Abstract
The increasingly widespread use of engineered nanoparticles in medical, industrial, and food applications has raised concerns regarding their potential toxicity to humans and the environment. Silicon dioxide nanoparticles (SiO2 NPs), which have relatively low direct toxicity, have been increasingly applied in both consumer products and biomedical applications, leading to significantly higher exposure for humans and the environment. We carried out a toxicity assessment of SiO2 NPs using the common water flea D. magna by focusing on physiological and behavioral indicators such as heart rate, swimming performance, and growth. Exposure to SiO2 NPs did not produce acute or chronic toxicity at limited concentrations (<100 μg/mL), but did have statistically significant negative effects on heart rate, swimming distance, and body size. The use of fluorescein isothiocyanate in a silica matrix allowed the tracing and visualization of clear SiO2 NP accumulation in D. magna, which was confirmed by ICP-MS. Although exposure to SiO2 NPs seemed to affect cardiac and swimming performance, such end-point experiments may be insufficient to fully understand the toxicity of these nanoparticles. However, the physiological and behavioral changes shown here suggest potential adverse effects on the aquatic environment by substances previously considered nontoxic.
1. Introduction
Engineered nanoparticles have been used in a broad range of fields due to their exceptional advantages compared to conventional bulk materials. Their diverse physicochemical, mechanical, and biocompatible properties have led to a tremendous increase in their production and usage [1,2,3]. Silicon dioxide nanostructures are among the most commonly produced nanoparticles globally due to their benefits, low toxicity and cost, and ease of production. Globally, ~1.5 million t of silicon nanoparticles (SiO2 NPs) have been produced, making them one of the most produced nanomaterials since 2013 [4,5,6]. These materials are widely used in consumer products such as ceramics, glass, medicine, and cosmetics, but have been extended to biomedical applications given their stabilization properties and low toxicity [7,8]. Given that the possibility of exposure to humans and leakage into the environment has increased significantly, their potential for environmental damage or adverse effects should also be considered with respect to their interactions with living organisms. Although the potential toxic effects on human health have been aggressively explored by in vitro and in vivo studies [9,10,11], toxicological research on environmental effects is lacking, despite growing exposure to various ecosystems.
In the last two decades, researchers have consistently assessed the aquatic toxicity of SiO2 NPs using key parameters such as size, function, and morphology [12], which are considered crucial factors because of their potential roles in toxicity. Surface modification techniques, such as functionalized and coated nanoparticles, give rise to different properties through covalent bonds and ionic coordination, resulting in alterations in toxicity [13,14,15]. Particle size is also an important factor in understanding toxicity because the surface-to-volume ratio and surface reactivity increase as particle size decreases. Previous studies have demonstrated that a decrease in size induces greater toxicity [16,17]. A wide range of particle sizes have been found absorbed into the gastrointestinal system of D. magna, a water flea widely used for aquatic toxicology tests, while nanoparticles have been observed in almost all tissues of this species [18]. In particular, similar size-dependent effects on immobilization and mortality have also been reported for titanium-, aluminum-, copper-, and other metal-based nanoparticles [19,20]. From a morphological perspective, shape-based toxicity assessments have received less attention than those of other key parameters, and these have tended to focus on amorphous forms given their usage and toxicity, such that pristine SiO2 NPs (e.g., spherical, nonporous, and unfunctionalized) have received less research focus than functionalized amorphous nanoparticles [21,22]. However, spherical SiO2 NPs should not be overlooked in toxicity assessments, as new possibilities for their use and production are being reported in a variety of fields [23,24].
As a test subject for biological laboratory studies, D. magna has many benefits, including high sensitivity to the surrounding environment, economical handling, and short lifespan for monitoring from neonates to adults [25]. D. magna is a key species with regard to its geographical distribution and role in the food webs of freshwater ecosystems, which elevates its importance as a key species in ecotoxicology [26]. Additionally, its transparency allows the observation of optical parameters efficiently via noninvasive methods [27]. Given these advantages, acute and reproductive toxicity tests for the use of D. magna as an environmental testing organism are well-established by the Organisation for Economic Co-operation and Development (OECD) and International Organization for Standardization (ISO) [28]. Furthermore, behavioral and physiological changes in D. magna have been reported with respect to the toxicity of various substances under sublethal concentrations [29,30,31,32]. For example, swimming performance and heart rate have been used to test for the effects of drugs, chemicals, and particles.
In this study, we observed physiological, behavioral, and chronological changes in D. magna to better understand the effects of SiO2 NPs at below sublethal concentrations, and used tracing to determine the relationship between SiO2 NPs accumulation and changes in D. magna. To enable visualization, fluorescein 5 (6)-isothiocyanate- (FITC) molecules were incorporated into the SiO2 NPs.
2. Materials and Methods
2.1. D. magna Culture
D. magna from ephippia were purchased from Micro Biotests Inc. (Belgium) and hatched under a 16/8 h light/dark regime using 7000 lux light for 72 h. The population was then maintained at <15 per glass beaker containing 1.5 L of Elendt M4 medium at 20.0 ± 1.0 °C under the same light cycle in a climate incubator. D. magna were fed daily on 0.1 mg C per individual with Chlorella vulgaris (~1.5 × 108 cells/mL) purchased from the Culture Collection of Algae at Cologne University (Germany); 0.5 μL/mL of yeast, cerophyll, and trout chow (YCT) was also supplied three times a week. The beakers and culture medium were replaced three times a week to ensure water quality, and new neonates were removed daily to prevent crowding. The pH and dissolved oxygen content of all culture and testing media were checked before replacement. The reliability of the test conditions was regularly checked using potassium dichromate (Sigma-Aldrich, St. Louis, MO, USA) as a reference substance.
2.2. Synthesis and Characterization of SiO2 NPs
The synthesis and mechanisms of dye-doped SiO2 NPs have been well-established [33]. FITC was chosen from among various conjugated fluorescent molecules for the advantages conveyed by its high quantum yield, biocompatibility, stability, and well-established synthesis with SiO2 NPs [33,34,35]. For synthesis, we used the same method as [34]. Scanning electron microscopy (SEM) and diffraction light scattering (DLS) were used to determine nanoparticle morphology and size. SEM images and hydrodynamic size were obtained using a HITACHI S-4800 and a Zetasizer Nano ZSP (Malvern), respectively. To determine the average particle size, 141 single particles were measured using ImageJ software.
2.3. Immobilization Test
The acute immobilization tests followed the procedure described in OECD guideline 202 [36]. The third brood of 60 neonates (<24 h) was exposed to various concentrations of two SiO2 NP sizes (20 and 50 nm) on a log scale (0.01, 0.1, 1.0, 10, and 100 μg/mL), as well as those of the control series. Groups were divided into six replicates of five daphnids for each concentration of SiO2 NPs in ISO medium, which was added to six-well culture plates filled with 10 mL of media in a group for 48 h. During the acute tests, immobilization and mortality were checked at designated times (3, 6, 12, 24, and 48 h). The experiment was performed in duplicate.
2.4. Reproduction Test
The reproduction tests were based on OECD guideline 211 [37] and performed using static tests. D. magna was randomly pooled from the third brood. Eight daphnids were individually held at the two concentrations (1.0 and 10 μg/mL) as well as in the control series for 21 d. We set the control series under the same conditions, except for SiO2 NP exposure. The glass vessels were filled with 60 mL of solution in a 100 mL volume beaker. The SiO2 NPs were sonicated for 20 min before addition to the new media. Neonates were removed and counted daily. Mothers were fed daily with C. vulgaris (~1.5 × 108 cells/mL, 0.1 mg C/D. magna) and thrice a week with YCT (0.5 μL/mL). The beakers and media were renewed three times per week.
2.5. Growth Test
D. magna length was measured one per week using an optical microscope (Model CKX41, Olympus Inc., Tokyo, Japan). The magnification, selected based on individual size, ranged from 2–4×. For this purpose, D. magna were transferred onto glass slides with a few drops of the originating medium. Body length was determined from the center of the eye to the base of the apical spine by using ImageJ program. In addition, ten neonates from the third brood in each condition were randomly pooled to check their size, following the same procedure.
2.6. Swimming Performance Monitoring
Swimming performance was monitored using a direct-read instrument (Model Zebrabox, View Point Life Science Inc., Lyon, France). This was measured after exposure to SiO2 NPs at various concentrations on the same log scale as above for 48 h. Daphnids were transferred into a 96-well plate separately and placed in the Zebrabox. To minimize the influence of sudden environmental changes, this plate was kept in the Zebrabox under complete darkness for 30 min before measurement. After stabilization, the swimming distance was recorded in tracking mode every minute for 80 min under darkness. A transparent background mode with a low detection threshold of 10 was used. Other threshold options for inactivity were 4 and 2. Real-time frequencies were analyzed using automated observation software (Zebralab-2, View Point Life Science Inc., Lyon, France). Swimming performance was calculated as distance per minute. Therefore, frames with no detected motion were excluded from the analysis.
2.7. Heart Rate Counting
Six neonates after the immobilization test were randomly selected for heartbeat counting under each exposure condition. Methylcellulose solution (4% v/v, Lot No. SLCC9072, Sigma-Aldrich Corp., St. Louis, MO, USA) was used to fix the neonates on a glass plate. Heart rate was recorded as a video file for 1 min using an optical microscope at 4× magnification (Model CKX41, Olympus Inc., Tokyo, Japan). The heart rate was counted manually using low-speed playback (×0.3) and repeated three times, with the mean values of each sample used for comparison.
2.8. Fluorescent Imaging
D. magna were rinsed three times and transferred to fresh medium for 5 min to avoid strong fluorescence from the stuck nanoparticles on the carapace. They were then rinsed three times again and transferred to a glass slide. Visualization was performed using a fluorescence microscope (ZEISS SteREO Discovery V8) with filter set 09 and ZEN 2.6 software. The filtration and camera exposure times were based on the control series to eliminate background autofluorescence. ImageJ software was used merge fluorescence images.
2.9. ICP-MS Analysis
To remove the influence of continuous adsorption due to the SiO2 NPs and the adhesive substances on the carapace surface, D. magna after observation for 24 d in fluorescent microscopy were placed in fresh media for another 3 d. Fresh media was renewed daily. Each group was thoroughly ground in 300 μL 5% HNO3 solution. The samples were then transferred to a glass container holding 1.5 mL 70% HNO3 (Sigma-Aldrich) and 5% H2O2 (Sigma-Aldrich, St. Louis, MO, USA) (1:3) mixture, and heated at 100 °C for 1 h for thorough sample digestion. The digested sample was diluted with 3 mL 5% HNO3. The amount of total silicon ions was measured by inductively coupled plasma mass spectroscopy (iCAP Q ICP-MS, Thermo Scientific) in triplicate. Before the measurement, the ICP-MS was washed with 5% HNO3 for 10 min. A calibration curve from 1–1000 μg/L was made with a silicon standard (FluKa, Munich, Germany). The measurement mode was STD, the detection dwell time was 10 ms, and the test was repeated 10 times for each sample.
3. Results
3.1. Characterization of FITC-Adopted SiO2 NPs
FITC-adopted SiO2 NPs were synthesized at 20 and 50 nm for visualization using a noninvasive method. To avoid functionalization on the SiO2 surface, a tetraethyl-orthosilicate (TEOS) precursor with covalent incorporation of FITC molecules was used to form a silica matrix. Both nanoparticle sizes were nearly spherical in shape, though slightly angular. The average sizes measured by DLS were slightly higher than those by SEM, but differences were negligible (Figure 1).
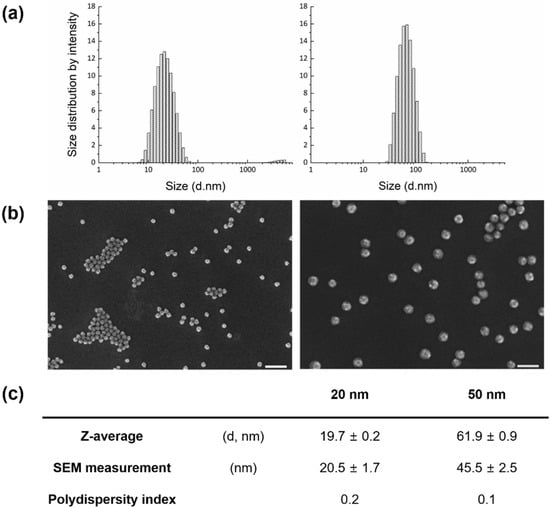
Figure 1.
Characterization of synthesized silicon dioxide nanoparticles (SiO2 NPs): (a) Hydrodynamic sizes measured by diffraction light scattering (DLS); (b) representative scanning electron microscopy (SEM) images; and (c) particle information (n = 141 particles).
3.2. Acute and Chronic Effects of SiO2 NPs on D. magna Mortality and Reproduction
Acute toxicity tests were carried out to better understand this dynamic and to determine concentrations in a long-term exposure test at low concentrations. There was no significant mortality observed among the different groups, including the control series, for exposures up to 48 h (Figure S1). The chronic test was performed for two concentrations (1.0 and 10.0 μg/mL) based on the results of the short-term test, i.e., acute, swimming performance and heart rate. The mean number of neonates in the control series over 21 d was 78.7 ± 12.7, validated according to OECD guideline 211. There was no difference in reproductive output between the experimental and control groups, demonstrating that SiO2 NPs did not affect reproductive outcomes under the applied conditions (Figure S2).
3.3. Effects of SiO2 NPs on D. magna Swimming Performance and Heart Rate
Six individuals were randomly selected from each group for heart rate testing. Although the heart rate did not show significant changes at low concentrations (0.01 and 0.1 μg/mL) compared to the control, heart rates for the 20 nm SiO2 NPs groups increased with dosage (Figure 2). The differences between the heart rate of the control series and that of high-concentration 20 nm groups (1.0 and 10 μg/mL) were statistically significant. Similarly, the heart rates of the 50 nm groups increased overall, and all but 0.01 μg/mL showed statistically significant differences from the control.
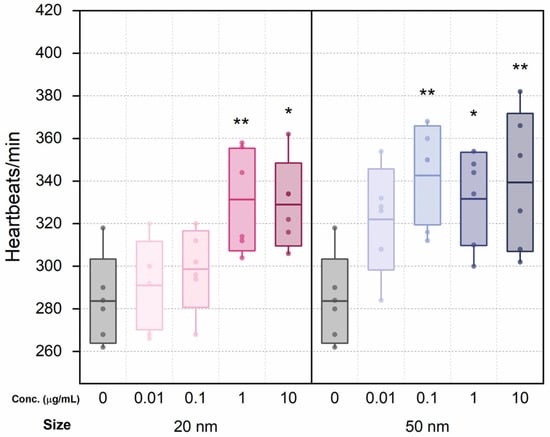
Figure 2.
Heart rate changes following treatment with 20 and 50 nm SiO2 NPs after 48 h exposure. Boxes express standard deviations; central line indicates the mean. * and ** indicate p < 0.05 and p < 0.01 relative to the control group, respectively.
Another five individuals were randomly selected from each group to test swimming performance, in which swimming distance was tracked individually, every minute for 80 min. There was a clear decrease in swimming performance with SiO2 NPs treatment, with the average swimming distance per minute for the control group (13.2 mm) dropping for both the 20 nm (8.99 mm) and 50 nm (5.52 mm) groups (Figure 3). However, the difference in swimming distance among concentrations within the same nanoparticle size was not significant or dose-dependent.
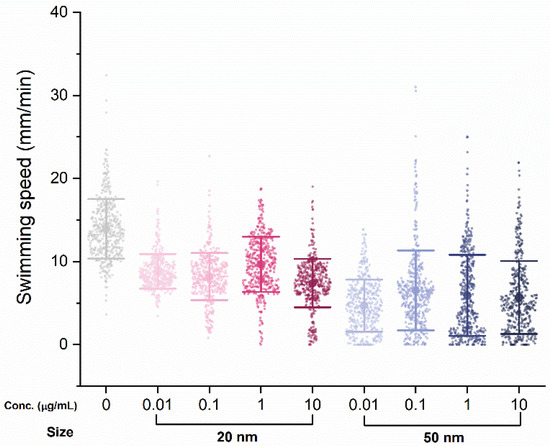
Figure 3.
Swimming speed changes upon exposure to SiO2 NPs after 48 h. Bars express standard deviations; small dots indicate swimming distance per minute (mm/min); central dot indicates the mean.
3.4. Chronic Effects on D. magna Growth
We hypothesized that sublethal concentrations of SiO2 NPs would induce food assimilation problems, affecting growth. Although dose dependency and nanoparticle size had no effect, the mere presence of SiO2 NPs affected the size of mothers (Figure 4) and neonates (Figure S3). The average lengths of the control series were 2.82 mm (7 d), 3.68 mm (14 d), and 3.79 mm (21 d), while the averages of both exposure groups were 2.76 mm (7 d), 3.36 mm (14 d), and 3.51 mm (21 d). At the 7 d mark, there were no statistically significant differences in growth between all groups (one-way ANOVA with Tukey comparisons test). However, at 14 d, body length between the control series and the 1 μg/mL groups showed statistically significant differences (p < 0.01 for both sizes), which was maintained at 21 d. The difference in growth increased over time in the low-concentration groups, but in the high-concentration groups, this was not statistically significant. Similarly, neonates from the control group had an average length of 0.949 ± 0.016 mm, as compared with the experimental group lengths of 0.915 ± 0.024 mm (20 nm, 1 μg/mL), 0.901 ± 0.019 mm (20 nm, 10 μg/mL, p <0.05), 0.898 ± 0.053 mm (50 nm, 1 μg/mL, p < 0.05), and 0.881 ± 0.055 mm (50 nm, 10 μg/mL, p < 0.01).
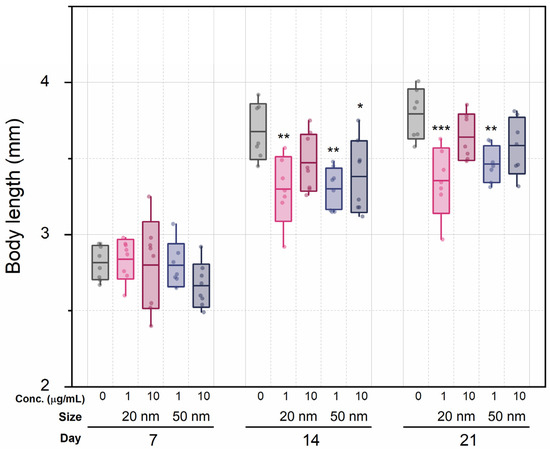
Figure 4.
Body length of individuals in each treatment group during 7, 14, or 21 d exposure to SiO2 NPs at two concentrations. Boxes express standard deviations; central line indicates the mean. *, ** and *** indicate p < 0.05, p < 0.01 and p < 0.001 relative to the control group, respectively.
3.5. Accumulation of SiO2 NPs in D. magna
After 14 d exposure, green fluorescence was observed in D. magna. The brightness increased proportionally with the applied concentration, but no remarkable change was observed by exposure time (Figure S4). D. magna were then transferred to an untreated condition identical to that of the control series, followed by 3 d incubation. Interestingly, fluorescence remained in D. magna exposed to SiO2 NPs (Figure 5). Next, the amount of SiO2 uptake in D. magna was measured using ICP-MS. After the visualization experiment, D. magna were transferred to fresh media and incubated for another 3 d to minimize the influence of SiO2 NPs. The measured relative amount of silicon ions in the exposure groups showed a > 1.5-fold increase compared to those of the control series (Figure 6). In the 20 nm SiO2 NPs, the relative intensity seemed to depend on the concentration, while the 10 μg/mL 50 nm Si nanoparticles had a lower intensity than those at 1 μg/mL, and the relative intensities showed negligible differences.
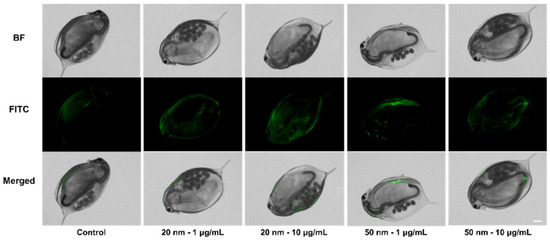
Figure 5.
Fluorescence images of D. manga exposed to SiO2 NPs for 21 d, followed by incubation in fresh media for another 3 d. Scale bar = 500 μM. BF = Bright field.
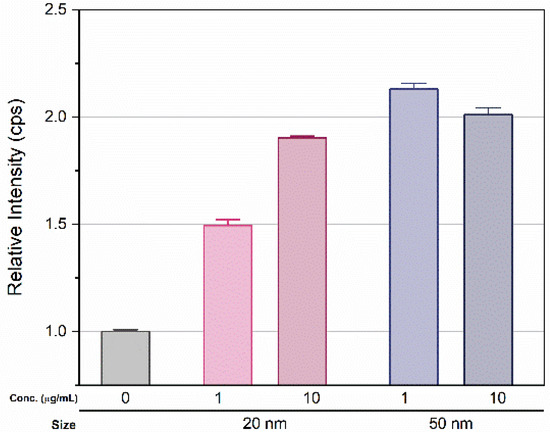
Figure 6.
Relative intensity of Si in D. magna (measured by ICP-MS) exposed to 1 and 10 μg/mL concentrations of 20 and 50 nm SiO2 NPs for 27 d, including washing in ISO medium. Error bar indicate standard deviation.
4. Discussion
4.1. Acute and Chronic Effects on D. magna Mortality, Reproduction, and Growth
The toxicity of SiO2 NPs can change significantly based on size, shape, and functionalization. Vicentini et al. [14] reported the acute toxicity of SiO2 NPs by comparing two morphologies, calculating 48 h half-maximal effective concentrations (EC50) of 4.9 mg/mL (tubular) and 2.2 mg/mL (spherical). A comparison between amine-functionalized and 50 nm spherical SiO2 NPs showed that the acute toxicity of the SiO2 NPs was 2.2 mg/mL (EC50) [13]. According to Clement et al. [15], the toxicity of amorphous 14 nm SiO2 NPs at 72 h was 29.7 mg/L (EC50), and these nanoparticles caused morphological alterations and cellular-level changes including mitochondrial deformation in tissues and deformed nuclei in D. magna eggs. It appears that a lack of direct lethality related to SiO2 NPs should not discourage the investigation of sublethal and chronic effects. Our acute toxicity tests were carried out under limited concentrations (<100 μg/mL) at 48 h, according to OECD test guideline 202. Spherical SiO2 NPs of both sizes would not affect immobilization on D. magna, as the acute toxicity of SiO2 NPs is lower for spherical and unfunctionalized conditions. In the duplication test, only eight daphnids showed mortality over a total of 660 individuals. Considering a few mortalities from the control series, the effect of SiO2 NPs on acute toxicity was negligible under the applied conditions.
Two studies have shown that SiO2 NPs lead to an increase in D. magna reproduction: this rose by 18% with a slurry of colloidal SiO2 NPs, while commercially available 7 and 10 nm SiO2 particles slightly increased reproduction [38]. On the other hand, Vicentini et al. [14] reported that reproduction decreased significantly upon exposure to concentrations of 50 mg/L 80 nm SiO2 particles. These conflicting results can be used to elucidate size and surface differences [13]. In our chronic toxicity test, SiO2 NPs in the 20 nm and 10 μg/mL groups gave rise to slightly higher reproduction compared to the control series. Nevertheless, the average number of offspring in all groups did not show statistically significant differences, demonstrating that the reproduction of D. magna was not affected by SiO2 NPs under the applied conditions. However, a growth analysis showed that both SiO2 NPs sizes caused growth retardation that was significant relative to the control, beginning from day 14 of exposure until study termination (day 21). Previous studies exploring this particular feature are rare, though Lee et al. [38] indicated no significant change in size with exposure to 7 and 10 nm particles. A comparison by Karimi et al. [39] of different silica oxides (colloidal and fumed silica) reported that the latter (120–140 nm particles) did not result in size changes with acute exposure, but the former (50–60 nm particles) increased the size of D. magna by up to 24%; on the other hand, chronic exposure (21 d) to both compounds increased the size by 10%. These changes were attributed to hormetic adaptation, and the authors concluded that exposure to silica oxides did not have a significant adverse effect on D. magna. Similar results (increased size with long-term exposure) and an almost identical conclusion were described by Lillicrap et al. [40], who investigated the effects of low-grade and high-grade silica fumes on D. magna.
4.2. Effects of SiO2 NPs on D. magna Swimming Performance and Heart Rate
Swimming performance has been used for the assessment of low-toxicity materials in cases where mortality and immobilization are difficult to identify. Observations of D. magna heart rate are also recognized as a sublethal endpoint for toxicity screening. Decreased speed or other adverse effects on swimming have been reported in previous studies exploring titanium, nano-C60, copper, and other metals or compounds [41,42,43,44]. Although we did not observe dose dependency, swimming speed decreased significantly within our exposure groups and the 50 nm groups showed slower swimming speeds than the 20 nm groups. Heart rate increased significantly in the 20 nm (1 and 10 µg/mL) and 50 nm (0.1, 1, and 10 µg/mL) groups relative to control. These changes are similar to those reported in prior studies using heartbeat as an endpoint for exploring fullerenes (nano-C60), graphene oxide, titanium oxide, and copper-based nanoparticles [30,41,42,45]. This may be caused by the absorption of SiO2 NPs because the toxic effect of nanoparticles on signal pathways has not been clearly demonstrated [44,46]. When considered alongside physiological/behavioral and growth changes, this may suggest that SiO2 NPs led to the obstruction of food assimilation and the physical restriction of movement [44,46,47,48,49].
4.3. Accumulation of SiO2 NPs in D. magna
Small zooplanktonic organisms are able to absorb micro- and nano- particles as food because they are filter feeders [49]. Although we found no dose-dependent fluorescence brightness, relatively strong fluorescence from exposed D. magna showed that small particles could be absorbed and accumulated through exposure. Given that the fluorescence brightness did not change significantly for over 21 d, the accumulation seemed to reach saturation at some point. The quantitative amount of silicon ion uptake was not precisely measured because of the fairly high intensity in the control series, possibly due to uptake from the cultural media. For this reason, we expressed the amount of silicon ions by normalizing the intensity from the control series. The measured relative amount of exposure samples showed more than a 1.5-fold increase over that of the control (Figure 6). The relative intensity of the silicon ions was affected by the size rather than the concentration, and increased with size. For the 20 nm particles, the relative intensity seemed to depend on the concentration, showing a difference of approximately 35% between the two concentrations. In contrast, 10 μg/mL of 50 nm particles had a lower intensity than 1 μg/mL, and the relative intensities had negligible differences. As a result, there was no significant consistency between nanoparticle uptake in D. magna and applied Si concentration due to saturation, but uptake and accumulation was clearly observed.
4.4. Conclusions
As expected from previous research, spherical SiO2 NPs did not lead to acute or severe chronic effects on D. magna. However, physiological and behavioral changes were observed in adults and neonates. The heart rate and swimming performance, which are strongly related to the health and survival of D. magna, were affected by SiO2 NPs in the environment. The length of D. magna mothers and neonates also decreased compared to that of the control group. Such growth factors could have been affected by food assimilation alterations by the accumulation of SiO2 NPs in the body. Accumulation trends were validated by ICP-MS and fluorescent imaging. Although the results did not demonstrate mortality, immobility, or reproductive adverse effects, as swimming performance and heart rate are regarded as reliable proxies for identifying toxicity in D. magna, these results appear to support the hypothesis that SiO2 NPs have toxic effects on this species. Physiological and behavioral endpoint experiments may not be sufficient to elucidate the toxicity of SiO2 NPs from an adverse outcome pathway or mechanistic point of view. However, for a better understanding of the effects of low-toxicity nanoparticles under sublethal concentrations, these endpoint tests provide an overview of sublethal toxicity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/mi12091105/s1, Figure S1: Survival (%) of D. magna upon exposure to SiO2 NPs after 48 h, Figure S2: The number of total neonates reproduced during 21 days, Figure S3: Body length of neonates for each treatment group, Figure S4: Fluorescence images of D. magna, Figure S5: The calibration curve of silicon ion measured with ICP-MS.
Author Contributions
Conceptualization, Y.K. and Y.J.K.; methodology, Y.K.; software, Y.K. and J.P. (Jayoung Park); validation, Y.K., A.S. and Y.J.K.; formal analysis, M.K., J.P. (Jayoung Park), J.P. (Jihoon Park) and E.H.G.; investigation, Y.K.; resources, Y.K.; data curation, Y.K.; writing—original draft preparation, Y.K.; writing—review and editing, A.S., E.H.G. and Y.J.K.; visualization, Y.K.; supervision, Y.J.K. and T.G.L.; project administration, Y.J.K.; funding acquisition, Y.J.K. and T.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nanomaterial Technology Development Program (NRF-2017M3A7B6052455) funded by the South Korean Ministry of Science.
Acknowledgments
The research support staffs, Zahra Adeli and Brenda T. Meupea, at KIST-Europe facilitated the maintenance of D. magna.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Braun, T.; Schubert, A.; Zsindely, S. Nanoscience and Nanotecnology on the Balance. Scientometrics 1997, 38, 321–325. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local. Environ. Sci. Technol. Lett. 2013, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Pulit-Prociak, J.; Banach, M. Silver Nanoparticles–A Material of the Future...? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakov, O. World Market for Nanomaterials: Structure and Trends. MATEC Web Conf. 2017, 129, 02013–02017. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Zhang, A.; Lu, D.; Li, G.; Zhang, Q.; Liu, Q.; Jiang, G. Distinguishing the Sources of Silica Nanoparticles by Dual Isotopic Fingerprinting and Machine Learning. Nat. Commun. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Adach, K.; Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Rusak, A.; Cwynar-Zając, Ł.; Machałowski, T.; Jesionowski, T.; Grzywacz, K.; Fijałkowski, M. Are Biogenic and Pyrogenic Mesoporous SiO2 Nanoparticles Safe for Normal Cells? Molecules 2021, 26, 1427. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, J.; Zhang, Y.; Gaurav, J.; Deloid, G.; Spyrogianni, A.; Sarosiek, K.A.; Bello, D.; Demokritou, P. Safer-by-Design Flame-Sprayed Silicon Dioxide Nanoparticles: The Role of Silanol Content on ROS Generation, Surface Activity and Cytotoxicity. Part. Fibre Toxicol. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Kim, S.H.; You, J.R.; Kim, W.H.; Jang, J.J.; Min, S.K.; Kim, H.C.; Chung, D.H.; Jeong, J.; Kang, B.C.; et al. Comparative Toxicity of Silicon Dioxide, Silver and Iron Oxide Nanoparticles after Repeated Oral Administration to Rats. J. Appl. Toxicol. 2015, 35, 681–693. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of Silica Nanoparticles: An Update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Puerari, R.C.; Ferrari, E.; Oscar, B.V.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Acute and Chronic Toxicity of Amine-Functionalized SiO2 Nanostructures toward Daphnia magna. Ecotoxicol. Environ. Saf. 2021, 212, 111979–111987. [Google Scholar] [CrossRef]
- Vicentini, D.S.; Puerari, R.C.; Oliveira, K.G.; Arl, M.; Melegari, S.P.; Matias, W.G. Toxicological Impact of Morphology and Surface Functionalization of Amorphous SiO2 Nanomaterials. NanoImpact 2017, 5, 6–12. [Google Scholar] [CrossRef]
- Clément, L.; Zenerino, A.; Hurel, C.; Amigoni, S.; de Givenchy, E.T.; Guittard, F.; Marmier, N. Toxicity Assessment of Silica Nanoparticles, Functionalised Silica Nanoparticles, and HASE-Grafted Silica Nanoparticles. Sci. Total Environ. 2013, 450–451, 120–128. [Google Scholar] [CrossRef]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The Size-Dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of In Vitro Studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef]
- Sun, D.; Gong, L.; Xie, J.; Gu, X.; Li, Y.; Cao, Q.; Li, Q.; Luodan, A.; Gu, Z.; Xu, H. Toxicity of Silicon Dioxide Nanoparticles with Varying Sizes on the Cornea and Protein Corona as a Strategy for Therapy. Sci. Bull. 2018, 63, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ye, R.; Han, B.; Wei, C.; Yang, X. Ecotoxicological Effect of Nano-Silicon Dioxide Particles on Daphnia magna. Integr. Ferroelectr. 2014, 154, 64–72. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio fischeri and Crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, L.; Chen, Y.; Tian, S. Acute Toxicities of Six Manufactured Nanomaterial Suspensions to Daphnia magna. J. Nanoparticle Res. 2009, 11, 67–75. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A Comparison between Sphere and Rod Nanoparticles Regarding Their in Vivo Biological Behavior and Pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Z.; Gao, Y.; Shi, J.; Li, Y. Intracellular Localization and Cytotoxicity of Spherical Mesoporous Silica Nano-and Microparticles. Small 2009, 5, 2722–2729. [Google Scholar] [CrossRef]
- Timpe, N.; Fullriede, H.; Borchers, L.; Stiesch, M.; Behrens, P.; Menzel, H. Nanoporous Silica Nanoparticles with Spherical and Anisotropic Shape as Fillers in Dental Composite Materials. BioNanoMaterials 2014, 15, 89–99. [Google Scholar] [CrossRef]
- Baryshnikova, K.V.; Petrov, M.I.; Babicheva, V.E.; Belov, P.A. Plasmonic and Silicon Spherical Nanoparticle Antireflective Coatings. Sci. Rep. 2016, 6, 22136–22141. [Google Scholar] [CrossRef]
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting Their Toxicity to Daphnia magna —a Call for Updating Toxicity Testing Policies. Proteomics 2020, 20, 1800412. [Google Scholar] [CrossRef] [Green Version]
- Grintzalis, K.; Lawson, T.N.; Nasser, F.; Lynch, I.; Viant, M.R. Metabolomic Method to Detect a Metabolite Corona on Amino-Functionalized Polystyrene Nanoparticles. Nanotoxicology 2019, 13, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Diger, R.; Paul, J.; Colmorgen, M.; Huè, S.; Tyroller, F.; Zinkler, D. Circulation and Respiratory Control in Millimetre-Sized Animals (Daphnia magna, Folsomia Candida) Studied by Optical Methods. J. Comp. Physiol. B 1997, 167, 399–408. [Google Scholar]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna Model in the Toxicity Assessment of Pharmaceuticals: A Review. Sci. Total Environ. 2021, 763, 143038–143055. [Google Scholar] [CrossRef] [PubMed]
- Farner, J.M.; Cheong, R.S.; Mahé, E.; Anand, H.; Tufenkji, N. Comparing TiO2 Nanoparticle Formulations: Stability and Photoreactivity Are Key Factors in Acute Toxicity to: Daphnia magna. Environ. Sci. Nano 2019, 6, 2532–2543. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; László, K.; Terebesi, C.; Gyarmati, B.S.; Farah, S.; Márton, R.; Molnár, M. Ecotoxicity Assessment of Graphene Oxide by Daphnia magna through a Multimarker Approach from the Molecular to the Physiological Level Including Behavioral Changes. Nanomaterials 2020, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jo, A.; Choi, J.; Kim, J.; Zoh, K.D.; Choi, K. Rapid Screening for Ecotoxicity of Plating and Semiconductor Wastewater Employing the Heartbeat of Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 186, 109721–109727. [Google Scholar] [CrossRef]
- Singh, G.; Kundu, A. Dopamine Synergizes with Caffeine to Increase the Heart Rate of Daphnia [Version 1; Referees: 1 Approved, 2 Approved with Reservations]. F1000Research 2018, 7, 254. [Google Scholar]
- Korzeniowska, B.; Nooney, R.; Wencel, D.; McDonagh, C. Silica Nanoparticles for Cell Imaging and Intracellular Sensing. Nanotechnology 2013, 24, 442002–442020. [Google Scholar] [CrossRef]
- Shin, H.R.; Kwak, M.; Lee, T.G.; Lee, J.Y. Quantifying the Level of Nanoparticle Uptake in Mammalian Cells Using Flow Cytometry. Nanoscale 2020, 12, 15743–15751. [Google Scholar] [CrossRef]
- Li, L.; Rao, G.; Lv, X.; Chen, R.; Cheng, X.; Wang, X.; Zeng, S.; Liu, X. Chemical Reactivation of Fluorescein Isothiocyanate Immunofluorescence-Labeled Resin-Embedded Samples. J. Biomed. Opt. 2018, 23, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Test No. 202: Daphnia Sp. Acute Immobilisation Test; OECD: Paris, France, 2004; ISBN 978-92-64-06994-7.
- Test No. 211: Daphnia magna Reproduction Test; OECD: Paris, France, 2012; ISBN 978-92-64-18520-3.
- Lee, S.W.; Kim, S.M.; Choi, J. Genotoxicity and Ecotoxicity Assays Using the Freshwater Crustacean Daphnia magna and the Larva of the Aquatic Midge Chironomus riparius to Screen the Ecological Risks of Nanoparticle Exposure. Environ. Toxicol. Pharmacol. 2009, 28, 86–91. [Google Scholar] [CrossRef]
- Karimi, S.; Troeung, M.; Wang, R.; Draper, R.; Pantano, P. Acute and Chronic Toxicity of Metal Oxide Nanoparticles in Chemical Mechanical Planarization Slurries with Daphnia magna. Environ. Sci. Nano 2018, 5, 1670–1684. [Google Scholar] [CrossRef]
- Lillicrap, A.; Allan, I.; Friede, B.; Garmo, O.; Macken, A. Is the Transformation/Dissolution Protocol Suitable for Ecotoxicity Assessments of Inorganic Substances Such as Silica Fume? Sci. Total Environ. 2014, 468–469, 358–367. [Google Scholar] [CrossRef]
- Lovern, S.B.; Strickler, J.R.; Klaper, R. Behavioral and Physiological Changes in Daphnia magna When Exposed to Nanoparticle Suspensions (Titanium Dioxide, Nano-C60, and C 60HxC70Hx). Environ. Sci. Technol. 2007, 41, 4465–4470. [Google Scholar] [CrossRef] [Green Version]
- Chung, W.; Song, M.; Lee, J. The Evaluation of Titanium Dioxide Nanoparticle Effects on Cardiac and Swimming Performance of Daphnia magna. Int. J. Appl. Environ. Sci. 2016, 11, 1375–1385. [Google Scholar]
- Untersteiner, H.; Kahapka, J.; Kaiser, H. Behavioural Response of the Cladoceran Daphnia magna STRAUS to Sublethal Copper Stress–Validation by Image Analysis. Aquat. Toxicol. 2003, 65, 435–442. [Google Scholar] [CrossRef]
- Bownik, A. Daphnia Swimming Behaviour as a Biomarker in Toxicity Assessment: A Review. Sci. Total Environ. 2017, 601–602, 194–205. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Chatterjee, N.; Lu, C.-H. Harmful Impact of ZnS Nanoparticles on Daphnia Sp. in the Western Part (Districts of Bankura and Purulia) of West Bengal, India. ISRN Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bownik, A. Physiological Endpoints in Daphnid Acute Toxicity Tests. Sci. Total Environ. 2020, 700, 134400–134420. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.M.; Maul, J.D.; Saed, M.; Shah, S.A.; Green, M.J.; Cañas-Carrell, J.E. Bioaccumulation, Stress, and Swimming Impairment in Daphnia magna Exposed to Multiwalled Carbon Nanotubes, Graphene, and Graphene Oxide. Environ. Toxicol. Chem. 2017, 36, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Laird, J.G.; Kennedy, A.J.; Steevens, J.A. Sublethal Effects of Multiwalled Carbon Nanotube Exposure in the Invertebrate Daphnia magna. Environ. Toxicol. Chem. 2016, 35, 200–204. [Google Scholar] [CrossRef]
- Danabas, D.; Ates, M.; Tastan, B.E.; Cimen, I.C.C.; Unal, I.; Aksu, O.; Kutlu, B. Effects of Zn and ZnO Nanoparticles on Artemia Salina and Daphnia magna Organisms: Toxicity, Accumulation and Elimination. Sci. Total Environ. 2020, 711, 134869–134878. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).