Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications
Abstract
:1. Introduction
2. Acoustic Biosensors
2.1. Bulk Acoustic Wave Biosensors
2.1.1. Quartz Crystal Microbalance (QCM)
2.1.1.1. QCM as Biosensors: Operating Principle
2.1.1.2. Challenges in QCM Biosensing
2.1.1.3. Piezoelectric Materials and Fabrication
2.1.1.4. Applications of QCM in Biosensing
- (i)
- Proteins and Biomolecular Detection
- (ii)
- Cell adhesion and cell detection
- (iii)
- DNA Biosensors
- (iv)
- Pathogenic Immunosensor: Virus and Bacterial Microorganism Detection
2.1.2. Film Bulk Acoustic Resonator (FBAR) Biosensors
2.1.2.1. Propagation Modes in FBAR
- (i)
- Thickness Extensional Mode (TE)
- (ii)
- Thickness Shear Mode (TS)
- (iii)
- Lateral Extensional Mode (LE)
2.1.2.2. FBAR as Biosensors: Operating Principle
2.1.2.3. Challenges in FBAR Biosensing
2.1.2.4. Piezoelectric Materials and Fabrication
2.1.2.5. Applications of FBAR in Biosensing
- (i)
- Proteins and Biomolecular Detection
- (ii)
- DNA Biosensor
2.2. Surface Acoustic Wave Biosensors
2.2.1. SAW Modes for Biosensors
- (i)
- Rayleigh Mode SAW (R-SAW)
- (ii)
- Love Wave Mode SAW (Shear Horizontal (SH) SAW Love)
- (iii)
- Lamb Wave Mode SAW
- (iv)
- Shear Horizontal Acoustic Plate Mode (SH-A PM)
2.2.2. SAW as Biosensors: Operating Principle
2.2.3. Piezoelectric Materials and Fabrication
2.2.4. Applications of SAW in Biosensing
- (i)
- Protein and Biomolecular Detection
- (ii)
- Cell Adhesion and Detection
- (iii)
- DNA Biosensors
- (iv)
- Pathogenic Immunosensor: Virus and Bacterial Microorganism Detection
3. Acoustic Microfluidic Actuation
3.1. BAW-Based Microfluidic Actuation: Operating Principle
3.1.1. BAW Based Microfluidic Biosensing Applications
3.2. SAW-Based Microfluidic Actuation: Operating Principle
3.2.1. SAW-Based Microfluidic Biosensing Applications
4. Future Perspectives
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LOC | Lab-on-a chip |
| POC | Point of Care |
| BAW | Bulk Acoustic Wave |
| SAW | Surface Acoustic Wave |
| QCM | Quartz Crystal Microbalance |
| FBAR | Film Bulk Acoustic Resonator |
| AC | Alternating Current |
| EF | Electric Field |
| RF | Radio Frequency |
| TSM | Thickness Shear Mode |
| BVD | Butterworth-Van-Dyke |
| LOD | Limit of Detection |
| NP | Nano Particles |
| TCF | Temperature Coefficient of Frequency |
| PDMS | Polydimethylsiloxane |
| DRIE | Deep Reactive Ion Etching |
| PCR | Polymerase Chain Reaction |
| ELISA | Enzyme Linked Immunosorbent Assay |
| MIP | Molecularly Imprinted Polymers |
| GO | Graphene Oxide |
| SLG | Single Layered Graphene |
| DNA | Deoxyribonucleic Acid |
| ssDNA | Single Stranded DNA |
| CEA | Carcinoembryonic antigen |
| PSA | Prostate specific antigen |
| PLA | Plasmin |
| HIV | Human Immunodeficiency Virus |
| HBsAg | Hepatitis B surface antigen |
| HBcAg | Hepatitis B core antigen |
| cTnI | Cardiac Troponin I |
| AMI | Acute Myocardial Infarction |
| DA | Dopamine |
| IgG | Immunoglobulin G |
| IgE | Immunoglobulin E |
| Trp | Tryptophan |
| PHEMA | Poly (2-hydroxyethyl methacrylate) |
| PMMA | Poly (methyl methacrylate) |
| SNP | Single Nucleotide Polymorphism |
| SBM | Single Base Mismatch |
| FC | Fully Complementary |
| NC | Non-Complementary |
| EGFR | Epidermal Growth Factor Receptor |
| CTC | Circulating Tumor cells |
| WBC | White Blood cells |
| SDR | Strand Displacement Reaction |
| dsDNA | double stranded DNA |
| HBV | Hepatitis B Virus |
| HPV | Human Papilloma Virus |
| AIV | Avian Influenza Virus |
| HAU | Hemagglutinating Unit |
| E-Coli | Escherichia coli |
| CFU | Colony Forming Unit |
| AFM | Atomic Force Microscopy |
| AlN | Aluminium Nitride |
| ZnO | Zinc Oxide |
| PZT | Lead Zirconate Titanate |
| PVDF | Polyvinylidene fluoride |
| CMOS | Complementary Metal Oxide Semiconductor |
| SMR | Solidly Mounted Resonators |
| TE | Thickness Extensional |
| TS | Thickness Shear |
| LE | Lateral Extensional |
| LFE | Lateral Field Excitation |
| PVD | Physical Vapor Deposition |
| CVD | Chemical Vapor Deposition |
| IDT | Interdigitated Transducer |
| R-SAW | Rayleigh Surface Acoustic Wave |
| SH-SAW | Shear Horizontal Surface Acoustic Wave |
| FPW | Flexural Plate Wave |
| SH-APM | Shear Horizontal Acoustic Plate Mode |
| ARF | Acoustic Radiation Force |
| TSAW | Travelling Surface Acoustic Wave |
| SSAW | Standing Surface Acoustic Wave |
| taSSAW | tilted angle Standing Surface Acoustic Wave |
| DMF | Digital Microfluidics |
References
- Fu, Y.Q.; Luo, J.; Nguyen, N.-T.; Walton, A.; Flewitt, A.J.; Zu, X.-T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Zhang, M.; Cui, W.; Chen, X.; Wang, C.; Pang, W.; Duan, X.; Zhang, D.; Zhang, H. Monolithic integrated system with an electrowetting-on-dielectric actuator and a film-bulk-acoustic-resonator sensor. J. Micromech. Microeng. 2015, 25, 025002. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Huang, X.; Chuan, T.K. MEMS-micropumps: A review. J. Fluids Eng. 2002, 124, 384–392. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-L.; Wang, Y.-N.; Fu, L.-M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [Green Version]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Rapp, B.E.; Gruhl, F.J.; Länge, K. Biosensors with label-free detection designed for diagnostic applications. Anal. Bioanal. Chem. 2010, 398, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of new label-free techniques for biosensors: A review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic device for single-cell analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ündar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Sonker, M.; Sahore, V.; Woolley, A.T. Recent advances in microfluidic sample preparation and separation techniques for molecular biomarker analysis: A critical review. Anal. Chim. Acta 2017, 986, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vigneshvar, S.; Sudhakumari, C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications—An overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Guo, X.; Wang, Y.; Duan, X.; Qu, H.; Zhang, H.; Zhang, D.; Pang, W. Microchip based electrochemical-piezoelectric integrated multi-mode sensing system for continuous glucose monitoring. Sens. Actuators B Chem. 2016, 223, 83–88. [Google Scholar] [CrossRef]
- Skládal, P. Piezoelectric biosensors. TrAC Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Oseev, A.; Lecompte, T.P.; Remy-Martin, F.; Mourey, G.; Chollet, F.; de Boiseaumarie, B.L.R.; Rouleau, A.; Bourgeois, O.; de Maistre, E.; Elie-Caille, C. Assessment of shear-dependent kinetics of primary haemostasis with a microfluidic acoustic biosensor. IEEE Trans. Biomed. Eng. 2020, 68, 2329–2338. [Google Scholar] [CrossRef]
- Wang, T.; Green, R.; Guldiken, R.; Mohapatra, S.; Mohapatra, S. Multiple-layer guided surface acoustic wave (SAW)-based pH sensing in longitudinal FiSS-tumoroid cultures. Biosens. Bioelectr. 2019, 124, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Kavita, V. DNA biosensors—A review. J. Bioeng. Biomed. Sci. 2017, 7, 222. [Google Scholar]
- Law, W.-C.; Yong, K.-T.; Baev, A.; Prasad, P.N. Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. ACS Nano 2011, 5, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic impedance biosensor: A review. Biosens. Bioelectr. 2017, 90, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.; Caruso, F. Biosensors: Recent advances. Rep. Prog. Phys. 1997, 60, 1397. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. The piezoelectric biosensors: Principles and applications. Int. J. Electrochem. Sci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Lec, R.M.; Lewin, P.A. Acoustic wave biosensors. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Biomedical Engineering Towards the Year 2000 and Beyond, Hong Kong, China, 1 November 1998; Volume 20, pp. 2779–2784. [Google Scholar]
- Teo, A.J.T. Active Droplet Control and Manipulation in Microfluidics. Ph.D. Thesis, Griffith University, Brisbane, Australia, November 2019. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Sun, Q.; Teo, A.J.; Wibowo, D.; Gao, Y.; Zhou, J.; Huang, Y.; Tan, S.H.; Zhao, C.-X. Development of a microfluidic droplet-based microbioreactor for microbial cultivation. ACS Biomater. Sci. Eng. 2020, 6, 3630–3637. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. In Microfluidics Based Microsystems: Fundamentals and Applications; Springer: New York, NY, USA, 2010; pp. 305–376. [Google Scholar]
- Fu, Y.Q.; Luo, J.; Du, X.; Flewitt, A.; Li, Y.; Markx, G.; Walton, A.; Milne, W. Recent developments on ZnO films for acoustic wave based bio-sensing and microfluidic applications: A review. Sens. Actuators B Chem. 2010, 143, 606–619. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Evans, S.; Cunningham, J.; O’Rorke, R.; Wälti, C.; Davies, A. Alignment of particles in microfluidic systems using standing surface acoustic waves. Appl. Phys. Lett. 2008, 92, 044104. [Google Scholar] [CrossRef]
- Shi, J.; Mao, X.; Ahmed, D.; Colletti, A.; Huang, T.J. Focusing microparticles in a microfluidic channel with standing surface acoustic waves (SSAW). Lab Chip 2008, 8, 221–223. [Google Scholar] [CrossRef]
- Teo, A.J.; Tan, S.H.; Nguyen, N.-T. On-demand droplet merging with an AC electric field for multiple-volume droplet generation. Anal. Chem. 2019, 92, 1147–1153. [Google Scholar] [CrossRef]
- Teo, A.J.; Yan, M.; Dong, J.; Xi, H.-D.; Fu, Y.; Tan, S.H.; Nguyen, N.-T. Controllable droplet generation at a microfluidic T-junction using AC electric field. Microfluid. Nanofluid. 2020, 24, 21. [Google Scholar] [CrossRef]
- Shi, J.; Ahmed, D.; Mao, X.; Lin, S.-C.S.; Lawit, A.; Huang, T.J. Acoustic tweezers: Patterning cells and microparticles using standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 2890–2895. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, J.; Zou, L.; Zou, Y.; Lang, L.; Gao, F.; Hu, N.; Wang, P. A novel sensitive cell-based Love Wave biosensor for marine toxin detection. Biosens. Bioelectr. 2016, 77, 573–579. [Google Scholar] [CrossRef]
- Andle, J.; Vetelino, J. Acoustic wave biosensors. Sens. Actuators A Phys. 1994, 44, 167–176. [Google Scholar] [CrossRef]
- Zu, H.; Wu, H.; Wang, Q.-M. High-temperature piezoelectric crystals for acoustic wave sensor applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Das, P.K.; Bhethanabotla, V.R. Surface acoustic waves in biosensing applications. Sens. Actuators Rep. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Janshoff, A.; Galla, H.J.; Steinem, C. Piezoelectric mass-sensing devices as biosensors—An alternative to optical biosensors? Angew. Chem. Int. Ed. 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Luong, J.H.; Guilbault, G.G. Analytical applications of piezoelectric crystal biosensors. Bios. Princ. Appl. 1991, 50, 107–114. [Google Scholar]
- White, R.M. Surface elastic waves. Proc. IEEE 1970, 58, 1238–1276. [Google Scholar] [CrossRef]
- Eom, C.-B.; Trolier-McKinstry, S. Thin-film piezoelectric MEMS. Mrs Bull. 2012, 37, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.A.; Halpern, J.M. Guide to selecting a biorecognition element for biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Ramos, M.; Díaz-Durán, B.; Escolano, J.-M.; Barba, M.; Mirea, T.; Olivares, J.; Clement, M.; Iborra, E. Gravimetric biosensor based on a 1.3 GHz AlN shear-mode solidly mounted resonator. Sens. Actuators B Chem. 2017, 239, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Mazouz, Z.; Fourati, N.; Zerrouki, C.; Ommezine, A.; Rebhi, L.; Yaakoubi, N.; Kalfat, R.; Othmane, A. Discriminating DNA mismatches by electrochemical and gravimetric techniques. Biosens. Bioelectr. 2013, 48, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Appel, J.; Chae, J. Real-time monitoring of whole blood coagulation using a microfabricated contour-mode film bulk acoustic resonator. J. Microelectromech. Syst. 2012, 21, 302–307. [Google Scholar] [CrossRef]
- Chen, D.; Song, S.; Ma, J.; Zhang, Z.; Wang, P.; Liu, W.; Guo, Q. Micro-electromechanical film bulk acoustic sensor for plasma and whole blood coagulation monitoring. Biosens. Bioelectr. 2017, 91, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Xiong, X.; Zu, H.; Wang, J.H.-C.; Wang, Q.-M. Theoretical analysis of a Love wave biosensor in liquid with a viscoelastic wave guiding layer. J. Appl. Phys. 2017, 121, 054501. [Google Scholar] [CrossRef]
- McHale, G.; Newton, M.I.; Banerjee, M.K.; Rowan, S.M. Interaction of surface acoustic waves with viscous liquids. Faraday Discuss. 1997, 107, 15–26. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. Highly sensitive Love wave acoustic biosensor for uric acid. Sens. Actuators B Chem. 2018, 261, 169–177. [Google Scholar] [CrossRef]
- Kondoh, J.; Matsui, Y.; Shiokawa, S. New biosensor using shear horizontal surface acoustic wave device. Jpn. J. Appl. Phys. 1993, 32, 2376. [Google Scholar] [CrossRef]
- Gray, E.R.; Turbé, V.; Lawson, V.E.; Page, R.H.; Cook, Z.C.; Ferns, R.B.; Nastouli, E.; Pillay, D.; Yatsuda, H.; Athey, D. Ultra-rapid, sensitive and specific digital diagnosis of HIV with a dual-channel SAW biosensor in a pilot clinical study. NPJ Digit. Med. 2018, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Pang, Y.; Li, D.; Huang, Z.; Zhang, Z.; Xue, N.; Xu, Y.; Mu, X. An aptamer-based shear horizontal surface acoustic wave biosensor with a CVD-grown single-layered graphene film for high-sensitivity detection of a label-free endotoxin. Microsyst. Nanoeng. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; de Vittorio, M. Conformable surface acoustic wave biosensor for E-coli fabricated on PEN plastic film. Biosens. Bioelectr. 2020, 163, 112164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef]
- Ferreira, G.N.; da Silva, A.-C.; Tomé, B. Acoustic wave biosensors: Physical models and biological applications of quartz crystal microbalance. Trends Biotechnol. 2009, 27, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Wingqvist, G. AlN-based sputter-deposited shear mode thin film bulk acoustic resonator (FBAR) for biosensor applications—A review. Surf. Coat. Technol. 2010, 205, 1279–1286. [Google Scholar] [CrossRef]
- He, H.; Yang, J.; Jiang, Q. Thickness-shear and thickness-twist vibrations of circular AT-cut quartz resonators. Acta Mech. Solida Sin. 2013, 26, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, M.; Stadler, H.; Weiss, T.; Ziegler, C. Thickness shear mode resonators (“mass-sensitive devices”) in bioanalysis. Fresenius J. Anal. Chem. 2000, 366, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Durmuş, N.G.; Lin, R.L.; Kozberg, M.; Dermici, D.; Khademhosseini, A.; Demirci, U. Acoustic-based biosensors. In Encyclopedia Microfluidics Nanofluidics; Springer: New York, NY, USA, 2014; pp. 1–15. [Google Scholar]
- Ballantine, D., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.; Frye, G.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design and Physico-Chemical Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Hu, J.; Huang, X.; Xue, S.; Yesilbas, G.; Knoll, A.; Schneider, O. Measurement of the mass sensitivity of QCM with ring electrodes using electrodeposition. Electrochem. Commun. 2020, 116, 106744. [Google Scholar] [CrossRef]
- Richter, R.P.; Rodenhausen, K.B.; Eisele, N.B.; Schubert, M. Coupling spectroscopic ellipsometry and quartz crystal microbalance to study organic films at the solid-liquid interface. In Ellipsometry of Functional Organic Surfaces and Films; Springer: New York, NY, USA, 2014; pp. 223–248. [Google Scholar]
- Huang, X.; Bai, Q.; Zhou, Q.; Hu, J. The Resistance-Amplitude-Frequency Effect of In–Liquid Quartz Crystal Microbalance. Sensors 2017, 17, 1476. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Thompson, M. Multiple chemical information from the thickness shear mode acoustic wave sensor in the liquid phase. Anal. Chem. 1993, 65, 1158–1168. [Google Scholar] [CrossRef]
- Martin, S.J.; Granstaff, V.E.; Frye, G.C. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal. Chem. 1991, 63, 2272–2281. [Google Scholar] [CrossRef]
- Tsortos, A.; Papadakis, G.; Mitsakakis, K.; Melzak, K.A.; Gizeli, E. Quantitative determination of size and shape of surface-bound DNA using an acoustic wave sensor. Biophys. J. 2008, 94, 2706–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, C. Development of quartz crystal oscillators for under-liquid sensing. Sens. Actuators A Phys. 1991, 29, 59–69. [Google Scholar] [CrossRef]
- Prakrankamanant, P.; Leelayuwat, C.; Promptmas, C.; Limpaiboon, T.; Wanram, S.; Prasongdee, P.; Pientong, C.; Daduang, J.; Jearanaikoon, P. The development of DNA-based quartz crystal microbalance integrated with isothermal DNA amplification system for human papillomavirus type 58 detection. Biosens. Bioelectr. 2013, 40, 252–257. [Google Scholar] [CrossRef]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. Influenza A virus molecularly imprinted polymers and their application in virus sub-type classification. J. Mater. Chem. B 2013, 1, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Chen, F.; Jiang, T.; Wang, H.; Slavik, M.; Wei, H.; Li, Y. QCM-based aptamer selection and detection of Salmonella typhimurium. Food Chem. 2017, 221, 776–782. [Google Scholar] [CrossRef]
- Fulgione, A.; Cimafonte, M.; Della Ventura, B.; Iannaccone, M.; Ambrosino, C.; Capuano, F.; Proroga, Y.T.R.; Velotta, R.; Capparelli, R. QCM-based immunosensor for rapid detection of Salmonella Typhimurium in food. Sci. Rep. 2018, 8, 16137. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.M.; Cheng, L.; Zhang, P.; Zhao, G.C. Label-free analytical performances of a peptide-based QCM biosensor for trypsin. Analyst 2020, 145, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Pang, W.; Zhao, H.; Kim, E.S.; Zhang, H.; Yu, H.; Hu, X. Piezoelectric microelectromechanical resonant sensors for chemical and biological detection. Lab Chip 2012, 12, 29–44. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. 2021, 33, 2005448. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Fogel, R.; Limson, J.; Seshia, A.A. Acoustic biosensors. Essays Biochem. 2016, 60, 101–110. [Google Scholar]
- Martin, S.J.; Frye, G.C.; Ricco, A.J.; Senturia, S.D. Effect of surface roughness on the response of thickness-shear mode resonators in liquids. Anal. Chem. 1993, 65, 2910–2922. [Google Scholar] [CrossRef]
- Etchenique, R.; Buhse, T. Anomalous behaviour of the quartz crystal microbalance in the presence of electrolytes. Analyst 2000, 125, 785–787. [Google Scholar] [CrossRef]
- Encarnaçao, J.M.; Stallinga, P.; Ferreira, G.N. Influence of electrolytes in the QCM response: Discrimination and quantification of the interference to correct microgravimetric data. Biosens. Bioelectr. 2007, 22, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; Höök, F.; Kasemo, B. QCM operation in liquids: An explanation of measured variations in frequency and Q factor with liquid conductivity. Anal. Chem. 1996, 68, 2219–2227. [Google Scholar] [CrossRef]
- Nunalee, F.N.; Shull, K.R.; Lee, B.P.; Messersmith, P.B. Quartz crystal microbalance studies of polymer gels and solutions in liquid environments. Anal. Chem. 2006, 78, 1158–1166. [Google Scholar] [CrossRef]
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanics approach. Phys. Scr. 1999, 59, 391. [Google Scholar] [CrossRef] [Green Version]
- Dunham, G.C.; Benson, N.H.; Petelenz, D.; Janata, J. Dual quartz crystal microbalance. Anal. Chem. 1995, 67, 267–272. [Google Scholar] [CrossRef]
- Hayden, O.; Bindeus, R.; Haderspöck, C.; Mann, K.-J.; Wirl, B.; Dickert, F.L. Mass-sensitive detection of cells, viruses and enzymes with artificial receptors. Sens. Actuators B Chem. 2003, 91, 316–319. [Google Scholar] [CrossRef]
- Jaruwongrungsee, K.; Maturos, T.; Wong-Ek, K.; Sangworasil, M.; Pintavirooj, C.; Wisitsora-At, A.; Tuantranont, A. Fabrication of QCM sensor array and PDMS micro chamber for biosensor applications. In Proceedings of the 6th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chonburi, Thailand, 6–9 May 2009; pp. 478–481. [Google Scholar]
- Tsortos, A.; Papadakis, G.; Gizeli, E. Shear acoustic wave biosensor for detecting DNA intrinsic viscosity and conformation: A study with QCM-D. Biosens. Bioelectr. 2008, 24, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef] [Green Version]
- Scrimin, P.; Prins, L.J. Sensing through signal amplification. Chem. Soc. Rev. 2011, 40, 4488–4505. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J.; Wang, R.; Li, Y.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Lu, H. A nanobeads amplified QCM immunosensor for the detection of avian influenza virus H5N1. Biosens. Bioelectr. 2011, 26, 4146–4154. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, P.; Zhu, M.; Li, B.; Yang, P.; Cai, J. Silver nanocluster based sensitivity amplification of a quartz crystal microbalance gene sensor. Microchim. Acta 2016, 183, 881–887. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, S.S. Sensitive detection of microRNA using QCM biosensors: Sandwich hybridization and signal amplification by TiO2 nanoparticles. Anal Methods 2020, 12, 5103–5109. [Google Scholar] [CrossRef]
- Pohanka, M. QCM biosensor for Prostate Specific Antigen assay using antibody–gold particle conjugate. Int. J. Electrochem. Sci. 2021, 16, 21051. [Google Scholar] [CrossRef]
- Zhou, B.; Hao, Y.; Chen, S.; Yang, P. A quartz crystal microbalance modified with antibody-coated silver nanoparticles acting as mass signal amplifiers for real-time monitoring of three latent tuberculosis infection biomarkers. Microchim. Acta 2019, 186, 212. [Google Scholar] [CrossRef]
- Rabe, J.; Buttgenbach, S.; Zimmermann, B.; Hauptmann, P. Design, manufacturing, and characterization of high-frequency thickness-shear mode resonators. In Proceedings of the IEEE/EIA International Frequency Control Symposium and Exhibition, Kansas City, MO, USA, 9 June 2000; pp. 106–112. [Google Scholar]
- Zhang, Y.; Luo, J.; Flewitt, A.J.; Cai, Z.; Zhao, X. Film bulk acoustic resonators (FBARs) as biosensors: A review. Biosens. Bioelectr. 2018, 116, 1–15. [Google Scholar] [CrossRef]
- Matko, V. Next generation AT-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.; Kang, Q.; Wang, Y.-E.; Hu, Q.; Du, J. New cut angle quartz crystal microbalance with low frequency-temperature coefficients in an aqueous phase. Talanta 2008, 76, 803–808. [Google Scholar] [CrossRef]
- Anderson, H.; Jönsson, M.; Vestling, L.; Lindberg, U.; Aastrup, T. Quartz crystal microbalance sensor design: I. Experimental study of sensor response and performance. Sens. Actuators B Chem. 2007, 123, 27–34. [Google Scholar] [CrossRef]
- Hao, R.; Wang, D.; Zuo, G.; Wei, H.; Yang, R.; Zhang, Z.; Cheng, Z.; Guo, Y.; Cui, Z.; Zhou, Y. Rapid detection of Bacillus anthracis using monoclonal antibody functionalized QCM sensor. Biosens. Bioelectr. 2009, 24, 1330–1335. [Google Scholar] [CrossRef]
- Michalzik, M.; Wilke, R.; Büttgenbach, S. Miniaturized QCM-based flow system for immunosensor application in liquid. Sens. Actuators B Chem. 2005, 111, 410–415. [Google Scholar] [CrossRef]
- Abe, T.; Hung, V.N.; Esashi, M. Inverted mesa-type quartz crystal resonators fabricated by deep-reactive ion etching. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, B.P.; Graz, I.M.; Schwödiauer, R.; Gruber, H.; Bauer, S. User-friendly, miniature biosensor flow cell for fragile high fundamental frequency quartz crystal resonators. Biosens. Bioelectr. 2009, 24, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; You, J.; Park, C.; Jang, K.; Na, S. In-situ and highly sensitive detection of epidermal growth factor receptor mutation using nano-porous quartz crystal microbalance. J. Mech. Sci. Technol. 2018, 32, 1927–1932. [Google Scholar] [CrossRef]
- Giamblanco, N.; Conoci, S.; Russo, D.; Marletta, G. Single-step label-free hepatitis B virus detection by a piezoelectric biosensor. RSC Adv. 2015, 5, 38152–38158. [Google Scholar] [CrossRef]
- Yatabe, Y.; Hida, T.; Horio, Y.; Kosaka, T.; Takahashi, T.; Mitsudomi, T. A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J. Mol. Diagn. 2006, 8, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Uludag, Y.; Tothill, I.E. Development of a sensitive detection method of cancer biomarkers in human serum (75%) using a quartz crystal microbalance sensor and nanoparticles amplification system. Talanta 2010, 82, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Jandas, P.J.; Luo, J.; Quan, A.; Li, C.; Fu, C.; Fu, Y.Q. Graphene oxide-Au nano particle coated quartz crystal microbalance biosensor for the real time analysis of carcinoembryonic antigen. RSC Adv. 2020, 10, 4118–4128. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectr. 2018, 99, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Piskin, A.K.; Yavuz, H.; Denizli, A. Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor. Talanta 2019, 204, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Q.; Pei, Y.; Dong, H.; Aastrup, T.; Pei, Z. Direct attachment of suspension cells to PDA surface and its application in suspension-cell QCM biosensor. Sens. Actuators B Chem. 2021, 326, 128823. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bakhshpour, M.; Göktürk, I.; Pişkin, A.K.; Denizli, A. Quartz Crystal Microbalance (QCM) Based Biosensor Functionalized by HER2/neu Antibody for Breast Cancer Cell Detection. Chemosensors 2021, 9, 80. [Google Scholar] [CrossRef]
- Afzal, A.; Mujahid, A.; Schirhagl, R.; Bajwa, S.; Latif, U.; Feroz, S. Gravimetric Viral Diagnostics: QCM Based Biosensors for Early Detection of Viruses. Chemosensors 2017, 5, 7. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Callaway, Z.T.; Lu, H.; Huang, T.J.; Li, Y. A nanowell-based QCM aptasensor for rapid and sensitive detection of avian influenza virus. Sens. Actuators B Chem. 2017, 240, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Uludag, Y.; Tothill, I.E. Cancer biomarker detection in serum samples using surface plasmon resonance and quartz crystal microbalance sensors with nanoparticle signal amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Suthar, J.; Parsons, E.S.; Hoogenboom, B.W.; Williams, G.R.; Guldin, S. Acoustic Immunosensing of Exosomes Using a Quartz Crystal Microbalance with Dissipation Monitoring. Anal. Chem. 2020, 92, 4082–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piovarci, I.; Melikishvili, S.; Tatarko, M.; Hianik, T.; Thompson, M. Detection of Sub-Nanomolar Concentration of Trypsin by Thickness-Shear Mode Acoustic Biosensor and Spectrophotometry. Biosensors 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Construction of a QCM Biosensor for free Hemoglobin Assay. Int. J. Electrochem. Sci. 2019, 14, 5237–5246. [Google Scholar] [CrossRef]
- Lu, C.-H.; Zhang, Y.; Tang, S.-F.; Fang, Z.-B.; Yang, H.-H.; Chen, X.; Chen, G.-N. Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens. Bioelectr. 2012, 31, 439–444. [Google Scholar] [CrossRef]
- Pirich, C.L.; de Freitas, R.A.; Torresi, R.M.; Picheth, G.F.; Sierakowski, M.R. Piezoelectric immunochip coated with thin films of bacterial cellulose nanocrystals for dengue detection. Biosens. Bioelectr. 2017, 92, 47–53. [Google Scholar] [CrossRef]
- Sharma, M.; Rao, V.; Merwyn, S.; Agarwal, G.; Upadhyay, S.; Vijayaraghavan, R. A novel piezoelectric immunosensor for the detection of malarial Plasmodium falciparum histidine rich protein-2 antigen. Talanta 2011, 85, 1812–1817. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S.; Park, S.J. Detection of HIV-1 antigen by quartz crystal microbalance using gold nanoparticles. Sens. Actuators B Chem. 2016, 237, 452–458. [Google Scholar] [CrossRef]
- Lim, S.L.; Ooi, C.-W.; Tan, W.S.; Chan, E.-S.; Ho, K.L.; Tey, B.T. Biosensing of hepatitis B antigen with poly (acrylic acid) hydrogel immobilized with antigens and antibodies. Sens. Actuators B Chem. 2017, 252, 409–417. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.J.; Luo, J.; Fu, C.; Wei, Q. Molecularly imprinted poly (methacrylic acid) based QCM biosensor for selective determination of L-tryptophan. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125859. [Google Scholar] [CrossRef]
- Asai, N.; Shimizu, T.; Shingubara, S.; Ito, T. Fabrication of highly sensitive QCM sensor using AAO nanoholes and its application in biosensing. Sens. Actuators B Chem. 2018, 276, 534–539. [Google Scholar] [CrossRef]
- Poturnayova, A.; Karpisova, I.; Castillo, G.; Mező, G.; Kocsis, L.; Csámpai, A.; Keresztes, Z.; Hianik, T. Detection of plasmin based on specific peptide substrate using acoustic transducer. Sens. Actuators B Chem. 2016, 223, 591–598. [Google Scholar] [CrossRef]
- Poturnayova, A.; Szabo, K.; Tatarko, M.; Hucker, A.; Kocsis, R.; Hianik, T. Determination of plasmin in milk using QCM and ELISA methods. Food Control 2021, 123, 107774. [Google Scholar] [CrossRef]
- Song, S.; Lu, Y.; Li, X.; Cao, S.; Pei, Y.; Aastrup, T.; Pei, Z. Optimization of 3D surfaces of dextran with different molecule weights for real-time detection of biomolecular interactions by a QCM biosensor. Polymers 2017, 9, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Song, S.; Hou, C.; Pang, S.; Li, X.; Wu, X.; Shao, C.; Pei, Y.; Pei, Z. Facile fabrication of branched-chain carbohydrate chips for studying carbohydrate-protein interactions by QCM biosensor. Chin. Chem. Lett. 2018, 29, 65–68. [Google Scholar] [CrossRef]
- Chi, L.; Xu, C.; Li, S.; Wang, X.; Tang, D.; Xue, F. In situ amplified QCM immunoassay for carcinoembryonic antigen with colorectal cancer using horseradish peroxidase nanospheres and enzymatic biocatalytic precipitation. Analyst 2020, 145, 6111–6118. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, M.; Muckley, E.S.; Subjakova, V.; Goswami, M.; Sumpter, B.G.; Hianik, T.; Ivanov, I.N. Machine learning enabled acoustic detection of sub-nanomolar concentration of trypsin and plasmin in solution. Sens. Actuators B Chem. 2018, 272, 282–288. [Google Scholar] [CrossRef]
- Bastian, E.D.; Brown, R.J. Plasmin in milk and dairy products: An update. Int. Dairy J. 1996, 6, 435–457. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, W.; Pan, Y.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta 2014, 126, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bai, H.; Luo, J.; Yang, P.; Cai, J. A recyclable chitosan-based QCM biosensor for sensitive and selective detection of breast cancer cells in real time. Analyst 2014, 139, 6259–6265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, R.; Hao, Y.; Yang, P. A CD44-biosensor for evaluating metastatic potential of breast cancer cells based on quartz crystal microbalance. Sci. Bull. 2017, 62, 923–930. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [Green Version]
- Oseev, A.; Mukhin, N.; Elie-Caille, C.; Boireau, W.; Lucklum, R.; Lecompte, T.; Remy-Martin, F.; Manceau, J.-F.; Chollet, F.; Leblois, T. Topology Challenge for the Assessment of Living Cell Deposits with Shear Bulk Acoustic Biosensor. Nanomaterials 2020, 10, 2079. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Limpanichpakdee, T.; Yang, M.-H.; Tyan, Y.-C. An electrode of quartz crystal microbalance decorated with CNT/chitosan/fibronectin for investigating early adhesion and deforming morphology of rat mesenchymal stem cells. Carbohydr. Polym. 2011, 85, 726–732. [Google Scholar] [CrossRef]
- Fohlerová, Z.; Turánek, J.; Skládal, P. The cell adhesion and cytotoxicity effects of the derivate of vitamin E compared for two cell lines using a piezoelectric biosensor. Sens. Actuators B Chem. 2012, 174, 153–157. [Google Scholar] [CrossRef]
- Stamov, D.R.; Pompe, T. Structure and function of ECM-inspired composite collagen type I scaffolds. Soft Matter 2012, 8, 10200–10212. [Google Scholar] [CrossRef]
- Liu, F.; Li, F.; Nordin, A.N.; Voiculescu, I. A novel cell-based hybrid acoustic wave biosensor with impedimetric sensing capabilities. Sensors 2013, 13, 3039–3055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Bai, H.; Yang, P. Real-time monitoring of mechanical changes during dynamic adhesion of erythrocytes to endothelial cells by QCM-D. Chem. Commun. 2015, 51, 11449–11451. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Saint-Guirons, J.; Käck, C.; Ingemarsson, B.; Aastrup, T. Real-time analysis of the carbohydrates on cell surfaces using a QCM biosensor: A lectin-based approach. Biosens. Bioelectr. 2012, 35, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, D.; Xu, Y.; Li, N.; Liu, F. A self-assembled DNA nanostructure-amplified quartz crystal microbalance with dissipation biosensing platform for nucleic acids. Chem. Commun. 2012, 48, 6678–6680. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- What Are Single Nucleotide Polymorphisms (SNPs). Genetics Home Reference–NIH. US National Library of Medicine. Available online: https://ghr.nlm.nih.gov/primer/genomicresearch/snp (accessed on 2 August 2021).
- Wang, D.; Tang, W.; Wu, X.; Wang, X.; Chen, G.; Chen, Q.; Li, N.; Liu, F. Highly selective detection of single-nucleotide polymorphisms using a quartz crystal microbalance biosensor based on the toehold-mediated strand displacement reaction. Anal. Chem. 2012, 84, 7008–7014. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Wang, H.; Tang, W.; Pan, W.; Li, N.; Liu, F. A reusable quartz crystal microbalance biosensor for highly specific detection of single-base DNA mutation. Biosens. Bioelectron. 2013, 48, 276–280. [Google Scholar] [CrossRef]
- Bunroddith, K.; Viseshakul, N.; Chansiri, K.; Lieberzeit, P. QCM-based rapid detection of PCR amplification products of Ehrlichia canis. Anal. Chim. Acta 2018, 1001, 106–111. [Google Scholar] [CrossRef]
- Wangmaung, N.; Chomean, S.; Promptmas, C.; Mas-oodi, S.; Tanyong, D.; Ittarat, W. Silver quartz crystal microbalance for differential diagnosis of Plasmodium falciparum and Plasmodium vivax in single and mixed infection. Biosens. Bioelectr. 2014, 62, 295–301. [Google Scholar] [CrossRef]
- Yao, C.; Xiang, Y.; Deng, K.; Xia, H.; Fu, W. Sensitive and specific HBV genomic DNA detection using RCA-based QCM biosensor. Sens. Actuators B Chem. 2013, 181, 382–387. [Google Scholar] [CrossRef]
- Kaewphinit, T.; Santiwatanakul, S.; Promptmas, C.; Chansiri, K. Detection of non-amplified Mycobacterium tuberculosis genomic DNA using piezoelectric DNA-based biosensors. Sensors 2010, 10, 1846–1858. [Google Scholar] [CrossRef] [Green Version]
- Garai-Ibabe, G.; Grinyte, R.; Golub, E.I.; Canaan, A.; de la Chapelle, M.L.; Marks, R.S.; Pavlov, V. Label free and amplified detection of cancer marker EBNA-1 by DNA probe based biosensors. Biosens. Bioelectr. 2011, 30, 272–275. [Google Scholar] [CrossRef]
- Kleo, K.; Kapp, A.; Ascher, L.; Lisdat, F. Detection of vaccinia virus DNA by quartz crystal microbalance. Anal. Biochem. 2011, 418, 260–266. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenzavirus. Biosens. Bioelectr. 2013, 42, 148–155. [Google Scholar] [CrossRef]
- Huang, X.; Xu, J.; Ji, H.-F.; Li, G.; Chen, H. Quartz crystal microbalance based biosensor for rapid and sensitive detection of maize chlorotic mottle virus. Anal. Methods 2014, 6, 4530–4536. [Google Scholar] [CrossRef]
- Salam, F.; Uludag, Y.; Tothill, I.E. Real-time and sensitive detection of Salmonella Typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta 2013, 115, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, R.; Sorokulova, I.; Olsen, E.; Globa, L.; Pustovyy, O.; Moore, T.; Chin, B.; Barbaree, J.; Vodyanoy, V. Detection and identification of methicillin resistant and sensitive strains of Staphylococcus aureus using tandem measurements. J. Microbiol. Methods 2012, 90, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, F.; Wang, R.; Li, Y. Whole-bacterium SELEX of DNA aptamers for rapid detection of E. coli O157: H7 using a QCM sensor. J. Biotechnol. 2018, 266, 39–49. [Google Scholar] [CrossRef]
- Ngo, V.K.T.; Nguyen, D.G.; Nguyen, H.P.U.; Nguyen, T.K.M.; Huynh, T.P.; Lam, Q.V.; Huynh, T.D.; Truong, T.N.L. Quartz crystal microbalance (QCM) as biosensor for the detecting of Escherichia coli O157: H7. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 045004. [Google Scholar]
- Lacour, V.; Herth, E.; Lardet-Vieudrin, F.; Dubowski, J.J.; Leblois, T. GaAs based on bulk acoustic wave sensor for biological molecules detection. Procedia Eng. 2015, 120, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhang, X.; Choi, S.; Chae, J. A high-quality-factor film bulk acoustic resonator in liquid for biosensing applications. J. Microelectromech. Syst. 2010, 20, 213–220. [Google Scholar] [CrossRef]
- Zhang, H.; Marma, M.S.; Kim, E.S.; McKenna, C.E.; Thompson, M.E. A film bulk acoustic resonator in liquid environments. J. Micromech. Microeng. 2005, 15, 1911–1916. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Xu, Y.; Li, D.; Li, Z.; Song, H. The pure-shear mode solidly mounted resonator based on c-axis oriented ZnO film. Appl. Surf. Sci. 2010, 256, 7638–7642. [Google Scholar] [CrossRef]
- Flewitt, A.; Luo, J.; Fu, Y.Q.; Garcia-Gancedo, L.; Du, X.; Lu, J.; Zhao, X.; Iborra, E.; Ramos, M.; Milne, W. ZnO based SAW and FBAR devices for bio-sensing applications. J. Non-Newton. Fluid Mech. 2015, 222, 209–216. [Google Scholar] [CrossRef]
- Zheng, D.; Xiong, J.; Guo, P.; Wang, S.; Gu, H. AlN-based film buck acoustic resonator operated in shear mode for detection of carcinoembryonic antigens. RSC Adv. 2016, 6, 4908–4913. [Google Scholar] [CrossRef]
- Bjurström, J.; Wingqvist, G.; Yantchev, V.; Katardjiev, I. Temperature compensation of liquid FBAR sensors. J. Micromech. Microeng. 2007, 17, 651. [Google Scholar] [CrossRef]
- Katardjiev, I.; Yantchev, V. Recent developments in thin film electro-acoustic technology for biosensor applications. Vacuum 2012, 86, 520–531. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Xu, Y.; Chen, D.; Li, D.; Zhang, L. Protein-modified shear mode film bulk acoustic resonator for bio-sensing applications. Appl. Phys. A 2014, 116, 1567–1572. [Google Scholar] [CrossRef]
- Ali, A.; Lee, J.-Y. Electrical characterization of piezoelectric-on-silicon contour mode resonators fully immersed in liquid. Sens. Actuators A Phys. 2016, 241, 216–223. [Google Scholar] [CrossRef]
- Auer, S.; Nirschl, M.; Schreiter, M.; Vikholm-Lundin, I. Detection of DNA hybridisation in a diluted serum matrix by surface plasmon resonance and film bulk acoustic resonators. Anal. Bioanal. Chem. 2011, 400, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, F.; Ashley, G.M.; Garcia-Gancedo, L.; Luo, J.; Flewitt, A.J.; Milne, W.I.; Lu, J.R. Label-free detection of human prostate-specific antigen (hPSA) using film bulk acoustic resonators (FBARs). Sens. Actuators B Chem. 2014, 190, 946–953. [Google Scholar] [CrossRef]
- Zheng, D.; Xiong, J.; Guo, P.; Li, Y.; Wang, S.; Gu, H. Detection of a carcinoembryonic antigen using aptamer-modified film bulk acoustic resonators. Mater. Res. Bull. 2014, 59, 411–415. [Google Scholar] [CrossRef]
- Zhang, H.; Marma, M.S.; Bahl, S.K.; Kim, E.S.; McKenna, C.E. Sequence specific label-free DNA sensing using film-bulk-acoustic-resonators. IEEE Sens. J. 2007, 7, 1587–1588. [Google Scholar] [CrossRef]
- Nirschl, M.; Blüher, A.; Erler, C.; Katzschner, B.; Vikholm-Lundin, I.; Auer, S.; Vörös, J.; Pompe, W.; Schreiter, M.; Mertig, M. Film bulk acoustic resonators for DNA and protein detection and investigation of in vitro bacterial S-layer formation. Sens. Actuators A Phys. 2009, 156, 180–184. [Google Scholar] [CrossRef]
- Lee, T.Y.; Song, J.T. Detection of carcinoembryonic antigen using AlN FBAR. Thin Solid Film. 2010, 518, 6630–6633. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, W.; Marma, M.S.; Lee, C.-Y.; Kamal-Bahl, S.; Kim, E.S.; McKenna, C.E. Label-free detection of protein-ligand interactions in real time using micromachined bulk acoustic resonators. Appl. Phys. Lett. 2010, 96, 123702. [Google Scholar] [CrossRef]
- Zhang, H.; Marma, M.S.; Kim, E.S.; McKenna, C.E.; Thompson, M.E. Implantable resonant mass sensor for liquid biochemical sensing. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems. Maastricht MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; pp. 347–350. [Google Scholar]
- Zhang, X.; Xu, W.; Abbaspour-Tamijani, A.; Chae, J. Thermal analysis and characterization of a high Q film bulk acoustic resonator (FBAR) as biosensers in liquids. In Proceedings of the IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009; pp. 939–942. [Google Scholar]
- Yu, H.; Pang, W.; Zhang, H.; Kim, E.S. Ultra temperature-stable bulk-acoustic-wave resonators with SiO2 compensation layer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Yu, H.; Zhang, H.; Kim, E.S. Temperature-compensated film bulk acoustic resonator above 2 GHz. IEEE Electron Device Lett. 2005, 26, 369–371. [Google Scholar] [CrossRef]
- García-Gancedo, L.; Pedros, J.; Zhao, X.; Ashley, G.; Flewitt, A.; Milne, W.; Ford, C.; Lu, J.; Luo, J. Dual-mode thin film bulk acoustic wave resonators for parallel sensing of temperature and mass loading. Biosens. Bioelectr. 2012, 38, 369–374. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Shih, W.-C.; Chang, W.-T.; Yang, C.-H.; Kao, K.-S.; Cheng, C.-C. Biosensor for human IgE detection using shear-mode FBAR devices. Nanoscale Res. Lett. 2015, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Cui, W.; Liang, J.; Zhang, D.; Pang, W.; Zhang, H. A single-chip biosensing platform integrating FBAR sensor with digital microfluidic device. In Proceedings of the IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 1521–1523. [Google Scholar]
- Garcia-Gancedo, L.; Al-Naimi, F.; Flewitt, A.J.; Milne, W.I.; Ashley, G.M.; Luo, J.K.; Zhao, X.; Lu, J.R. ZnO-based FBAR resonators with carbon nanotube electrodes. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2438–2445. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Xu, Y. Highly sensitive lateral field excited piezoelectric film acoustic enzyme biosensor. IEEE Sens. J. 2013, 13, 2217–2222. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Li, D.; Li, Z. Film bulk acoustic resonator based biosensor for detection of cancer serological marker. Electron. Lett. 2011, 47, 1169–1170. [Google Scholar] [CrossRef]
- Guo, P.; Xiong, J.; Zheng, D.; Zhang, W.; Liu, L.; Wang, S.; Gu, H. A biosensor based on a film bulk acoustic resonator and biotin–avidin system for the detection of the epithelial tumor marker mucin 1. RSC Adv. 2015, 5, 66355–66359. [Google Scholar] [CrossRef]
- Lin, A.; Li, Y.-J.; Wang, L.; Chen, S.-J.; Gross, M.E.; Kim, E.S. Label-free detection of prostate-specific antigen with FBAR-based sensor with oriented antibody immobilization. In Proceedings of the IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 797–800. [Google Scholar]
- Zhao, X.; Pan, F.; Cowsill, B.; Lu, J.R.; Garcia-Gancedo, L.; Flewitt, A.J.; Ashley, G.M.; Luo, J. Interfacial immobilization of monoclonal antibody and detection of human prostate-specific antigen. Langmuir 2011, 27, 7654–7662. [Google Scholar] [CrossRef]
- Kim, E.; Choi, Y.-K.; Song, J.; Lee, J. Detection of various self-assembled monolayers by AlN-based film bulk acoustic resonator. Mater. Res. Bull. 2013, 48, 5076–5079. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Guo, P.; Xiong, J.; Wang, S. Streptavidin modified ZnO film bulk acoustic resonator for detection of tumor marker mucin 1. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Appel, J.; Chae, J. Contour-mode film bulk acoustic resonator for monitoring blood coagulation in real-time. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 2871–2874. [Google Scholar]
- Lin, A.; Sahin, F.E.; Chen, S.-J.; Pham, P.; Kim, E.S. Real-time label-free detection of DNA synthesis by FBAR-based mass sensing. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 1286–1289. [Google Scholar]
- Zhou, Z.; Tan, J.; Zhang, J.; Qin, M. Structural optimization and analysis of surface acoustic wave biosensor based on numerical method. Int. J. Distrib. Sens. Netw. 2019, 15, 1550147719875648. [Google Scholar] [CrossRef]
- Rayleigh, L. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 1885, 1, 4–11. [Google Scholar] [CrossRef]
- Khattri, K. Earthquake focal mechanism studies—A review. Earth-Sci. Rev. 1973, 9, 19–63. [Google Scholar] [CrossRef]
- White, R.M.; Voltmer, F.W. Direct Piezoelectric Coupling to Surface Elastic Waves. Appl. Phys. Lett. 1965, 7, 314–316. [Google Scholar] [CrossRef]
- Milstein, L.; Das, P. Spread spectrum receiver using surface acoustic wave technology. IEEE Trans. Commun. 1977, 25, 841–847. [Google Scholar] [CrossRef]
- Smith, W.R.; Gerard, H.M.; Collins, J.H.; Reeder, T.M.; Shaw, H.J. Design of surface wave delay lines with interdigital transducers. IEEE Trans. Microw. Theory Tech. 1969, 17, 865–873. [Google Scholar] [CrossRef]
- Rocha-Gaso, M.-I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface generated acoustic wave biosensors for the detection of pathogens: A review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Molecularly imprinted polymers for sensors: Comparison of optical and mass-sensitive detection. In Molecularly Imprinted Sensors; Elsevier: Amsterdam, The Netherlands, 2012; pp. 125–159. [Google Scholar]
- Luo, J.; Luo, P.; Xie, M.; Du, K.; Zhao, B.; Pan, F.; Fan, P.; Zeng, F.; Zhang, D.; Zheng, Z. A new type of glucose biosensor based on surface acoustic wave resonator using Mn-doped ZnO multilayer structure. Biosens. Bioelectr. 2013, 49, 512–518. [Google Scholar] [CrossRef]
- Reusch, M.; Holc, K.; Žukauskaitė, A.; Lebedev, V.; Kurz, N.; Ambacher, O. Flexural plate wave sensors with buried IDT for sensing in liquids. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Tang, P.; Wang, Y.; Huo, J.; Lin, X. Love wave sensor for prostate-specific membrane antigen detection based on hydrophilic molecularly-imprinted polymer. Polymers 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Yang, C.; Zhang, F.; Shang, Z.; Xu, Y.; Chen, Y.; Chen, M.; Mu, X. A high sensitive SH-SAW biosensor based 36° YX black LiTaO3 for label-free detection of Pseudomonas Aeruginosa. Sens. Actuators B Chem. 2019, 281, 757–764. [Google Scholar] [CrossRef]
- Jeng, M.-J.; Sharma, M.; Li, Y.-C.; Lu, Y.-C.; Yu, C.-Y.; Tsai, C.-L.; Huang, S.-F.; Chang, L.-B.; Lai, C.-S. Surface acoustic wave sensor for c-reactive protein detection. Sensors 2020, 20, 6640. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Walk, C.; Greifendorf, D.; Weidenmueller, J.; Jupe, A.; Seidl, K. Development of a multi channel piezoelectric flexural plate wave biomems-sensor for rapid point-of-care diagnostics. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1082–1085. [Google Scholar]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [Green Version]
- Takai, T.; Iwamoto, H.; Takamine, Y.; Yamazaki, H.; Fuyutsume, T.; Kyoya, H.; Nakao, T.; Kando, H.; Hiramoto, M.; Toi, T. High-performance SAW resonator on new multilayered substrate using LiTaO 3 crystal. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Neculoiu, D.; Bunea, A.-C.; Dinescu, A.M.; Farhat, L.A. Band pass filters based on GaN/Si lumped-element SAW resonators operating at frequencies above 5 GHz. IEEE Access 2018, 6, 47587–47599. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, Y.; Lam, C. A novel structure to suppress transverse modes in radio frequency TC-SAW resonators and filters. IEEE Microw. Wirel. Compon. Lett. 2019, 29, 249–251. [Google Scholar] [CrossRef]
- Plessky, V.P.; Reindl, L.M. Review on SAW RFID tags. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 654–668. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Surface acoustic wave (SAW) for chemical sensing applications of recognition layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, J.; da Cunha, M.P. High temperature LGS SAW gas sensor. Sens. Actuators B Chem. 2006, 113, 816–822. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. ZnO/ST-Quartz SAW resonator: An efficient NO2 gas sensor. Sens. Actuators B Chem. 2017, 252, 840–845. [Google Scholar] [CrossRef]
- Qiu, X.; Tang, R.; Chen, S.J.; Zhang, H.; Pang, W.; Yu, H. pH measurements with ZnO based surface acoustic wave resonator. Electrochem. Commun. 2011, 13, 488–490. [Google Scholar] [CrossRef]
- Bruckner, G.; Bardong, J.; Postl, M.; Klug, A. Experimental investigations of the acousto-electric effect in high temperature SAW gas sensing. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Vlassov, Y.N.; Kozlov, A.; Pashchin, N.; Yakovkin, I. Precision SAW pressure sensors. In Proceedings of the 1993 IEEE International Frequency Control Symposium, Salt Lake City, UT, USA, 2–4 June 1993; pp. 665–669. [Google Scholar]
- Tang, Z.; Wu, W.; Gao, J. A wireless passive SAW delay line temperature and pressure sensor for monitoring water distribution system. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Lamanna, L.; Rizzi, F.; Bhethanabotla, V.R.; de Vittorio, M. GHz AlN-based multiple mode SAW temperature sensor fabricated on PEN substrate. Sens. Actuators A Phys. 2020, 315, 112268. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Gao, Y.; Made, D.I.; Sun, C.; Je, M.; Gu, A.Y. An energy autonomous 400 MHz active wireless SAW temperature sensor powered by vibration energy harvesting. IEEE Trans. Circuits Syst. I Regul. Pap. 2015, 62, 976–985. [Google Scholar] [CrossRef]
- Malocha, D.; Humphries, J.; Figueroa, J.; Lamothe, M.; Weeks, A. 915 MHz SAW wireless passive sensor system performance. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Shu, L.; Peng, B.; Yang, Z.; Wang, R.; Deng, S.; Liu, X. High-temperature SAW wireless strain sensor with langasite. Sensors 2015, 15, 28531–28542. [Google Scholar] [CrossRef] [Green Version]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef]
- Tigli, O.; Zaghloul, M.E. Surface acoustic wave (SAW) biosensors. In Proceedings of the 2010 53rd IEEE International Midwest Symposium on Circuits and Systems, Seattle, WA, USA, 1–4 August 2010; pp. 77–80. [Google Scholar]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Grate, J.W.; Martin, S.J.; White, R.M. Acoustic wave microsensors. Anal. Chem. 1993, 65, 940A–948A. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. A Rayleigh surface acoustic wave (R-SAW) resonator biosensor based on positive and negative reflectors with sub-nanomolar limit of detection. Sens. Actuators B Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef]
- De Simoni, G.; Signore, G.; Agostini, M.; Beltram, F.; Piazza, V. A surface-acoustic-wave-based cantilever bio-sensor. Biosens. Bioelectr. 2015, 68, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Vellekoop, M.J. Acoustic wave sensors and their technology. Ultrasonics 1998, 36, 7–14. [Google Scholar] [CrossRef]
- Gizeli, E.; Bender, F.; Rasmusson, A.; Saha, K.; Josse, F.; Cernosek, R. Sensitivity of the acoustic waveguide biosensor to protein binding as a function of the waveguide properties. Biosens. Bioelectr. 2003, 18, 1399–1406. [Google Scholar] [CrossRef]
- Herrmann, F.; Weihnacht, M.; Buttgenbach, S. Properties of sensors based on shear-horizontal surface acoustic waves in LiTaO/sub 3//SiO/sub 2/and quartz/SiO/sub 2/structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 268–273. [Google Scholar] [CrossRef]
- Water, W.; Chen, S.-E. Using ZnO nanorods to enhance sensitivity of liquid sensor. Sens. Actuators B Chem. 2009, 136, 371–375. [Google Scholar] [CrossRef]
- Gizeli, E.; Stevenson, A.C.; Goddard, N.J.; Lowe, C.R. A novel Love-plate acoustic sensor utilizing polymer overlayers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.W.; Brozik, S.M. Low-level detection of a Bacillus anthracis simulant using Love-wave biosensors on 36 YX LiTaO3. Biosens. Bioelectr. 2004, 19, 849–859. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, Y.J. Implementation of guiding layers of surface acoustic wave devices: A review. Biosens. Bioelectr. 2018, 99, 500–512. [Google Scholar] [CrossRef]
- Huang, I.-Y.; Lee, M.-C.; Chang, Y.-W. Development of a novel flexural plate wave biosensor for immunoglobulin-E detection by using SAM and MEMS technologies. In Proceedings of the SENSORS, 2006 IEEE, Daegu, Korea, 22–25 October 2006; pp. 70–73. [Google Scholar]
- Huang, I.-Y.; Lee, M.-C. Development of a FPW allergy biosensor for human IgE detection by MEMS and cystamine-based SAM technologies. Sens. Actuators B Chem. 2008, 132, 340–348. [Google Scholar] [CrossRef]
- Déjous, C.; Savart, M.; Rebière, D.; Pistré, J. A shear-horizontal acoustic plate mode (SH-APM) sensor for biological media. Sens. Actuators B Chem. 1995, 27, 452–456. [Google Scholar] [CrossRef]
- Andle, J.; Weaver, J.; Vetelino, J.; McAllister, D. Selective acoustic plate mode DNA sensor. Sens. Actuators B Chem. 1995, 24, 129–133. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Lee, J.; Lee, Y.; Kwak, J.; Suk Lee, S. Increase in detection sensitivity of surface acoustic wave biosensor using triple transit echo wave. Appl. Phys. Lett. 2018, 113, 083702. [Google Scholar] [CrossRef]
- Länge, K.; Blaess, G.; Voigt, A.; Götzen, R.; Rapp, M. Integration of a surface acoustic wave biosensor in a microfluidic polymer chip. Biosens. Bioelectr. 2006, 22, 227–232. [Google Scholar] [CrossRef]
- Jandas, P.; Luo, J.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800. [Google Scholar] [CrossRef]
- Lo, X.-C.; Li, J.-Y.; Lee, M.-T.; Yao, D.-J. Frequency shift of a SH-SAW biosensor with glutaraldehyde and 3-aminopropyltriethoxysilane functionalized films for detection of epidermal growth factor. Biosensors 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.-B.; Kim, Y.-I. Love wave SAW biosensors for detection of antigen-antibody binding and comparison with SPR biosensor. Food Sci. Biotechnol. 2011, 20, 1413. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Romashko, R.V.; Zaporozhets, T.S.; Efimov, T.A. Label-free biosensors for laboratory-based diagnostics of infections: Current achievements and new trends. Biosensors 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanik, S.; Pingguan-Murphy, B.; Osman, N.A. Developments of immobilized surface modified piezoelectric crystal biosensors for advanced applications. Int. J. Electrochem. Sci. 2013, 8, 8863–8892. [Google Scholar]
- Galipeau, D.W.; Story, P.R.; Vetelino, K.A.; Mileham, R.D. Surface acoustic wave microsensors and applications. Smart Mater. Struct. 1997, 6, 658. [Google Scholar] [CrossRef]
- Li, S.; Wan, Y.; Su, Y.; Fan, C.; Bhethanabotla, V.R. Gold nanoparticle-based low limit of detection Love wave biosensor for carcinoembryonic antigens. Biosens. Bioelectr. 2017, 95, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohltjen, H. Mechanism of operation and design considerations for surface acoustic wave device vapour sensors. Sens. Actuators 1984, 5, 307–325. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Jin, D.; Yu, Y.; Yang, F.; Zhang, Y.; Yao, Q.; Zhang, G.-J. AuNP-amplified surface acoustic wave sensor for the quantification of exosomes. ACS Sens. 2020, 5, 362–369. [Google Scholar] [CrossRef]
- Lan, J.-W.; Huang, I.-Y.; Lin, Y.-C.; Lin, C.-Y.; Chen, J.-L.; Hsieh, C.-H. Development of an FPW biosensor with low insertion loss and high fabrication yield for detection of carcinoembryonic antigen. Sensors 2016, 16, 1729. [Google Scholar] [CrossRef] [Green Version]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Girasole, M.; Fosca, M.; Dinarelli, S.; Staiano, M.; Marzullo, V.; Capo, A. A Shear horizontal surface acoustic wave biosensor for a rapid and specific detection of d-serine. Sens. Actuators B Chem. 2016, 226, 1–6. [Google Scholar] [CrossRef]
- Chang, K.; Pi, Y.; Lu, W.; Wang, F.; Pan, F.; Li, F.; Jia, S.; Shi, J.; Deng, S.; Chen, M. Label-free and high-sensitive detection of human breast cancer cells by aptamer-based leaky surface acoustic wave biosensor array. Biosens. Bioelectr. 2014, 60, 318–324. [Google Scholar] [CrossRef]
- Shimizu, Y. Current status of piezoelectric substrate and propagation characteristics for SAW devices. Jpn. J. Appl. Phys. 1993, 32, 2183. [Google Scholar] [CrossRef]
- Zida, S.I.; Lin, Y.D.; Khung, Y.L. Current Trends on Surface Acoustic Wave Biosensors. Adv. Mater. Technol. 2021, 6, 2001018. [Google Scholar] [CrossRef]
- Berkenpas, E.; Bitla, S.; Millard, P.; Da Cunha, M.P. Pure shear horizontal SAW biosensor on langasite. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 1404–1411. [Google Scholar] [CrossRef]
- Tigli, O.; Zaghloul, M.E. A novel SAW device in CMOS: Design, modeling, and fabrication. IEEE Sens. J. 2007, 7, 219–227. [Google Scholar] [CrossRef]
- Rapp, M.; Wessa, T.; Ache, H. Modification of commercially available low-loss SAW devices towards an immunosensor for in-situ measurements in water. In Proceedings of the 1995 IEEE Ultrasonics Symposium, an International Symposium, Seattle, WA, USA, 7–10 November 1995; pp. 433–436. [Google Scholar]
- Chen, Y.-C.; Chang, W.-T.; Cheng, C.-C.; Shen, J.-Y.; Kao, K.-S. Development of human IgE biosensor using Sezawa-mode SAW devices. Curr. Appl. Phys. 2014, 14, 608–613. [Google Scholar] [CrossRef]
- Zakaria, M.; Hamzah, F.; Omar, M.; Hashim, U.; Ayub, R.M.; Farehanim, M.; Al-Mufti, A.W. Preparation of zinc oxide piezoelectric substrate for saw biosensor device. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 388–391. [Google Scholar]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. A miniaturized immunosensor platform for automatic detection of carcinoembryonic antigen in EBC. Sens. Actuators B Chem. 2014, 205, 94–101. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Cao, K.; Wang, P.; Su, Y.; Zhu, X.; Wan, Y. A Microfluidic Love-Wave Biosensing Device for PSA Detection Based on an Aptamer Beacon Probe. Sensors 2015, 15, 13839–13850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. Sensitive detection of carcinoembryonic antigen in exhaled breath condensate using surface acoustic wave immunosensor. Sens. Actuators B Chem. 2015, 217, 100–106. [Google Scholar] [CrossRef]
- Huang, I.-Y.; Lee, M.-C.; Chang, Y.-W.; Huang, R.-S. Development and characterization of FPW based allergy biosensor. In Proceedings of the 2007 IEEE International Symposium on Industrial Electronics, Vigo, Spain, 4–7 June 2007; pp. 2736–2740. [Google Scholar]
- Shiokawa, S.; Moriizumi, T. Design of SAW sensor in liquid. Jpn. J. Appl. Phys. 1988, 27, 142. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Sun, Z.; Li, Y.-T.; Zhang, G.-J. A surface acoustic wave biosensor synergizing DNA-mediated in situ silver nanoparticle growth for a highly specific and signal-amplified nucleic acid assay. Analyst 2017, 142, 3468–3476. [Google Scholar] [CrossRef]
- Yao, H.; Fernández, C.S.; Xu, X.; Wynendaele, E.; de Spiegeleer, B. A Surface Acoustic Wave (SAW) biosensor method for functional quantification of E. coli l-asparaginase. Talanta 2019, 203, 9–15. [Google Scholar] [CrossRef]
- Han, J.; Li, M.; Li, H.; Li, H.; Li, C.; Li, H.; Qian, L.; Yang, B. A 433-MHz surface acoustic wave sensor with Ni-TiO 2-poly (L-lysine) composite film for dopamine determination. Microchim. Acta 2020, 187, 671. [Google Scholar] [CrossRef] [PubMed]
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Öpik, A. Molecularly imprinted polymer film interfaced with Surface Acoustic Wave technology as a sensing platform for label-free protein detection. Anal. Chim. Acta 2016, 902, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y.; Miyachi, S.; Nagasaki, Y. High-performance surface acoustic wave immunosensing system on a PEG/aptamer hybridized surface. Langmuir 2013, 29, 7369–7376. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Park, J.-Y.; Seo, H.; Lee, T.; Lee, W.; Kim, S.K.; Hahn, Y.K.; yeon Jung, J.; Kim, S. Sensitive and reproducible detection of cardiac troponin I in human plasma using a surface acoustic wave immunosensor. Sens. Actuators B Chem. 2013, 178, 19–25. [Google Scholar] [CrossRef]
- Jeng, M.-J.; Li, Y.-C.; Sharma, M.; Chen, C.-W.; Tsai, C.-L.; Lin, Y.-H.; Huang, S.-F.; Chang, L.-B.; Lai, C.-S. A Surface Acoustic Wave Sensor with a Microfluidic Channel for Detecting C-Reactive Protein. Chemosensors 2021, 9, 106. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, C.Y.; Tan, S.Y.; Wong, M.S.F.; Chen, Y.F.; Zhang, L.; Yao, K.; Gan, S.K.E.; Verma, C.; Tan, Y.-J. SAW sensor for Influenza A virus detection enabled with efficient surface functionalization. Sens. Actuators B Chem. 2015, 209, 78–84. [Google Scholar] [CrossRef]
- Turbé, V.; Gray, E.R.; Lawson, V.E.; Nastouli, E.; Brookes, J.C.; Weiss, R.A.; Pillay, D.; Emery, V.C.; Verrips, C.T.; Yatsuda, H. Towards an ultra-rapid smartphone-connected test for infectious diseases. Sci. Rep. 2017, 7, 6522. [Google Scholar]
- Jupe, A.; Livshits, P.; Kahnert, S.; Figge, M.; Mross, S.; Goertz, M.; Kappert, H.; Vogt, H.; Goehlich, A. Development of a piezoelectric flexural plate-wave (FPW) biomems-sensor for rapid point-of-care diagnostics. In Nanostructured Materials for the Detection of CBRN; Springer: Berlin/Heidelberg, Germany, 2018; pp. 199–212. [Google Scholar]
- Onen, O.; Ahmad, A.A.; Guldiken, R.; Gallant, N.D. Surface modification on acoustic wave biosensors for enhanced specificity. Sensors 2012, 12, 12317–12328. [Google Scholar] [CrossRef] [Green Version]
- Jandas, P.; Luo, J.; Quan, A.; Qiu, C.; Cao, W.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.-S.; Lee, Y.; Lee, H.J.; Lee, J.N.; Kim, S.K.; Han, K.Y.; Cho, E.C.; Park, J.C.; Lee, S.S. Sensitive and simultaneous detection of cardiac markers in human serum using surface acoustic wave immunosensor. Anal. Chem. 2011, 83, 8629–8635. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.M. Cardiac markers and their point-of-care testing for diagnosis of acute myocardial infarction. Clin. Biochem. 2006, 39, 771–780. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Komatsu, N.; Watanabe, H.; Shinoda, N.; Nakazato, M.; Kumakiri, C.; Okada, S.-i.; Hasegawa, H. Decreased serum levels of D-serine in patients with schizophrenia: Evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry 2003, 60, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.S.; Holmes, C.; Lopez, G.J.; Wu, T.; Sharabi, Y. Cerebrospinal fluid biomarkers of central dopamine deficiency predict Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 50, 108–112. [Google Scholar] [CrossRef]
- Maouche, N.; Ktari, N.; Bakas, I.; Fourati, N.; Zerrouki, C.; Seydou, M.; Maurel, F.; Chehimi, M.M. A surface acoustic wave sensor functionalized with a polypyrrole molecularly imprinted polymer for selective dopamine detection. J. Mol. Recognit. 2015, 28, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Saarma, M.; Syritski, V. Molecularly imprinted polymer-based SAW sensor for label-free detection of cerebral dopamine neurotrophic factor protein. Sens. Actuators B Chem. 2020, 308, 127708. [Google Scholar] [CrossRef]
- Fournel, F.; Baco, E.; Mamani-Matsuda, M.; Degueil, M.; Bennetau, B.; Moynet, D.; Mossalayi, D.; Vellutini, L.; Pillot, J.-P.; Dejous, C. Love wave biosensor for real-time detection of okadaic acid as DSP phycotoxin. Sens. Actuators B Chem. 2012, 170, 122–128. [Google Scholar] [CrossRef]
- Stamp, M.E.; Brugger, M.S.; Wixforth, A.; Westerhausen, C. Acoustotaxis–in vitro stimulation in a wound healing assay employing surface acoustic waves. Biomater. Sci. 2016, 4, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.-C.; Chang, H.-Y.; Wang, T.-P.; Yao, D.-J. Detection of cells captured with antigens on shear horizontal surface-acoustic-wave sensors. J. Lab. Autom. 2013, 18, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zu, H.; Wang, J.H.-C.; Wang, Q.-M. A study of Love wave acoustic biosensors monitoring the adhesion process of tendon stem cells (TSCs). Eur. Biophys. J. 2019, 48, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Brugger, M.S.; Schnitzler, L.G.; Nieberle, T.; Wixforth, A.; Westerhausen, C. Shear-horizontal surface acoustic wave sensor for non-invasive monitoring of dynamic cell spreading and attachment in wound healing assays. Biosens. Bioelectr. 2021, 173, 112807. [Google Scholar] [CrossRef]
- Hardin, J.; Bertoni, G.; Kleinsmith, L. Becker’s World of the Cell; Pearson Education: London, UK, 2012. [Google Scholar]
- Wu, H.; Zu, H.; Wang, Q.-M.; Zhao, G.; Wang, J.H. Monitoring the adhesion process of tendon stem cells using shear-horizontal surface acoustic wave sensors. In Proceedings of the 2015 Joint Conference of the IEEE International Frequency Control Symposium & the European Frequency and Time Forum, Denver, CO, USA, 12–16 April 2015; pp. 310–315. [Google Scholar]
- Hao, H.-C.; Yao, D.-J. A sensitive, rapid and specific technique for the detection of antigen-specific cells on shear horizontal surface acoustic wave (SH-SAW) sensors. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 2117–2121. [Google Scholar]
- Lianidou, E.S.; Markou, A. Circulating tumor cells in breast cancer: Detection systems, molecular characterization, and future challenges. Clin. Chem. 2011, 57, 1242–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, M.; Zhang, L.; Ding, Y.; Luo, Y.; Xu, Q.; Shi, J.; Cao, L.; Fu, W. Rapid detection of human papilloma virus using a novel leaky surface acoustic wave peptide nucleic acid biosensor. Biosens. Bioelectr. 2009, 24, 3455–3460. [Google Scholar] [CrossRef]
- Cai, H.-L.; Yang, Y.; Zhang, Y.-H.; Zhou, C.-J.; Guo, C.-R.; Liu, J.; Ren, T.-L. A high sensitivity wireless mass-loading surface acoustic wave DNA biosensor. Mod. Phys. Lett. B 2014, 28, 1450056. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Turner, A.P.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectr. 2004, 19, 515–530. [Google Scholar] [CrossRef]
- Shastry, B.S. SNPs in disease gene mapping, medicinal drug development and evolution. J. Hum. Genet. 2007, 52, 871–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, J.-Y.; Mao, X.-B.; Ning, Y.; Zhang, G.-J. Single-shot analytical assay based on graphene-oxide-modified surface acoustic wave biosensor for detection of single-nucleotide polymorphisms. Anal. Chem. 2015, 87, 9352–9359. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, K.; Lu, W.; Chen, W.; Ding, Y.; Jia, S.; Zhang, K.; Li, F.; Shi, J.; Cao, L. Detection of single-nucleotide polymorphisms with novel leaky surface acoustic wave biosensors, DNA ligation and enzymatic signal amplification. Biosens. Bioelectr. 2012, 33, 274–278. [Google Scholar] [CrossRef]
- Ji, J.; Pang, Y.; Li, D.; Wang, X.; Xu, Y.; Mu, X. Single-layered graphene/Au-nanoparticles-based love wave biosensor for highly sensitive and specific detection of Staphylococcus aureus gene sequences. ACS Appl. Mater. Interfaces 2020, 12, 12417–12425. [Google Scholar] [CrossRef] [PubMed]
- Kordas, A.; Papadakis, G.; Milioni, D.; Champ, J.; Descroix, S.; Gizeli, E. Rapid Salmonella detection using an acoustic wave device combined with the RCA isothermal DNA amplification method. Sens. Bio-Sens. Res. 2016, 11, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Ten, S.; Hashim, U.; Gopinath, S.; Liu, W.; Foo, K.; Sam, S.; Rahman, S.; Voon, C.; Nordin, A. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectr. 2017, 93, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ovejero, C.; Benito-Lopez, F.; Díez, P.; Casulli, A.; Siles-Lucas, M.; Fuentes, M.; Manzano-Román, R. Sensing parasites: Proteomic and advanced bio-detection alternatives. J. Proteom. 2016, 136, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Bisoffi, M.; Severns, V.; Branch, D.W.; Edwards, T.L.; Larson, R.S. Rapid detection of human immunodeficiency virus types 1 and 2 by use of an improved piezoelectric biosensor. J. Clin. Microbiol. 2013, 51, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baca, J.T.; Severns, V.; Lovato, D.; Branch, D.W.; Larson, R.S. Rapid detection of Ebola virus with a reagent-free, point-of-care biosensor. Sensors 2015, 15, 8605–8614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisoffi, M.; Hjelle, B.; Brown, D.; Branch, D.; Edwards, T.; Brozik, S.; Bondu-Hawkins, V.; Larson, R. Detection of viral bioagents using a shear horizontal surface acoustic wave biosensor. Biosens. Bioelectr. 2008, 23, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Matatagui, D.; Fontecha, J.L.; Fernández, M.J.; Gràcia, I.; Cané, C.; Santos, J.P.; Horrillo, M.C. Love-wave sensors combined with microfluidics for fast detection of biological warfare agents. Sensors 2014, 14, 12658–12669. [Google Scholar] [CrossRef] [Green Version]
- Lamanna, L.; Rizzi, F.; Guido, F.; Algieri, L.; Marras, S.; Mastronardi, V.M.; Qualtieri, A.; De Vittorio, M. Flexible and Transparent Aluminum-Nitride-Based Surface-Acoustic-Wave Device on Polymeric Polyethylene Naphthalate. Adv. Electron. Mater. 2019, 5, 1900095. [Google Scholar] [CrossRef]
- Luo, J.K.; Fu, Y.Q.; Milne, W.I. Acoustic wave based microfluidics and lab-on-a-chip. In Modeling and Measurement Methods for Acoustic Waves and for Acoustic Microdevices; IntechOpen: London, UK, 2013. [Google Scholar]
- Yeo, L.Y.; Friend, J.R. Surface Acoustic Wave Microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef] [Green Version]
- Franke, T.A.; Wixforth, A. Microfluidics for miniaturized laboratories on a chip. ChemPhysChem 2008, 9, 2140–2156. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic microfluidic separation techniques and bioapplications: A review. Micromachines 2020, 11, 921. [Google Scholar] [CrossRef]
- Gupta, S.; Bit, A. Acoustophoresis-based biomedical device applications. In Bioelectronics and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. [Google Scholar]
- Reboud, J.; Bourquin, Y.; Wilson, R.; Pall, G.S.; Jiwaji, M.; Pitt, A.R.; Graham, A.; Waters, A.P.; Cooper, J.M. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc. Natl. Acad. Sci. USA 2012, 109, 15162–15167. [Google Scholar] [CrossRef] [Green Version]
- Taller, D.; Richards, K.; Slouka, Z.; Senapati, S.; Hill, R.; Go, D.B.; Chang, H.-C. On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis. Lab Chip 2015, 15, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, X.; Mao, Z.; Chen, Y.; Nama, N.; Guo, F.; Li, P.; Wang, L.; Cameron, C.E.; Huang, T.J. Standing surface acoustic wave (SSAW)-based cell washing. Lab Chip 2015, 15, 331–338. [Google Scholar] [CrossRef]
- Wu, M.; Huang, P.H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia coli bacteria from peripheral blood mononuclear cells using standing surface acoustic waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Ding, X.; Peng, Z.; Lin, S.-C.S.; Geri, M.; Li, S.; Li, P.; Chen, Y.; Dao, M.; Suresh, S.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2014, 111, 12992–12997. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Eldosoky, M.A.; Taha, T.E. The separation of blood components using standing surface acoustic waves (SSAWs) microfluidic devices: Analysis and simulation. Bioengineering 2017, 4, 28. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Gautam, G.P.; Burger, T.; Wilcox, A.; Cumbo, M.J.; Graves, S.W.; Piyasena, M.E. Simple and inexpensive micromachined aluminum microfluidic devices for acoustic focusing of particles and cells. Anal. Bioanal. Chem. 2018, 410, 3385–3394. [Google Scholar] [CrossRef]
- Leibacher, I.; Reichert, P.; Dual, J. Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2015, 15, 2896–2905. [Google Scholar] [CrossRef] [Green Version]
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Fornell, A.; Cushing, K.; Nilsson, J.; Tenje, M. Binary particle separation in droplet microfluidics using acoustophoresis. Appl. Phys. Lett. 2018, 112, 063701. [Google Scholar] [CrossRef]
- Gautam, G.P.; Gurung, R.; Fencl, F.A.; Piyasena, M.E. Separation of sub-micron particles from micron particles using acoustic fluid relocation combined with acoustophoresis. Anal. Bioanal. Chem. 2018, 410, 6561–6571. [Google Scholar] [CrossRef] [PubMed]
- Devendran, C.; Gralinski, I.; Neild, A. Separation of particles using acoustic streaming and radiation forces in an open microfluidic channel. Microfluid. Nanofluid. 2014, 17, 879–890. [Google Scholar] [CrossRef]
- Xu, Y.; Hashmi, A.; Yu, G.; Lu, X.; Kwon, H.-J.; Chen, X.; Xu, J. Microbubble array for on-chip worm processing. Appl. Phys. Lett. 2013, 102, 023702. [Google Scholar] [CrossRef] [Green Version]
- Antfolk, M.; Antfolk, C.; Lilja, H.; Laurell, T.; Augustsson, P. A single inlet two-stage acoustophoresis chip enabling tumor cell enrichment from white blood cells. Lab Chip 2015, 15, 2102–2109. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Liu, Y.; Tan, P.L.; Then, X.Y.S.; Yung, L.-Y.L.; Lim, K.-M. On-chip measurements of cell compressibility via acoustic radiation. Lab Chip 2011, 11, 4072–4080. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Zhang, H.; Huang, J.; Wang, Z.; Yun, Z.; Wang, Y.; Pang, W.; Duan, X.; Zhang, H. On-chip acoustic mixer integration of electro-microfluidics towards in-situ and efficient mixing in droplets. Microfluid. Nanofluid. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Collins, D.J.; Neild, A.; Ai, Y. Highly focused high-frequency travelling surface acoustic waves (SAW) for rapid single-particle sorting. Lab Chip 2016, 16, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a microfluidic channel via standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef]
- Wu, M.; Ozcelik, A.; Rufo, J.; Wang, Z.; Fang, R.; Huang, T.J. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 2019, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.-C.; Hsu, C.-H.; Huang, Y.-W. Acoustophoretic control of microparticle transport using dual-wavelength surface acoustic wave devices. Micromachines 2019, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Lin, S.-C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.-K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Shi, J.; Lin, S.-C.S.; Yazdi, S.; Kiraly, B.; Huang, T.J. Tunable patterning of microparticles and cells using standing surface acoustic waves. Lab Chip 2012, 12, 2491–2497. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.; Cunningham, J.; O’Rorke, R.; Wälti, C.; Linfield, E.; Davies, A.; Evans, S. Formation and manipulation of two-dimensional arrays of micron-scale particles in microfluidic systems by surface acoustic waves. Appl. Phys. Lett. 2009, 94, 054101. [Google Scholar] [CrossRef]
- Barani, A.; Paktinat, H.; Janmaleki, M.; Mohammadi, A.; Mosaddegh, P.; Fadaei-Tehrani, A.; Sanati-Nezhad, A. Microfluidic integrated acoustic waving for manipulation of cells and molecules. Biosens. Bioelectr. 2016, 85, 714–725. [Google Scholar] [CrossRef]
- Nam, J.; Lim, H.; Kim, D.; Shin, S. Separation of platelets from whole blood using standing surface acoustic waves in a microchannel. Lab Chip 2011, 11, 3361–3364. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ma, F.; Bachman, H.; Cameron, C.E.; Zeng, X.; Huang, T.J. Acoustofluidic bacteria separation. J. Micromech. Microeng. 2016, 27, 015031. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [Green Version]
- Murphy, O.H.; Bahmanyar, M.R.; Borghi, A.; McLeod, C.N.; Navaratnarajah, M.; Yacoub, M.H.; Toumazou, C. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed. Microdevices 2013, 15, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, M.; Zhao, G. Point-of-Care Assessment of Hemostasis with a Love-Mode Surface Acoustic Wave Sensor. ACS Sens. 2020, 5, 282–291. [Google Scholar] [CrossRef]
- Reichert, P.; Deshmukh, D.; Lebovitz, L.; Dual, J. Thin film piezoelectrics for bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2018, 18, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Wang, Z.; Liu, P.; Becker, R.; Yang, S.; Yang, K.; Pei, Z.; Zhang, P.; Xia, J.; Shen, L. Acoustofluidic multimodal diagnostic system for Alzheimer’s disease. Biosens. Bioelectr. 2021, 196, 113730. [Google Scholar] [CrossRef]
- Hao, N.; Pei, Z.; Liu, P.; Bachman, H.; Naquin, T.D.; Zhang, P.; Zhang, J.; Shen, L.; Yang, S.; Yang, K. Acoustofluidics-Assisted Fluorescence-SERS Bimodal Biosensors. Small 2020, 16, 2005179. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Reboud, J.; Torun, H.; McHale, G.; Dodd, L.E.; Wu, Q.; Tao, K.; Yang, X.; Luo, J.T.; Todryk, S. Integrating microfluidics and biosensing on a single flexible acoustic device using hybrid modes. Lab Chip 2020, 20, 1002–1011. [Google Scholar] [CrossRef]
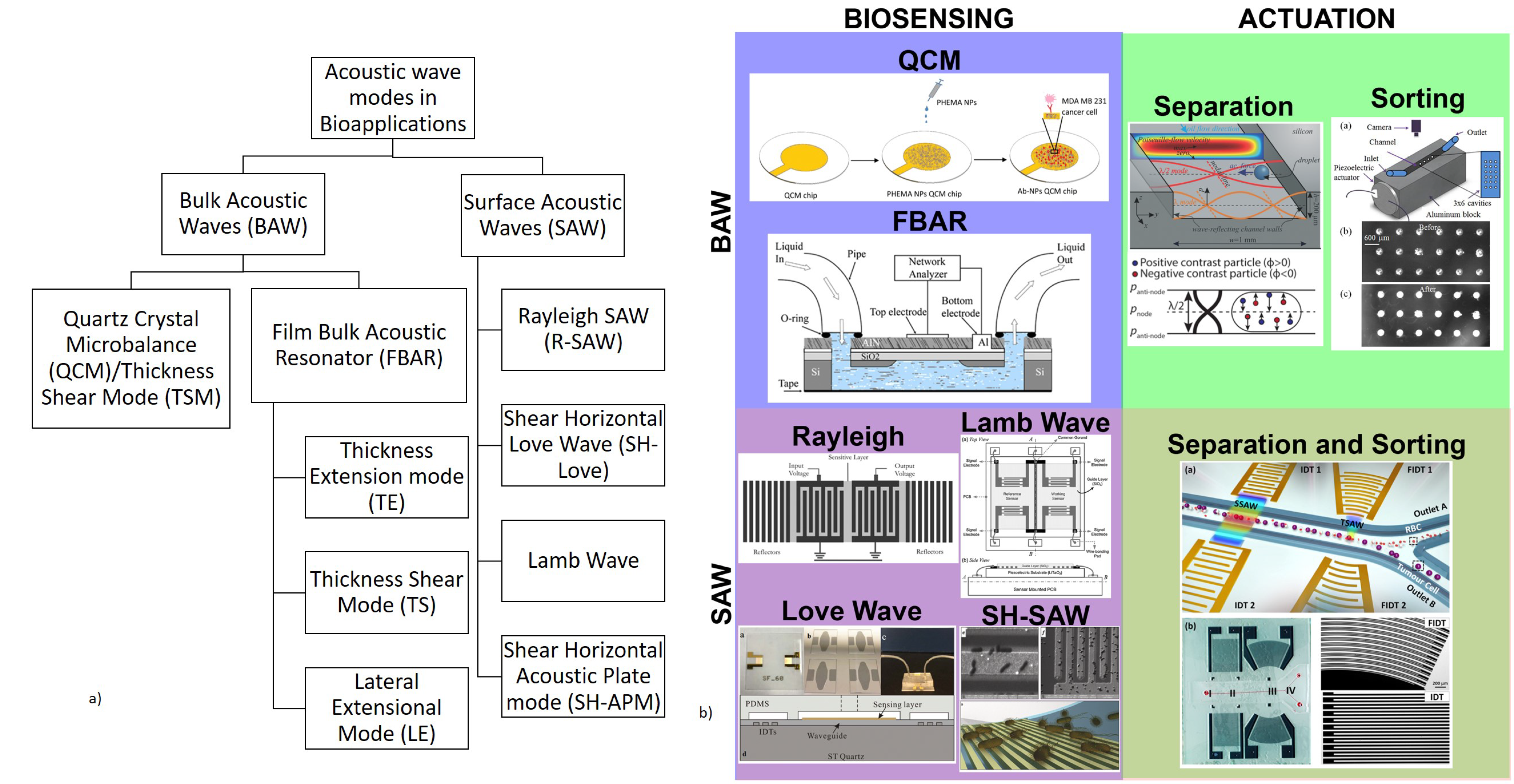








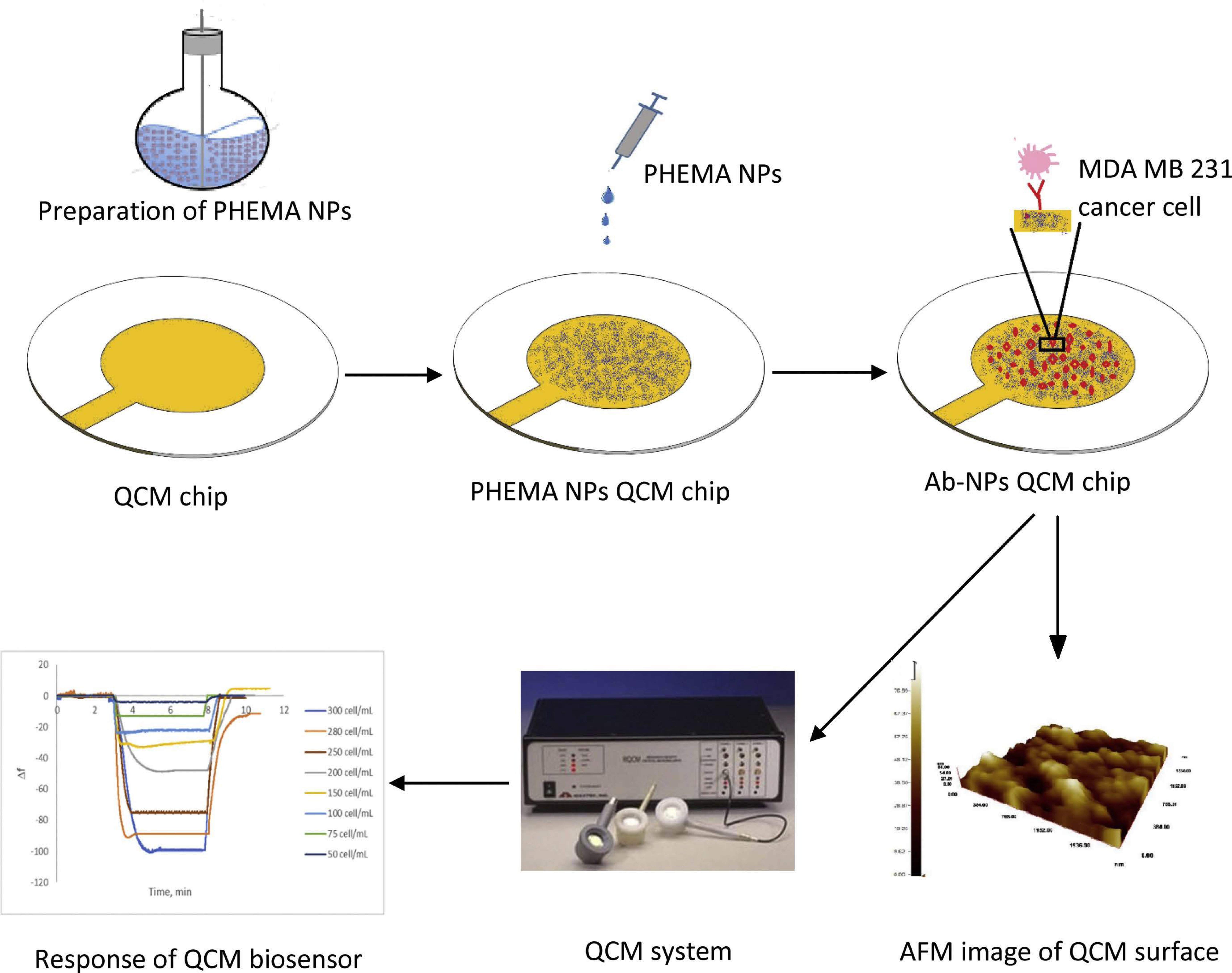
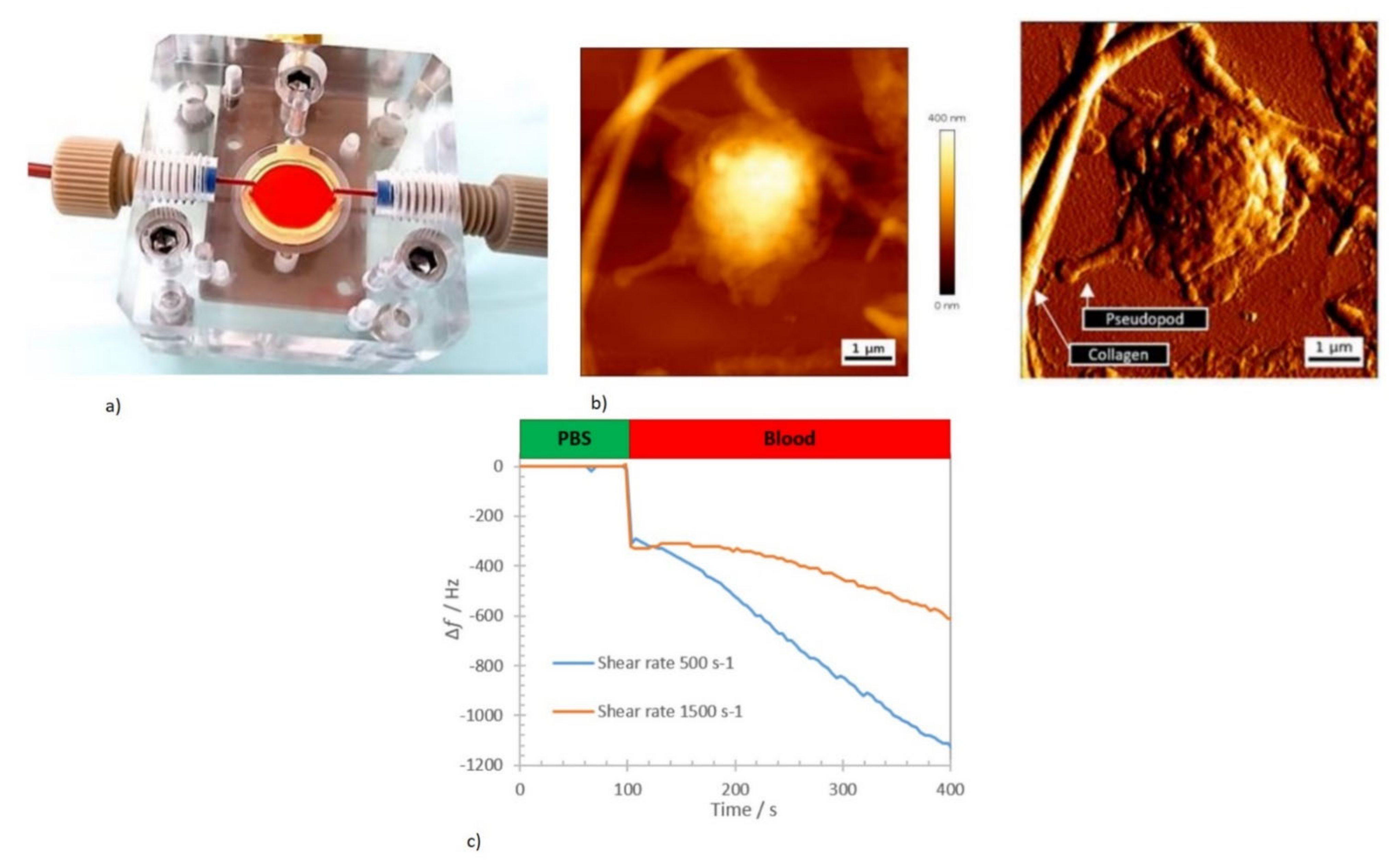
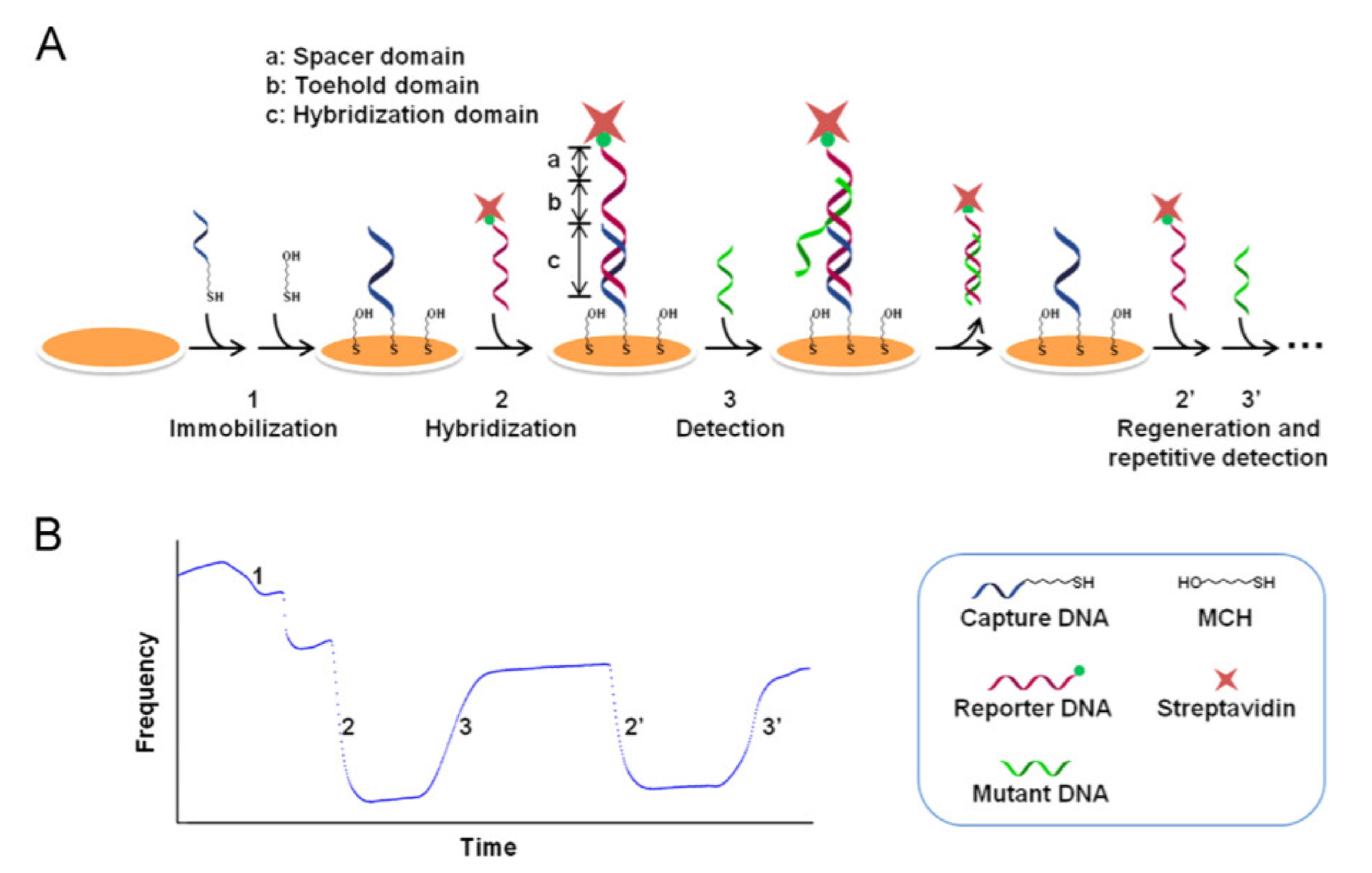
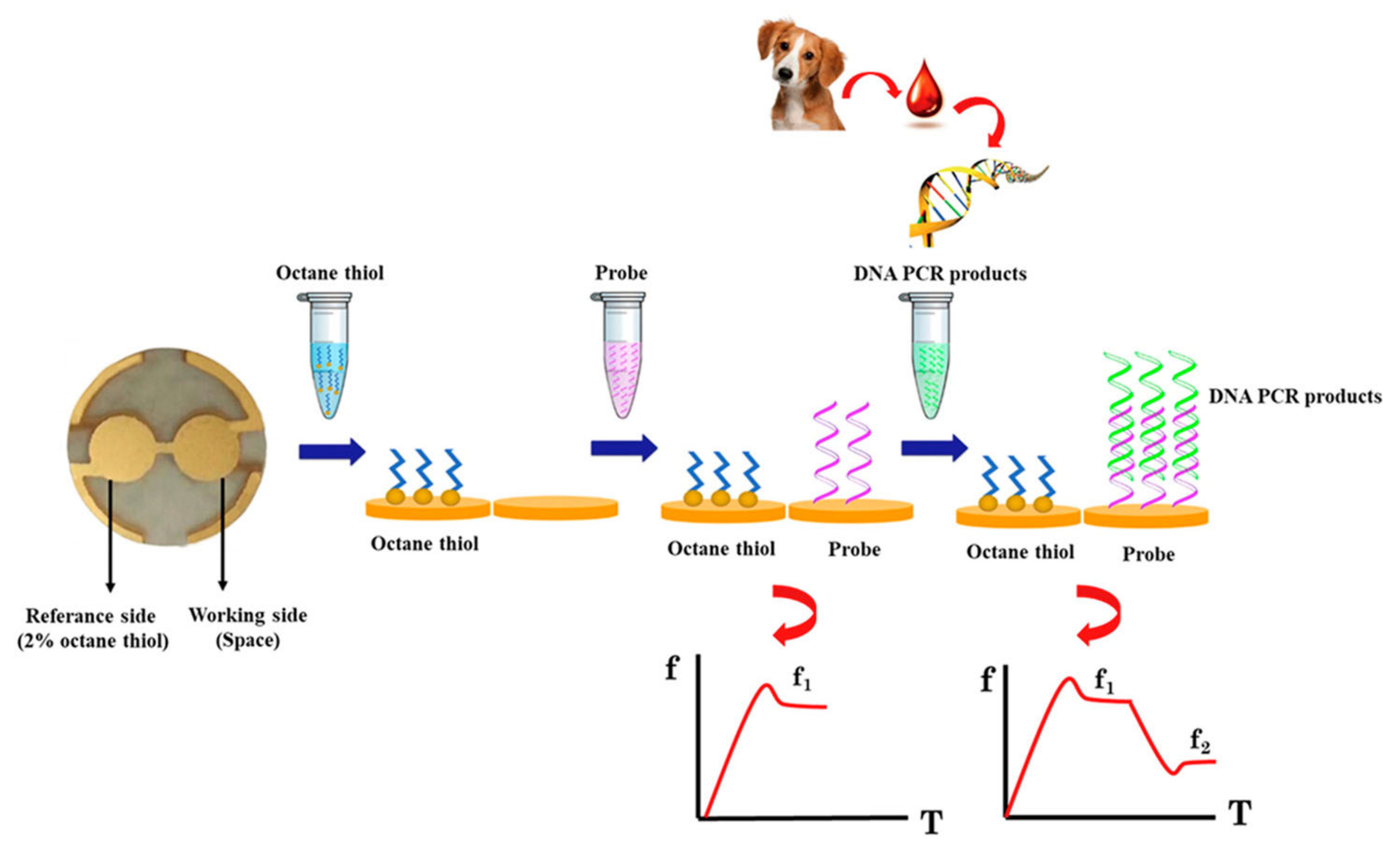
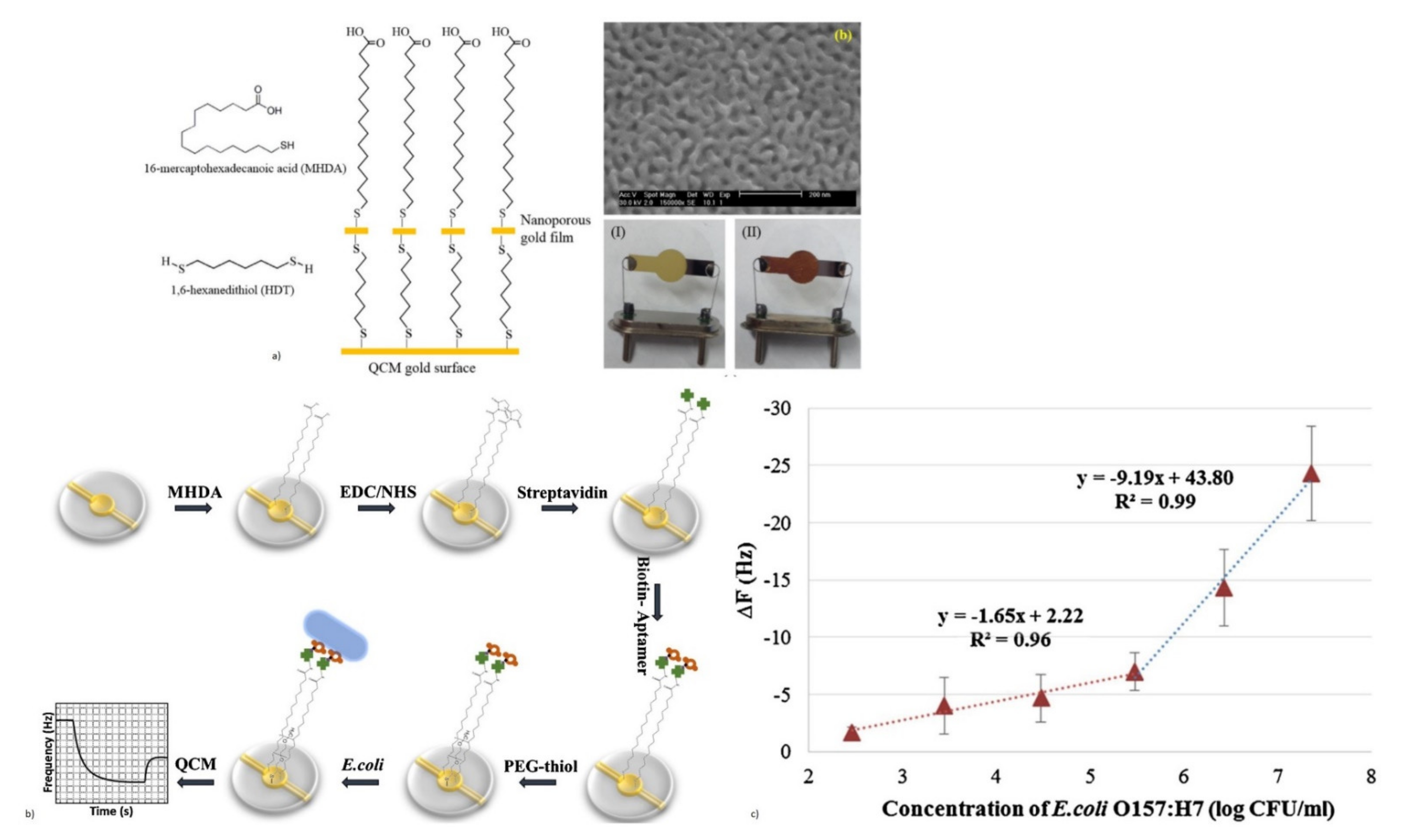


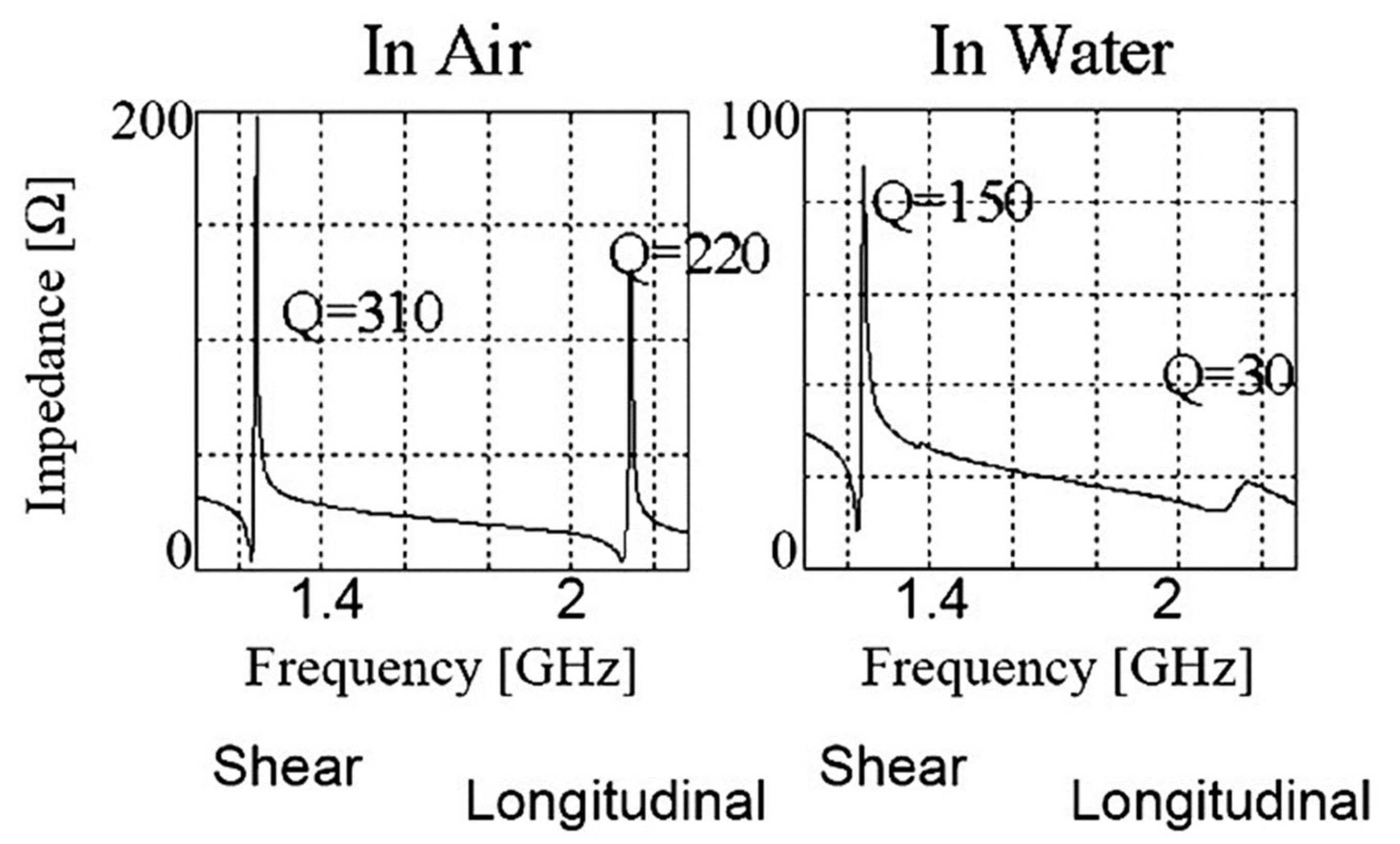
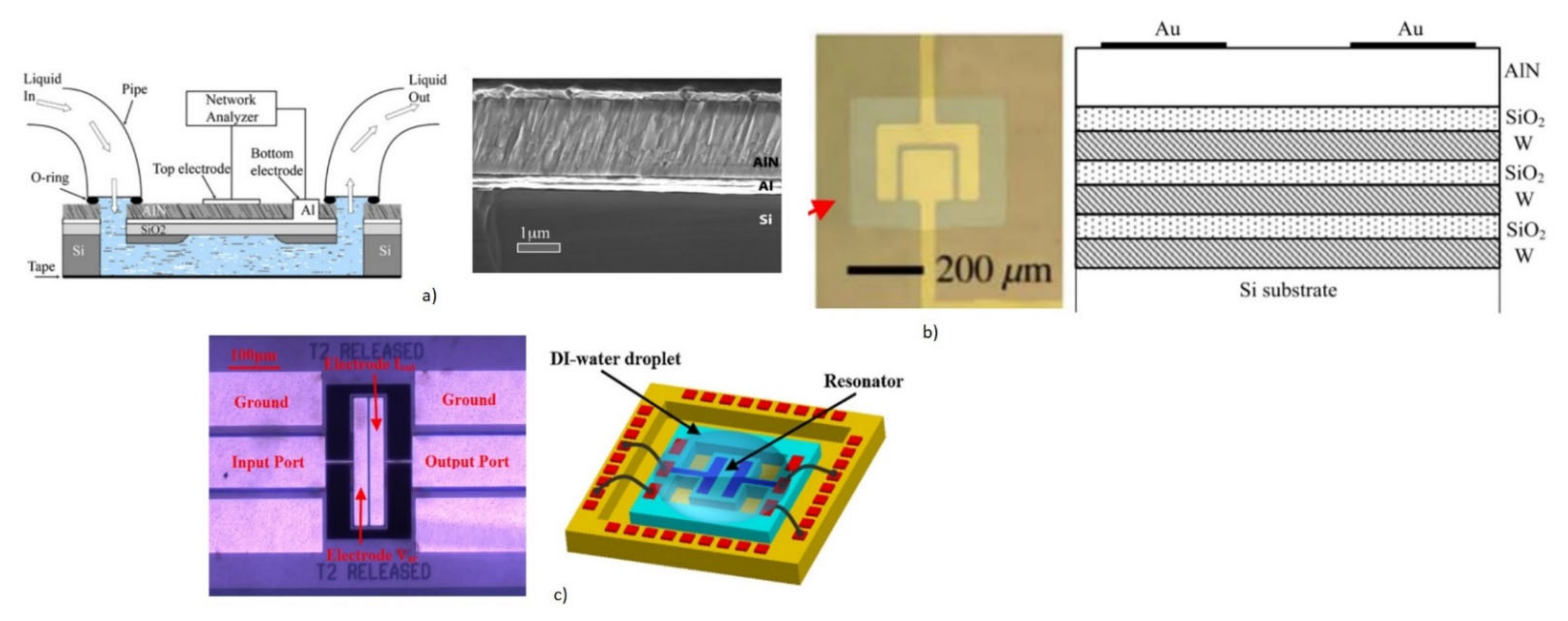

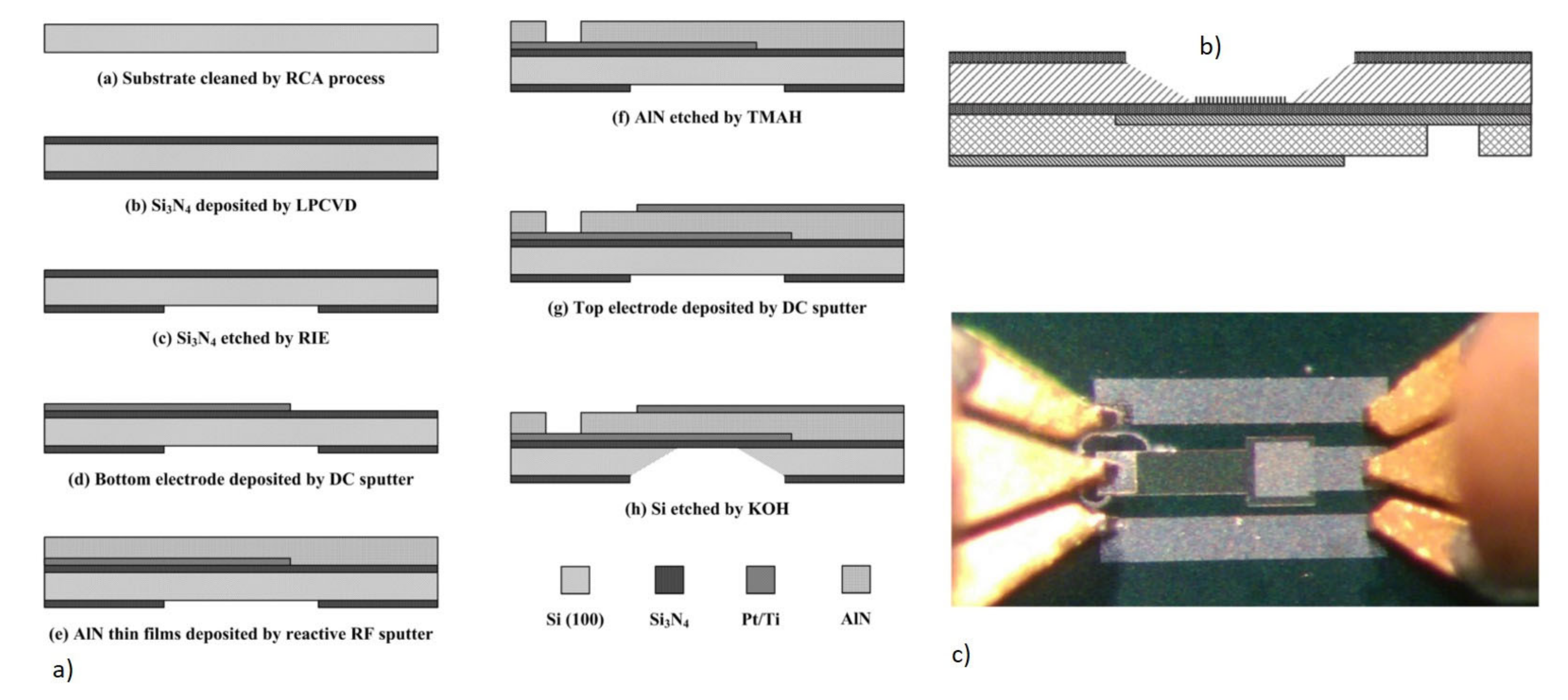


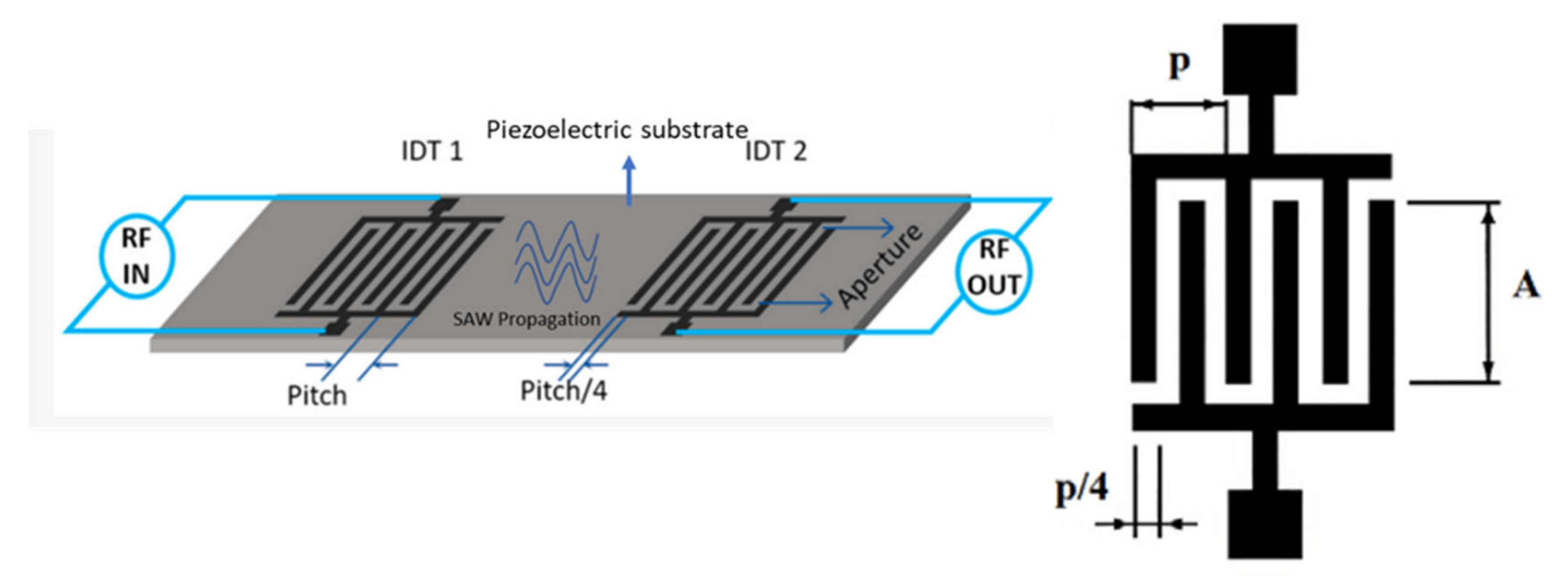






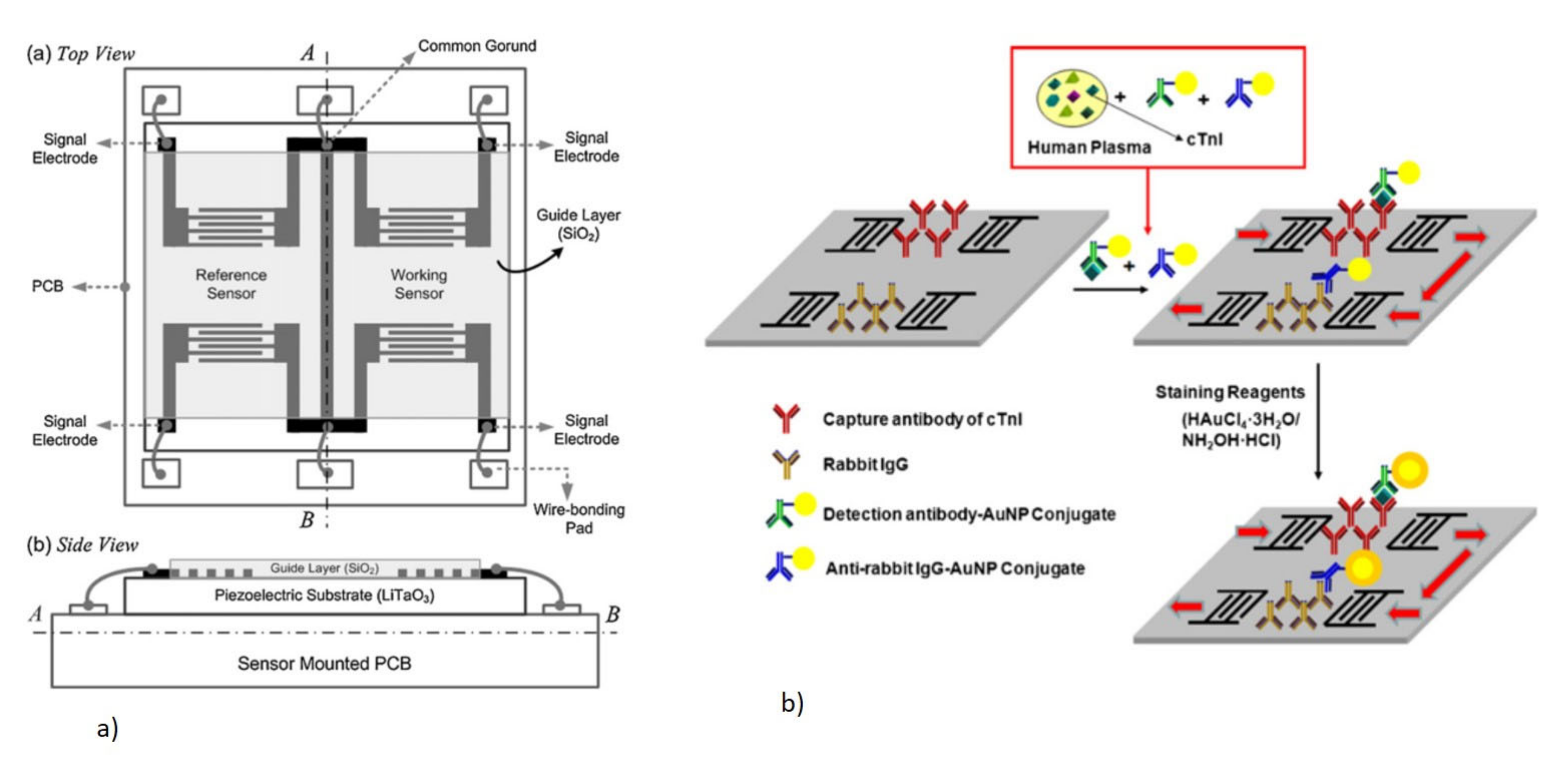
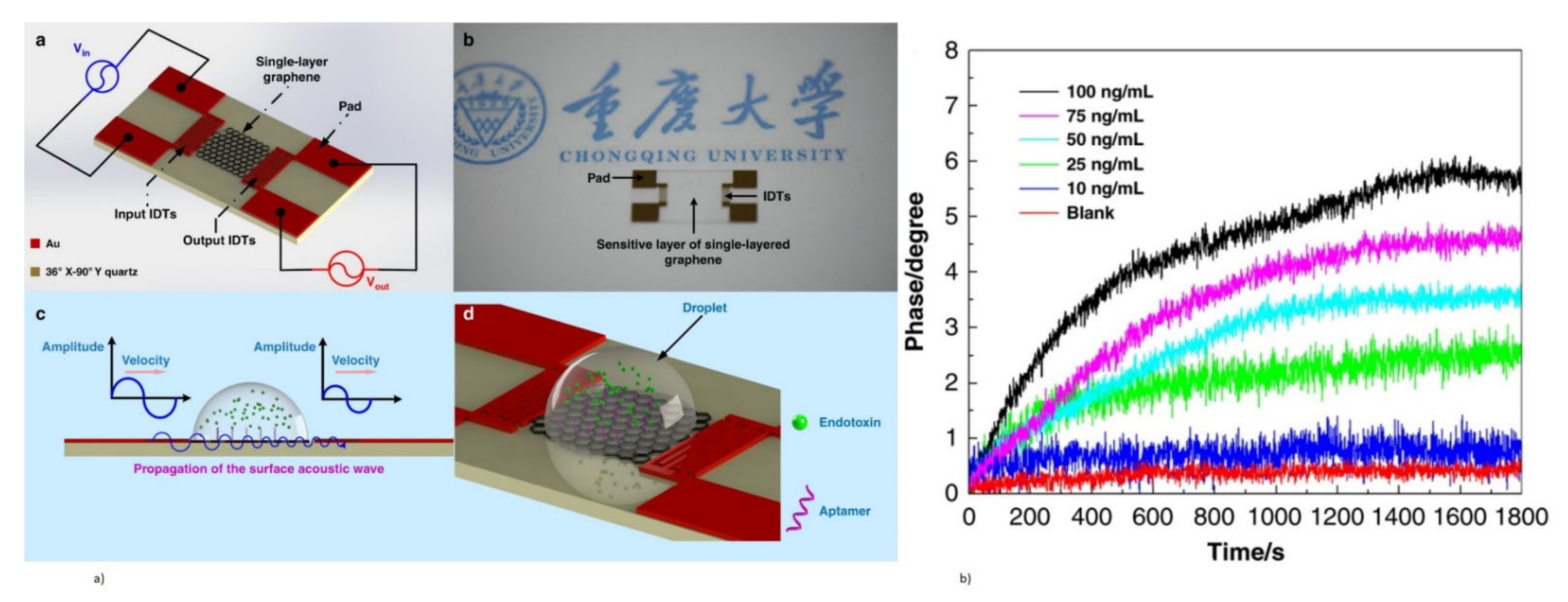





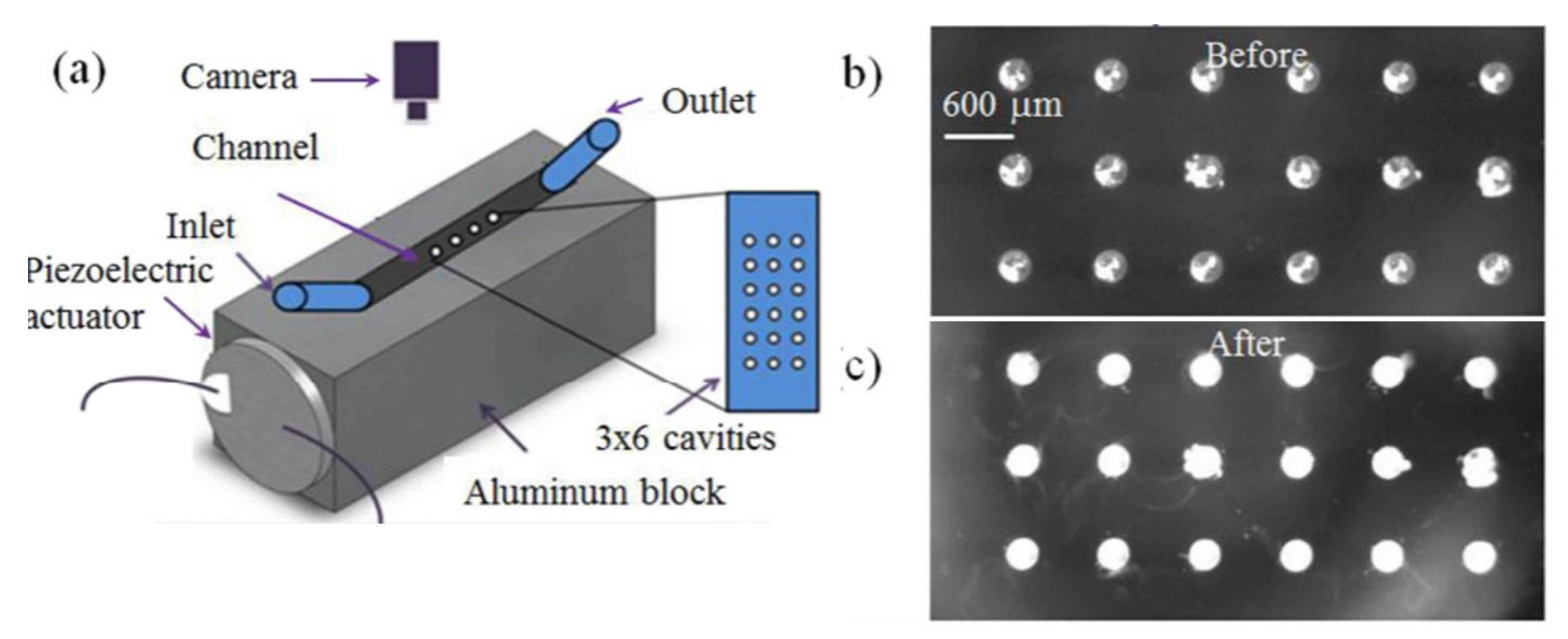
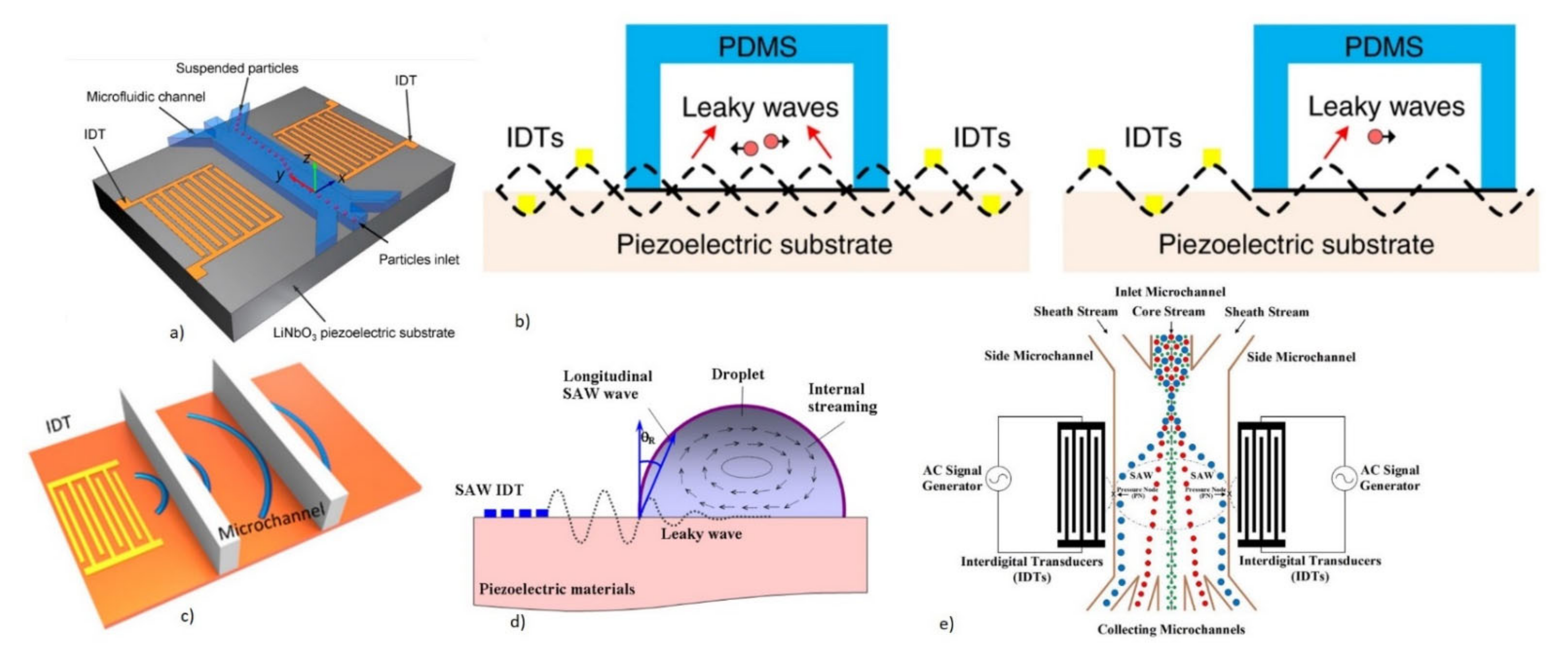
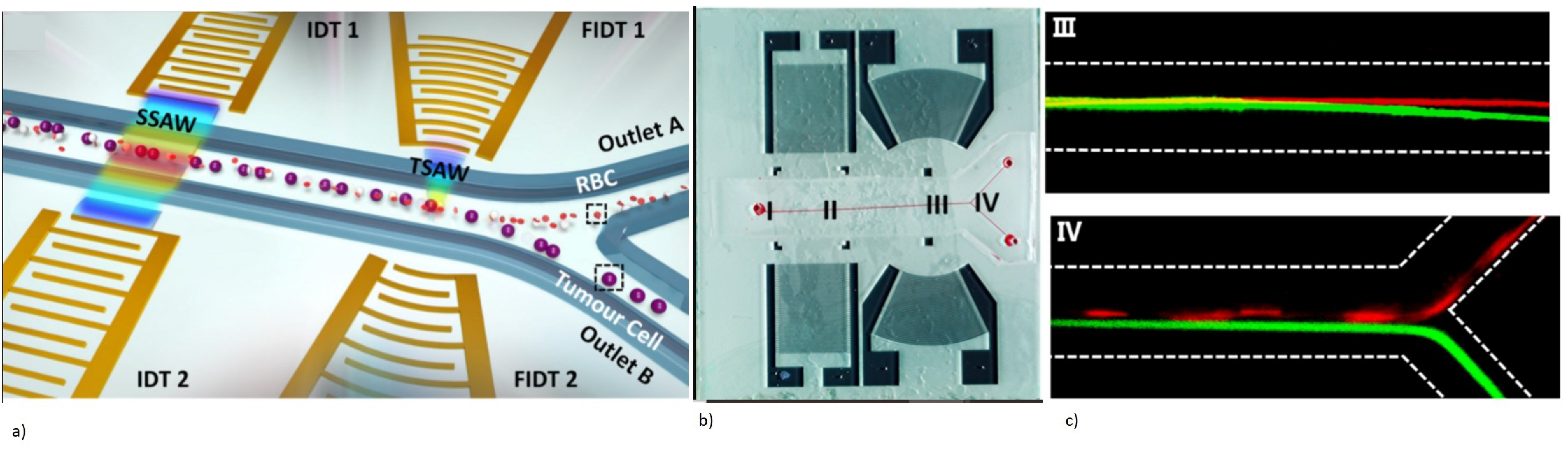
| Substrate | Electromechanical Coupling (%) | Acoustic Wave Velocity (m/s) | TCF (p pM/°C) | Dielectric Constant |
|---|---|---|---|---|
| 64° YX LiNbO3 | 11.0–11.5 | 4330–4742 | 70–81 | 85.2 |
| 36° YX LiTaO3 | 5.0–7.6 | 4100–4212 | 28–35 | 43 |
| X-112° Y cut LiTaO3 | 3300 | 18 | 43 | |
| ST-X Cut Quartz | 0.0016 | 3159 | 0 | 3.8 |
| Langasite (La3Ga5SiO14) | 0.8 | 2734 | 0 | 18.23 |
| ZnO | 1.5–1.7 | 6336 | −40~−60 | 8.66 |
| AlN | 3.1–8 | 11,050 | 19 | 8.5–10 |
| PZT | 20–35 | 4500 | - | 380 |
| PVDF | 2.9 | 2600 | - | 6–8 |
| SAW Mode | Device Configuration and Particle Displacement Profile | Substrate | Guide Layer | Operating Frequency | Substrate Thickness | Application |
|---|---|---|---|---|---|---|
| R-SAW |  | Quartz, LiNbO3, ZnO | NA | 30–300 MHz | >>λ | Gas sensing |
| SH-SAW- Love Mode |  | 36° YX LiTaO3, ST-Quartz, 64° YX LiNbO3 | SiO2, ZnO, PMMA, Photoresist | 30–500 MHz | >>λ | Gas sensing, liquid sensing |
| Lamb Wave (FPW) |  | ZnO, AlN, PZT | NA | 2–20 MHz | <<λ | Gas sensing, liquid sensing |
| SH-A PM |  | ST-Quartz ZX LiNbO3 | NA | 25–200 MHz | 3–10 λ | Gas sensing, liquid sensing |
| Factors | BAW | SAW | |
|---|---|---|---|
| QCM | FBAR | ||
| Advantages |
|
|
|
| Limitations |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, M.P.; Teo, A.J.T.; Li, K.H.H. Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines 2022, 13, 24. https://doi.org/10.3390/mi13010024
Nair MP, Teo AJT, Li KHH. Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines. 2022; 13(1):24. https://doi.org/10.3390/mi13010024
Chicago/Turabian StyleNair, Minu Prabhachandran, Adrian J. T. Teo, and King Ho Holden Li. 2022. "Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications" Micromachines 13, no. 1: 24. https://doi.org/10.3390/mi13010024
APA StyleNair, M. P., Teo, A. J. T., & Li, K. H. H. (2022). Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines, 13(1), 24. https://doi.org/10.3390/mi13010024








