stretching Force Control-Based Cyclic Loading Method for the Evaluation of Mechanical Properties of Gelation Methacrylate (GelMA) Microfibers. Micromachines 2022, 13, 1703. https://doi.org/10.3390/mi13101703
Liang Q, Yu X, Chen X, Huang Q, Sun T. A Stretching Force Control-Based Cyclic Loading Method for the Evaluation of Mechanical Properties of Gelation Methacrylate (GelMA) Microfibers. Micromachines. 2022; 13(10):1703. https://doi.org/10.3390/mi13101703
Chicago/Turabian StyleLiang, Qian, Xiao Yu, Xie Chen, Qiang Huang, and Tao Sun. 2022. "A Stretching Force Control-Based Cyclic Loading Method for the Evaluation of Mechanical Properties of Gelation Methacrylate (GelMA) Microfibers" Micromachines 13, no. 10: 1703. https://doi.org/10.3390/mi13101703
APA StyleLiang, Q., Yu, X., Chen, X., Huang, Q., & Sun, T. (2022). A Stretching Force Control-Based Cyclic Loading Method for the Evaluation of Mechanical Properties of Gelation Methacrylate (GelMA) Microfibers. Micromachines, 13(10), 1703. https://doi.org/10.3390/mi13101703







