Thermodynamic Modeling of Solvent-Assisted Lipid Bilayer Formation Process
Abstract
:1. Introduction
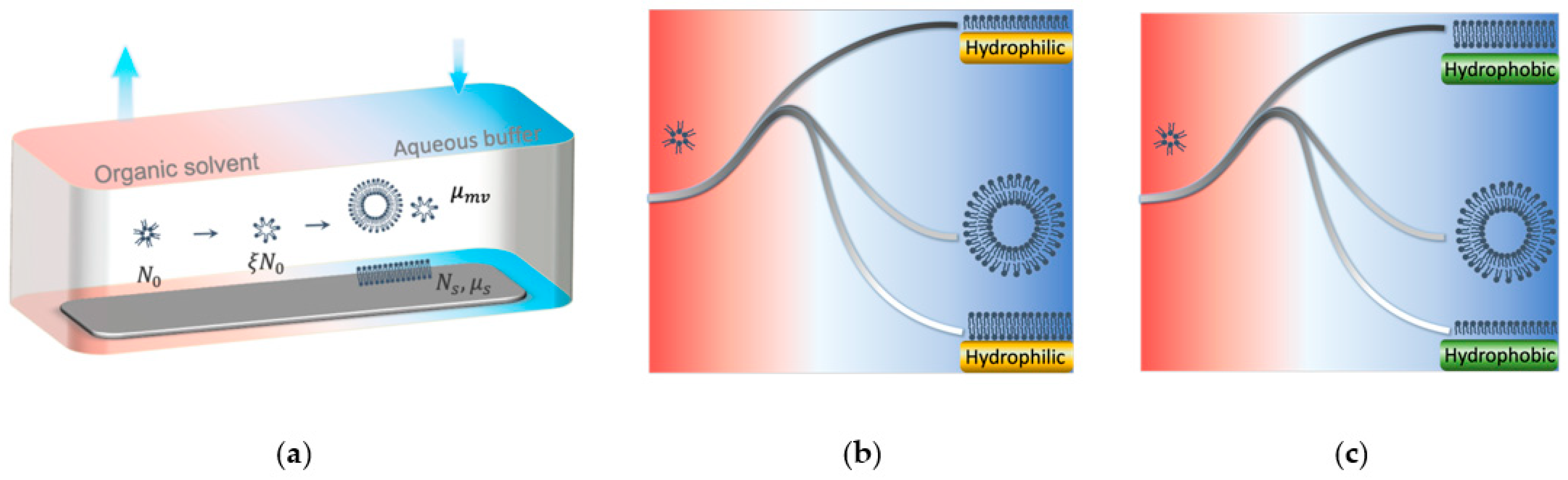
2. SALB Formation–Estimation of Energetics and a Thermodynamics Model
2.1. Estimation of Energetics of SALB Formation
2.2. Thermodynamic Analysis of SALB Formation
3. Results
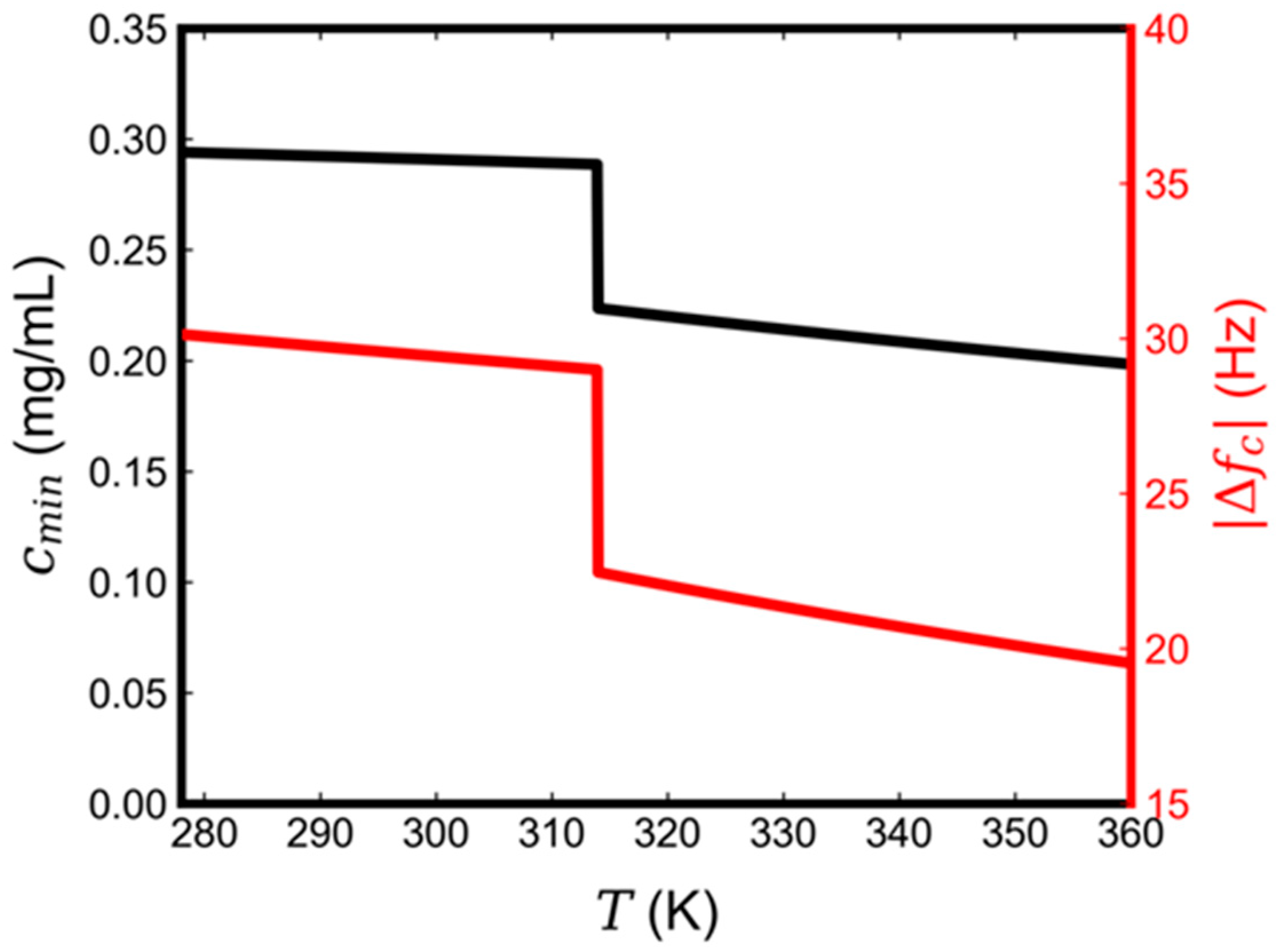
3.1. Lipid Concentration
3.2. Effect of Temperature
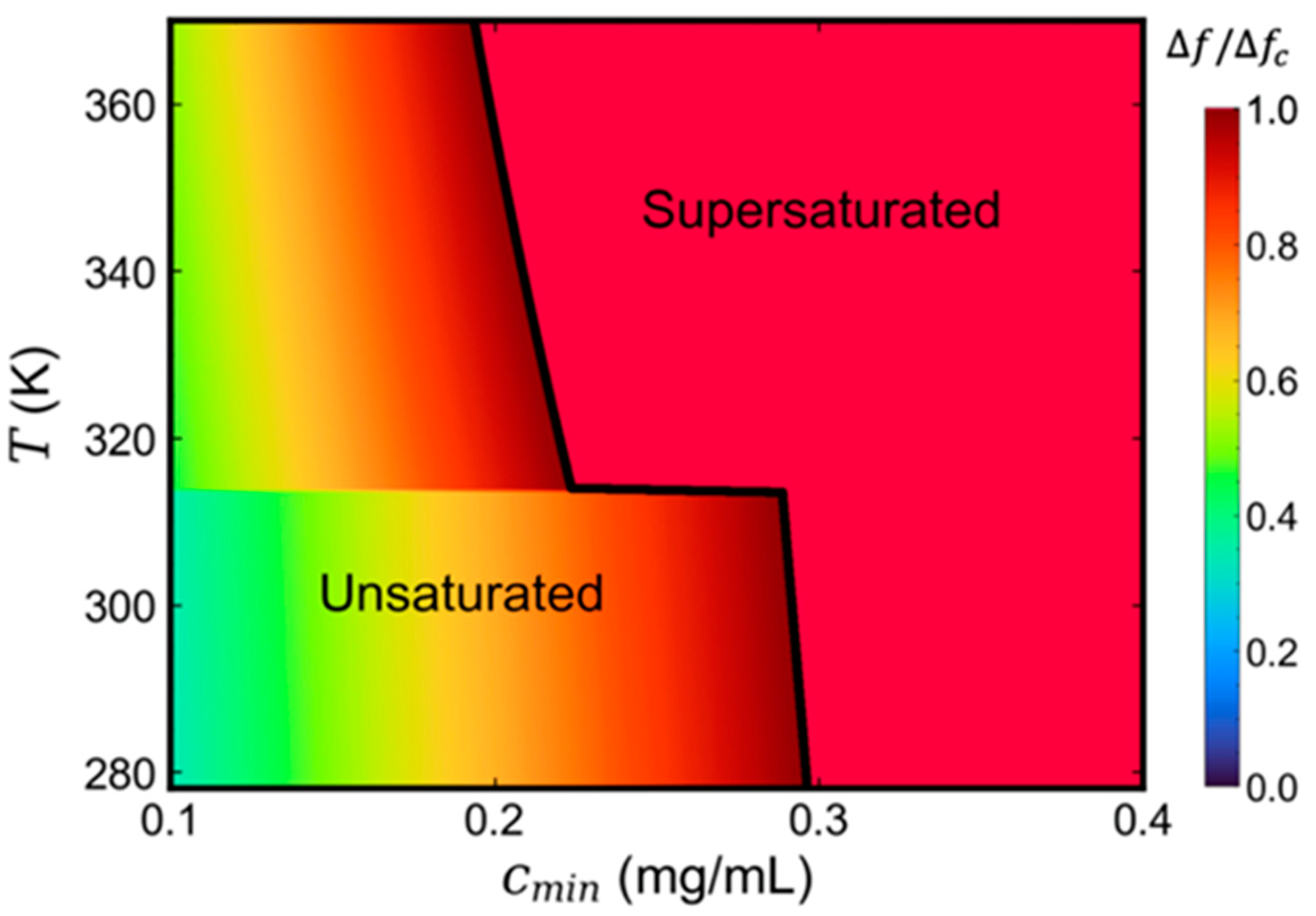
3.3. Phase Diagram Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Chan, Y.-H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, J.A.; Cho, N.J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef]
- Tayebi, L.; Ma, Y.; Vashaee, D.; Chen, G.; Sinha, S.K.; Parikh, A.N. Long-range interlayer alignment of intralayer domains in stacked lipid bilayers. Nat. Mater. 2012, 11, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.; Tang, W.L.; Li, S.D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics 2019, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Sut, T.N.; Yoon, B.K.; Jeon, W.-Y.; Jackman, J.A.; Cho, N.-J. Supported lipid bilayer coatings: Fabrication, bioconjugation, and diagnostic applications. Appl. Mater. Today 2021, 25, 101183. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Thostrup, P.; Rouiller, I.; Lucido, A.L.; Belkaïd, W.; Colman, D.R.; Lennox, R.B. Lipid Bilayer Membrane-Triggered Presynaptic Vesicle Assembly. ACS Chem. Neurosci. 2010, 1, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Siontorou, C.G.; Nikoleli, G.P.; Nikolelis, D.P.; Karapetis, S.K. Artificial Lipid Membranes: Past, Present, and Future. Membranes 2017, 7, 38. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophys J. 1985, 47, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Mennicke, U.; Salditt, T. Preparation of Solid-Supported Lipid Bilayers by Spin-Coating. Langmuir 2002, 18, 8172–8177. [Google Scholar] [CrossRef]
- Kalb, E.; Frey, S.; Tamm, L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta Biomembr. 1992, 1103, 307–316. [Google Scholar] [CrossRef]
- Rinia, H.A.; Demel, R.A.; van der Eerden, J.P.J.M.; de Kruijff, B. Blistering of Langmuir-Blodgett Bilayers Containing Anionic Phospholipids as Observed by Atomic Force Microscopy. Biophys. J. 1999, 77, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Sondhi, P.; Lingden, D.; Stine, K.J. Structure, Formation, and Biological Interactions of Supported Lipid Bilayers (SLB) Incorporating Lipopolysaccharide. Coatings 2020, 10, 981. [Google Scholar] [CrossRef]
- Dols-Perez, A.; Fumagalli, L.; Simonsen, A.C.; Gomila, G. Ultrathin Spin-Coated Dioleoylphosphatidylcholine Lipid Layers in Dry Conditions: A Combined Atomic Force Microscopy and Nanomechanical Study. Langmuir 2011, 27, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.C.; Bagatolli, L.A. Structure of spin-coated lipid films and domain formation in supported membranes formed by hydration. Langmuir 2004, 20, 9720–9728. [Google Scholar] [CrossRef]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Nayar, R.; Hope, M.J.; Cullis, P.R. Generation of large unilamellar vesicles from long-chain saturated phosphatidylcholines by extrusion technique. Biochim. Biophys. Acta Biomembr. 1989, 986, 200–206. [Google Scholar] [CrossRef]
- Reviakine, I.; Rossetti, F.F.; Morozov, A.N.; Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 2005, 122, 204711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, J.A.; Zan, G.H.; Zhdanov, V.P.; Cho, N.J. Rupture of lipid vesicles by a broad-spectrum antiviral peptide: Influence of vesicle size. J. Phys. Chem. B 2013, 117, 16117–16128. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.J. Solvent-assisted preparation of supported lipid bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Vafaei, S.; Cho, N.J. Fabrication of charged membranes by the solvent-assisted lipid bilayer (SALB) formation method on SiO2 and Al2O3. Phys. Chem. Chem. Phys. 2015, 17, 11546–11552. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, M.; Yorulmaz, S.; Vafaei, S.; Cho, N.J. Biomembrane Fabrication by the Solvent-assisted Lipid Bilayer (SALB) Method. J. Vis. Exp. 2015, 106, 53073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, J.A.; Tabaei, S.R.; Zhao, Z.; Yorulmaz, S.; Cho, N.J. Self-assembly formation of lipid bilayer coatings on bare aluminum oxide: Overcoming the force of interfacial water. ACS Appl. Mater. Interfaces 2015, 7, 959–968. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.O.; Liedberg, B.; Knoll, W.; Parikh, A.N.; Cho, N.J. Formation of cholesterol-rich supported membranes using solvent-assisted lipid self-assembly. Langmuir 2014, 30, 13345–13352. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Liedberg, B.; Parikh, A.N.; Cho, N.J. Observation of Stripe Superstructure in the β-Two-Phase Coexistence Region of Cholesterol-Phospholipid Mixtures in Supported Membranes. J. Am. Chem. Soc. 2014, 136, 16962–16965. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Jackman, J.A.; Kim, S.-O.; Zhdanov, V.P.; Cho, N.-J. Solvent-Assisted Lipid Self-Assembly at Hydrophilic Surfaces: Factors Influencing the Formation of Supported Membranes. Langmuir 2015, 31, 3125–3134. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, J.J.; Tabaei, S.R.; Cho, N.J. A phenomenological model of the solvent-assisted lipid bilayer formation method. Phys. Chem. Chem. Phys. 2016, 18, 24157–24163. [Google Scholar] [CrossRef] [Green Version]
- Tabaei, S.R.; Choi, J.H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.J. Solvent-assisted lipid bilayer formation on silicon dioxide and gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef]
- Efremov, A.; Mauro, J.C.; Raghavan, S. Macroscopic model of phospholipid vesicle spreading and rupture. Langmuir 2004, 20, 5724–5731. [Google Scholar] [CrossRef] [PubMed]
- Arsene, M.-L.; Răut, I.; Călin, M.; Jecu, M.-L.; Doni, M.; Gurban, A.-M. Versatility of Reverse Micelles: From Biomimetic Models to Nano (Bio)Sensor Design. Processes 2021, 9, 345. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.J.; Tassieri, M.; Reboud, J.; Wilson, R.; Cooper, J.M. Lipid Topology and Electrostatic Interactions Underpin Lytic Activity of Linear Cationic Antimicrobial Peptides in Membranes. Proc. Natl. Acad. Sci. USA 2017, 114, E8324–E8332. [Google Scholar] [CrossRef] [Green Version]
- Wnętrzak, A.; Łątka, K.; Dynarowicz-Łątka, P. Interactions of Alkylphosphocholines with Model Membranes—The Langmuir Monolayer Study. J. Membr. Biol. 2013, 246, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Czolkos, I.; Hakonen, B.; Orwar, O.; Jesorka, A. High-Resolution Micropatterned Teflon AF Substrates for Biocompatible Nanofluidic Devices. Langmuir 2012, 28, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Erkan, Y.; Halma, K.; Czolkos, I.; Jesorka, A.; Dommersnes, P.; Kumar, R.; Brown, T.; Orwar, O. Controlled Release of Chol-TEG-DNA from Nano- and Micropatterned SU-8 Surfaces by a Spreading Lipid Film. Nano Lett. 2008, 8, 227–231. [Google Scholar] [CrossRef]
- Janshoff, A.; Galla, H.J.; Steinem, C. Piezoelectric Mass-Sensing Devices as Biosensors-An Alternative to Optical Biosensors? Angew. Chem. Int. Ed. Engl. 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Edvardsson, M.; Svedhem, S.; Wang, G.; Richter, R.; Rodahl, M.; Kasemo, B. QCM-D and Reflectometry Instrument: Applications to Supported Lipid Structures and Their Biomolecular Interactions. Anal. Chem. 2009, 81, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Wilke, N. Energetics of the Phase Transition in Free-Standing versus Supported Lipid Membranes. J. Phys. Chem. B 2015, 119, 8718–8724. [Google Scholar] [CrossRef]
- Marsh, D. Equation of State for Phospholipid Self-Assembly. Biophys. J. 2016, 110, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Pinto, O.A.; Disalvo, E.A. A new model for lipid monolayer and bilayers based on thermodynamics of irreversible processes. PLoS ONE 2019, 14, e0212269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braganza, L.F.; Worcester, D.L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry 1986, 25, 2591–2596. [Google Scholar] [CrossRef]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Eicher, B.; Marquardt, D.; Heberle, F.A.; Letofsky-Papst, I.; Rechberger, G.N.; Appavou, M.S.; Katsaras, J.; Pabst, G. Intrinsic Curvature-Mediated Transbilayer Coupling in Asymmetric Lipid Vesicles. Biophys J. 2018, 114, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leekumjorn, S.; Sum, A.K. Molecular studies of the gel to liquid-crystalline phase transition for fully hydrated DPPC and DPPE bilayers. Biochim. Biophys. Acta 2007, 1768, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Tae, H.; Cho, N.-J.; Huang, C.; Hsia, K.J. Thermodynamic Modeling of Solvent-Assisted Lipid Bilayer Formation Process. Micromachines 2022, 13, 134. https://doi.org/10.3390/mi13010134
Xu H, Tae H, Cho N-J, Huang C, Hsia KJ. Thermodynamic Modeling of Solvent-Assisted Lipid Bilayer Formation Process. Micromachines. 2022; 13(1):134. https://doi.org/10.3390/mi13010134
Chicago/Turabian StyleXu, Hongmei, Hyunhyuk Tae, Nam-Joon Cho, Changjin Huang, and K. Jimmy Hsia. 2022. "Thermodynamic Modeling of Solvent-Assisted Lipid Bilayer Formation Process" Micromachines 13, no. 1: 134. https://doi.org/10.3390/mi13010134
APA StyleXu, H., Tae, H., Cho, N.-J., Huang, C., & Hsia, K. J. (2022). Thermodynamic Modeling of Solvent-Assisted Lipid Bilayer Formation Process. Micromachines, 13(1), 134. https://doi.org/10.3390/mi13010134







