Non-Cavitation Targeted Microbubble-Mediated Single-Cell Sonoporation
Abstract
:1. Introduction
2. Materials and Methods
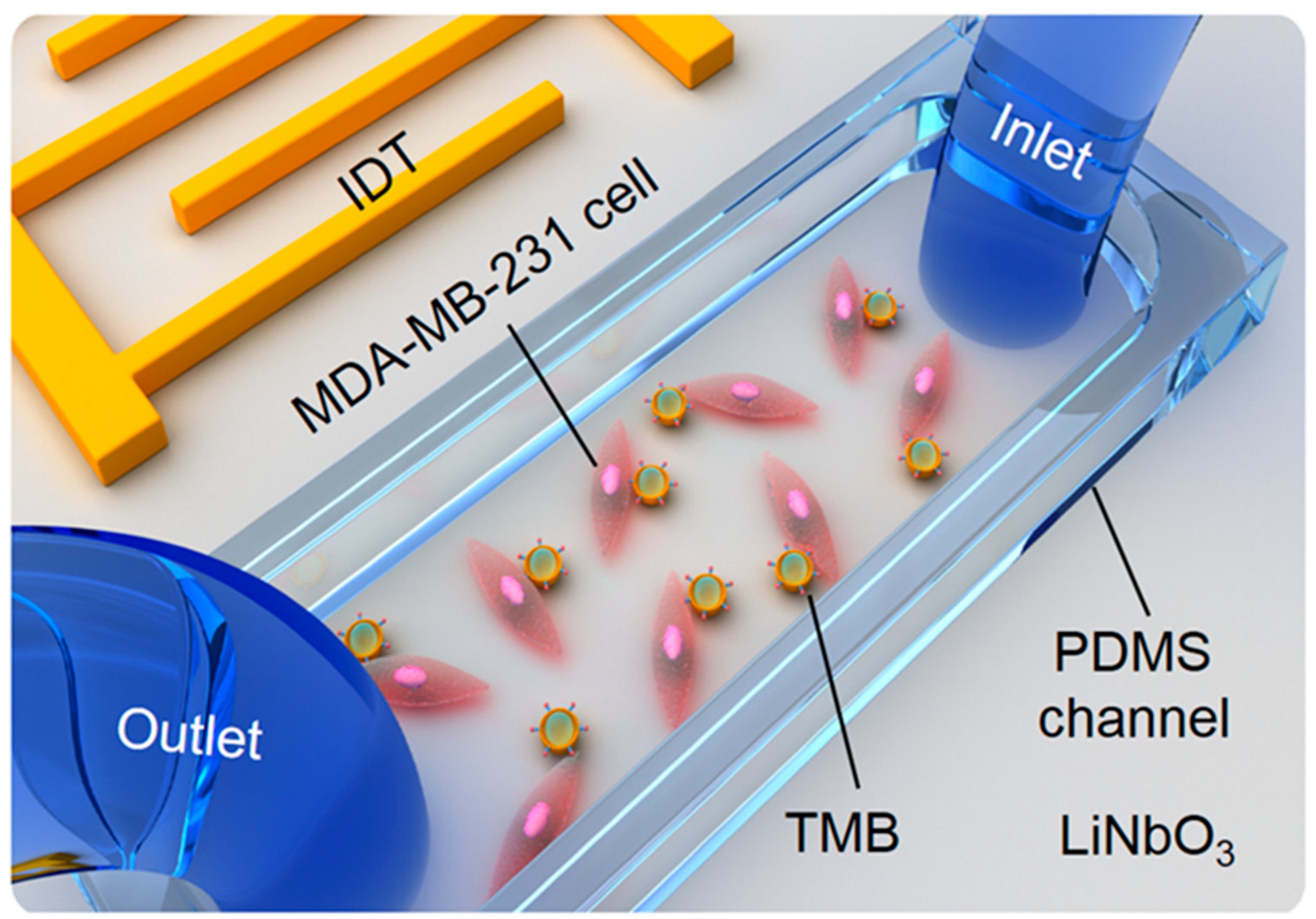
2.1. The Fabrication of the TSAW Device
2.2. Cell Culture in the PDMS Channel
2.3. Adhesion of Cells and TMBs
2.4. Cell Sonoporation
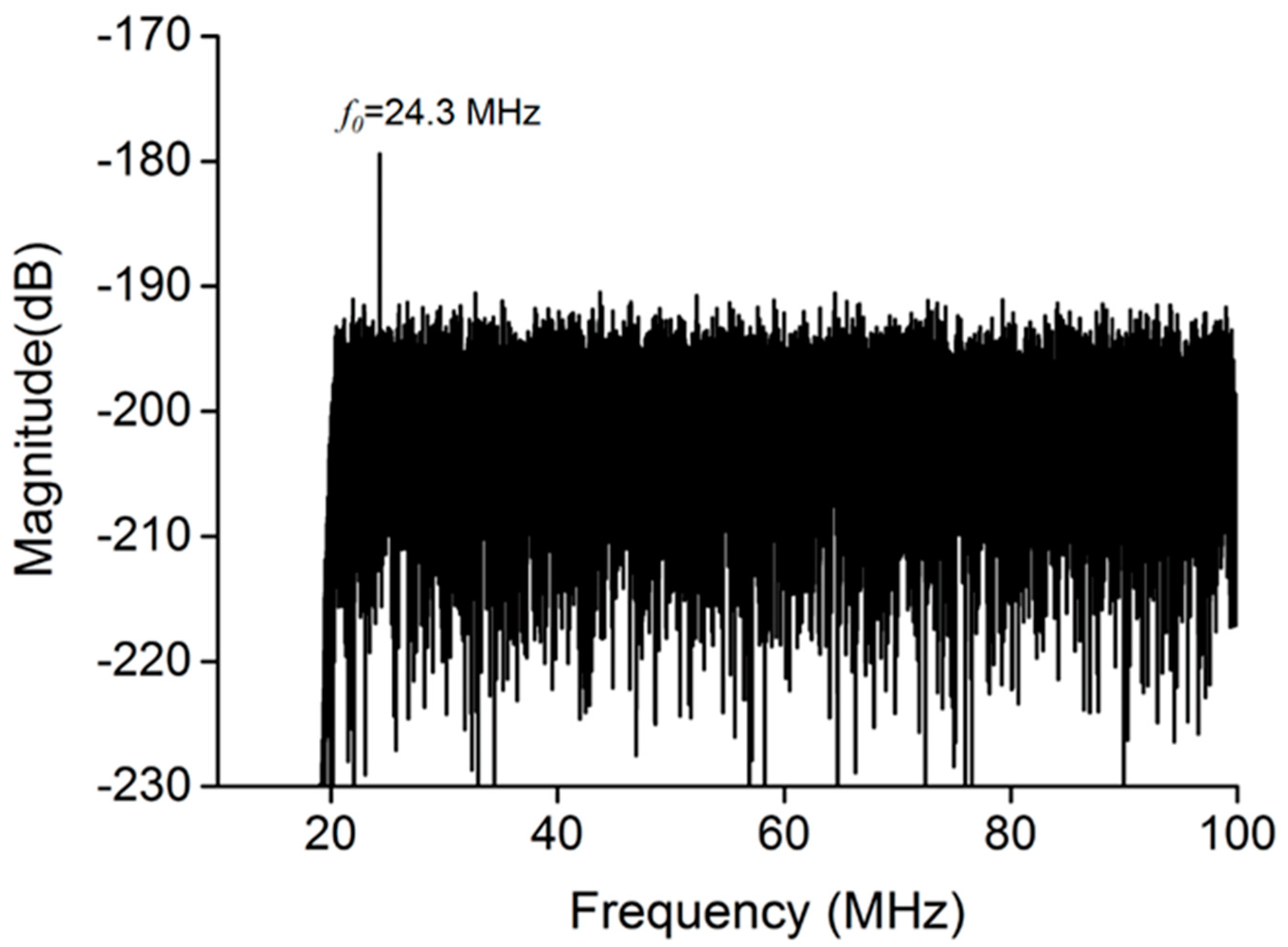
2.5. Bubble Cavitation Detection
2.6. Statistical Analysis
3. Results and Discussion
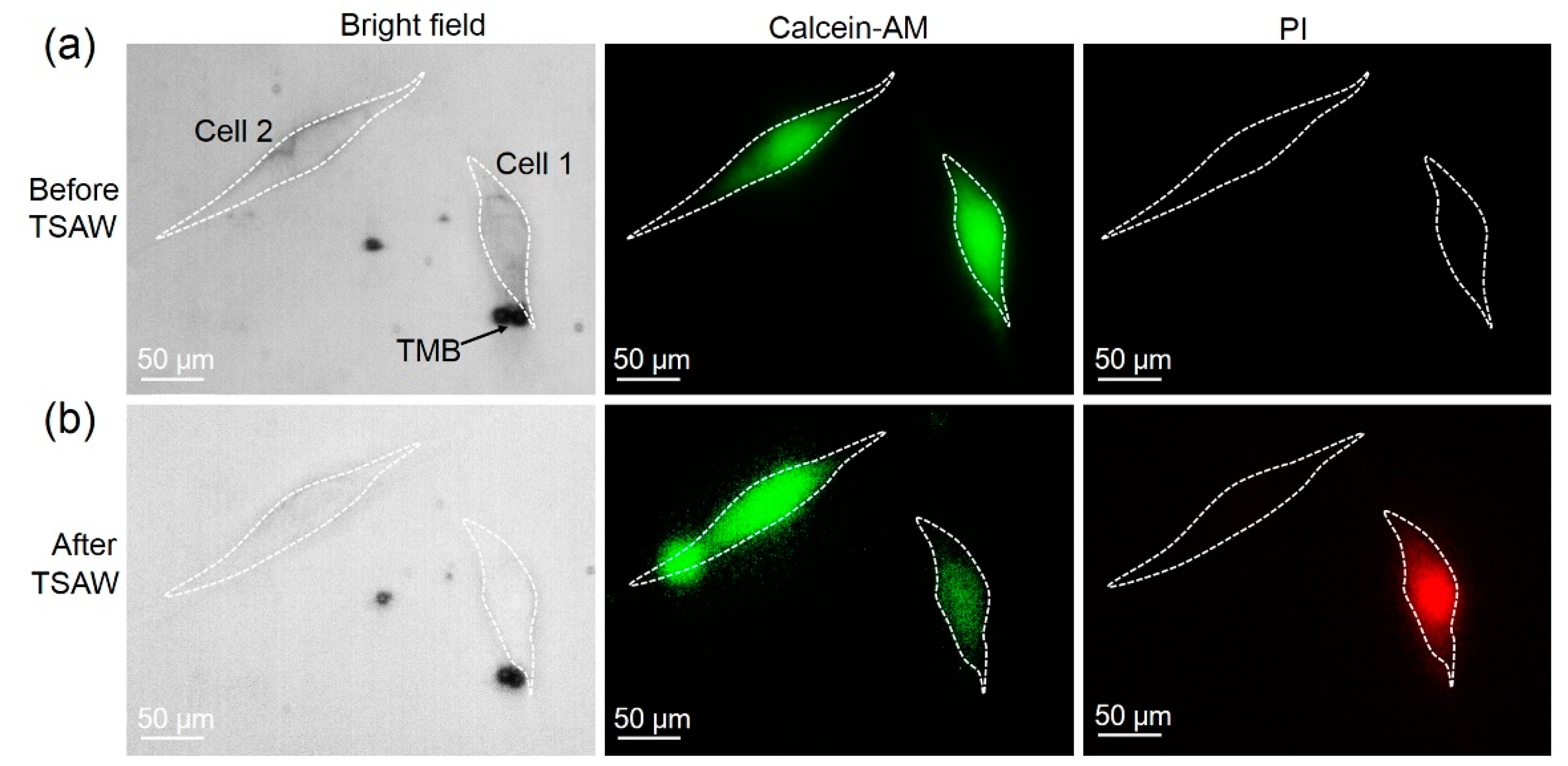
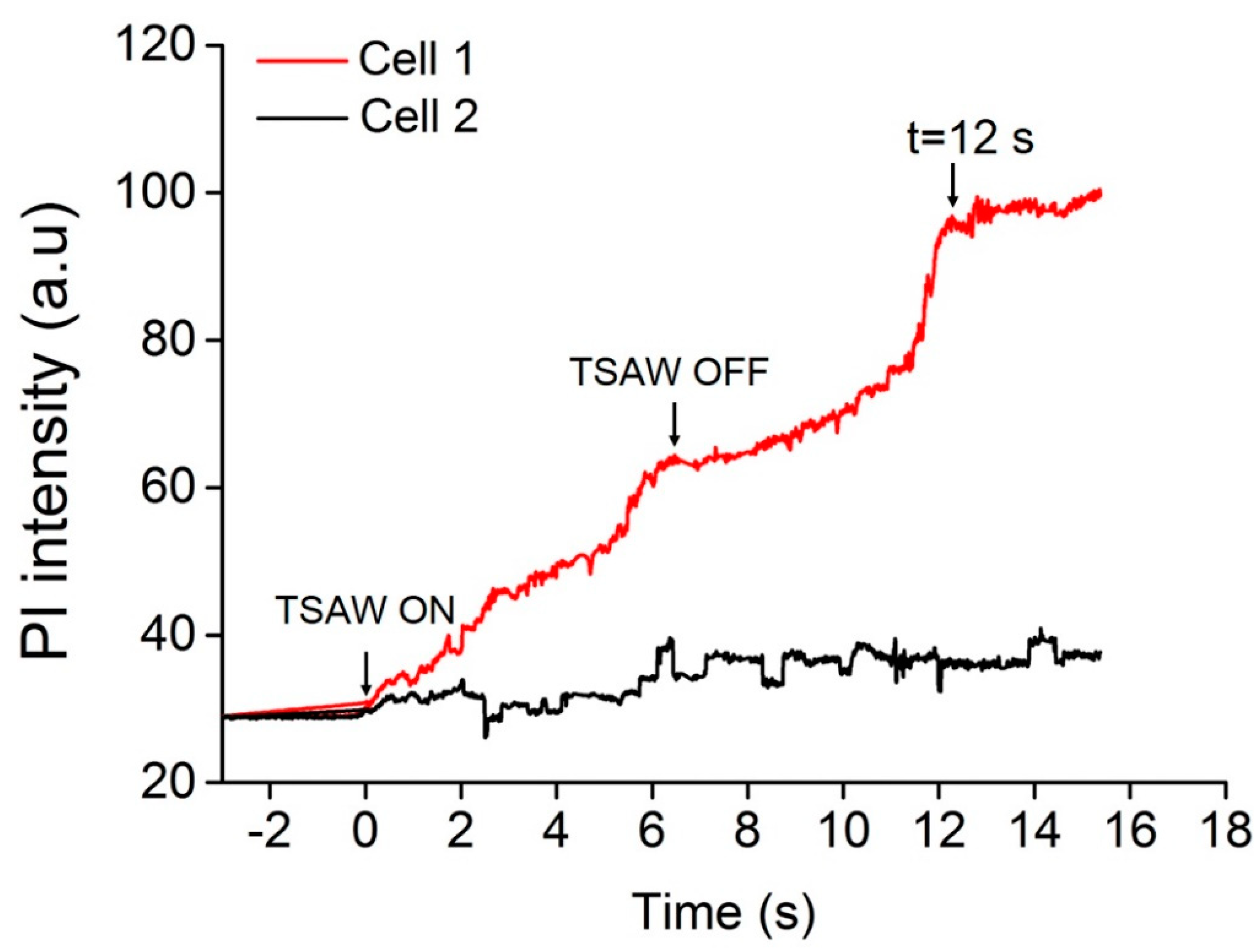
3.1. Sonoporation at a Single-Cell Level
3.2. TMBs’ Translational Movement Induced by the ARF
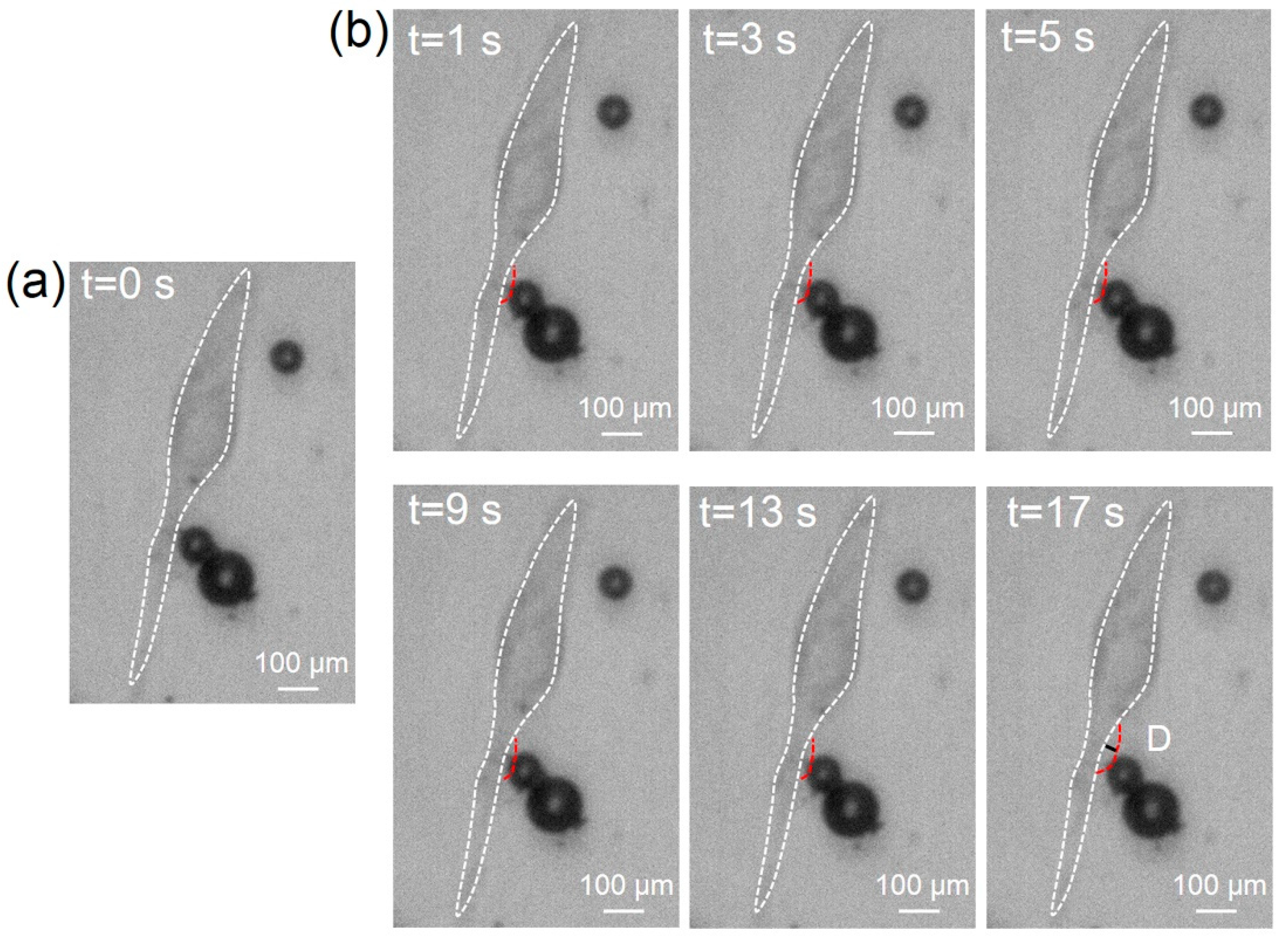
3.3. Cell Sonoporation Induced by Cell Membrane Deformation
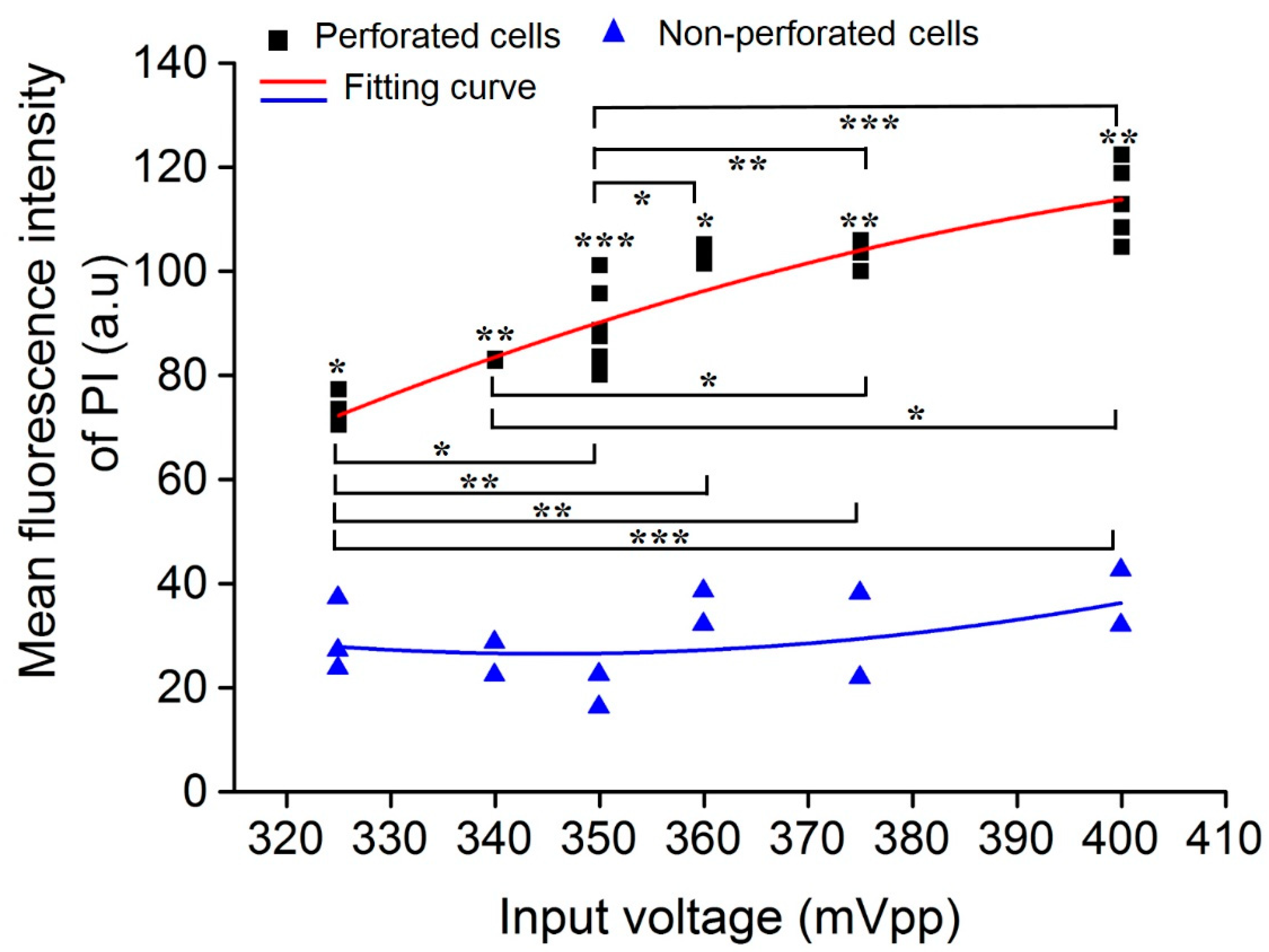
3.4. Impact of Acoustic Input Voltage on Cell Sonoporation
3.5. Impact of TMB Number on Cell Sonoporation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stewart, M.P.; Sharei, A.R.; Ding, X.S.; Sahay, G.; Langer, R.S.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef] [Green Version]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waleed, M.; Hwang, S.-U.; Kim, J.-D.; Shabbir, I.; Shin, S.-M.; Lee, Y.-G. Single-cell optoporation and transfection using femtosecond laser and optical tweezers. Biomed. Opt. Express 2013, 4, 1533–1547. [Google Scholar] [CrossRef]
- Sharei, A.; Cho, N.; Mao, S.; Jackson, E.; Poceviciute, R.; Adamo, A.; Zoldan, J.; Langer, R.; Jensen, K.F. Cell Squeezing as a Robust, Microfluidic Intracellular Delivery Platform. J. Vis. Exp. 2013, 81, e50980. [Google Scholar] [CrossRef]
- Xing, R.; Liu, G.; Zhu, J.; Hou, Y.; Chen, X. Functional Magnetic Nanoparticles for Non-Viral Gene Delivery and MR Imaging. Pharm. Res. 2013, 31, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, H.; Kim, B.; Kim, M.K.; Wie, D.S.; Lee, H.S.; Kim, D.R.; Lee, C.H. Flexible elastomer patch with vertical silicon nanoneedles for intracellular and intratissue nanoinjection of biomolecules. Sci. Adv. 2018, 4, eaau6972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. USA 2012, 109, 16486–16491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaladharan, K.; Kumar, A.; Gupta, P.; Illath, K.; Santra, T.; Tseng, F.-G. Microfluidic Based Physical Approaches towards Single-Cell Intracellular Delivery and Analysis. Micromachines 2021, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control. Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Meng, L.; Cai, F.; Jiang, P.; Deng, Z.; Li, F.; Niu, L.; Chen, Y.; Wu, J.; Zheng, H. On-chip targeted single cell sonoporation with microbubble destruction excited by surface acoustic waves. Appl. Phys. Lett. 2014, 104, 073701. [Google Scholar] [CrossRef]
- Kooiman, K.; Foppen-Harteveld, M.; De Jong, N. Sonoporation of endothelial cells with CD31-targeted microbubbles at low acoustic pressures. In Proceedings of the 2009 IEEE International Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 13–15. [Google Scholar]
- van Wamel, A.; Kooiman, K.; Harteveld, M.; Emmer, M.; Cate, F.T.; Versluis, M.; de Jong, N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J. Control. Release 2006, 112, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L. Sonoporation of cells by a parallel stable cavitation microbubble array. Adv. Sci. 2019, 6, 1900557. [Google Scholar] [CrossRef] [Green Version]
- Horsley, H.; Owen, J.; Browning, R.; Carugo, D.; Malone-Lee, J.; Stride, E.; Rohn, J. Ultrasound-activated microbubbles as a novel intracellular drug delivery system for urinary tract infection. J. Control. Release 2019, 301, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, T.; Skachkov, I.; Beekers, I.; Lattwein, K.R.; Voorneveld, J.D.; Kokhuis, T.J.; Bera, D.; Luan, Y.; van der Steen, A.F.; de Jong, N.; et al. Viability of endothelial cells after ultrasound-mediated sonoporation: Influence of targeting, oscillation, and displacement of microbubbles. J. Control. Release 2016, 238, 197–211. [Google Scholar] [CrossRef]
- Kooiman, K.; Foppen-Harteveld, M.; van der Steen, A.F.; de Jong, N. Sonoporation of endothelial cells by vibrating targeted microbubbles. J. Control. Release 2011, 154, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Cai, F.; Li, F.; Zhou, W.; Niu, L.; Zheng, H. Acoustic tweezers. J. Phys. D. Appl. Phys. 2019, 52, 273001. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647–704. [Google Scholar] [CrossRef] [Green Version]
- Dayton, P.A.; Allen, J.S.; Ferrara, K.W. The magnitude of radiation force on ultrasound contrast agents. J. Acoust. Soc. Am. 2002, 112, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, K.; Cui, J.; Ye, J.; Deng, C. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release 2012, 157, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Carugo, D.; Ankrett, D.N.; Glynnejones, P.; Capretto, L.; Boltryk, R.J.; Zhang, X.; Townsend, P.A.; Hill, M. The use of ultrasonic standing wave microfluid system for the delivery of pharmaceutical agents. Biomicrofluidics 2011, 5, 044108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Cai, F.; Jin, Q.; Niu, L.; Jiang, C.; Wang, Z.; Wu, J.; Zheng, H. Acoustic aligning and trapping of microbubbles in an enclosed PDMS microfluidic device. Sens. Actuators B Chem. 2011, 160, 1599–1605. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Farooq, U.; Haider, Z.; Liang, X.M.; Memon, K.; Hossain, S.M.C.; Zheng, Y.; Xu, H.; Qadir, A.; Panhwar, F.; Dong, S.; et al. Surface-Acoustic-Wave-Based Lab-on-Chip for Rapid Transport of Cryoprotectants across Cell Membrane for Cryopreservation with Significantly Improved Cell Viability. Small 2019, 15, e1805361. [Google Scholar] [CrossRef]
- Farooq, U.; Liu, Y.; Li, P.; Deng, Z.; Liu, X.; Zhou, W.; Yi, S.; Rong, N.; Meng, L.; Niu, L.; et al. Acoustofluidic dynamic interfacial tensiometry. J. Acoust. Soc. Am. 2021, 150, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, S.; Zwaan, E.B.; Ohl, C.-D. Sonoporation of suspension cells with a single cavitation bubble in a microfluidic confinement. Lab Chip 2007, 7, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Zhang, L.; Huang, X.; Farooq, U.; Pang, N.; Zhou, W.; Qi, L.; Xu, L.; Niu, L.; et al. Cell Lysis Based on an Oscillating Microbubble Array. Micromachines 2020, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Sun, Y.; Mao, Y.; Wu, M.; Deng, Z.; Li, S.; Liu, X.; Xue, L.; Zheng, H. Ultrasound Molecular Imaging of Atherosclerosis for Early Diagnosis and Therapeutic Evaluation through Leucocyte-like Multiple Targeted Microbubbles. Theranostics 2018, 8, 1879–1891. [Google Scholar] [CrossRef]
- Yan, F.; Li, L.; Deng, Z.; Jin, Q.; Chen, J.; Yang, W.; Yeh, C.-K.; Wu, J.; Shandas, R.; Liu, X.; et al. Paclitaxel-liposome–microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers—ScienceDirect. J. Control. Release 2013, 166, 246–255. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, W.; Jing, Y.; Yi, S.; Farooq, U.; Shi, J.; Pang, N.; Rong, N.; Xu, L. Non-Cavitation Targeted Microbubble-Mediated Single-Cell Sonoporation. Micromachines 2022, 13, 113. https://doi.org/10.3390/mi13010113
Liu X, Zhang W, Jing Y, Yi S, Farooq U, Shi J, Pang N, Rong N, Xu L. Non-Cavitation Targeted Microbubble-Mediated Single-Cell Sonoporation. Micromachines. 2022; 13(1):113. https://doi.org/10.3390/mi13010113
Chicago/Turabian StyleLiu, Xiufang, Wenjun Zhang, Yanshu Jing, Shasha Yi, Umar Farooq, Jingyao Shi, Na Pang, Ning Rong, and Lisheng Xu. 2022. "Non-Cavitation Targeted Microbubble-Mediated Single-Cell Sonoporation" Micromachines 13, no. 1: 113. https://doi.org/10.3390/mi13010113
APA StyleLiu, X., Zhang, W., Jing, Y., Yi, S., Farooq, U., Shi, J., Pang, N., Rong, N., & Xu, L. (2022). Non-Cavitation Targeted Microbubble-Mediated Single-Cell Sonoporation. Micromachines, 13(1), 113. https://doi.org/10.3390/mi13010113






