Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Chondrocyte Isolation for Dielectrophoresis
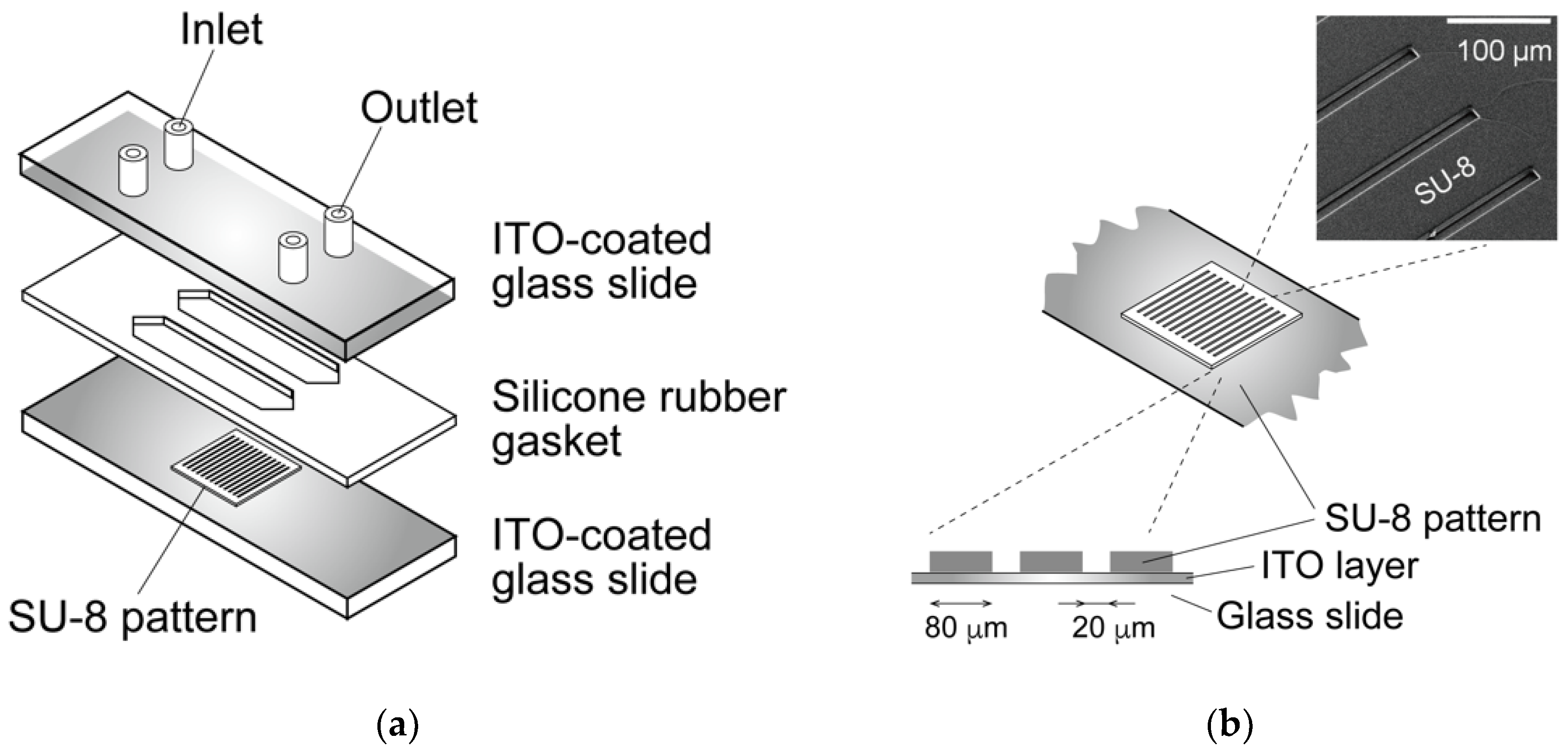
2.2. Dielectrophoresis Chamber for Living Cells
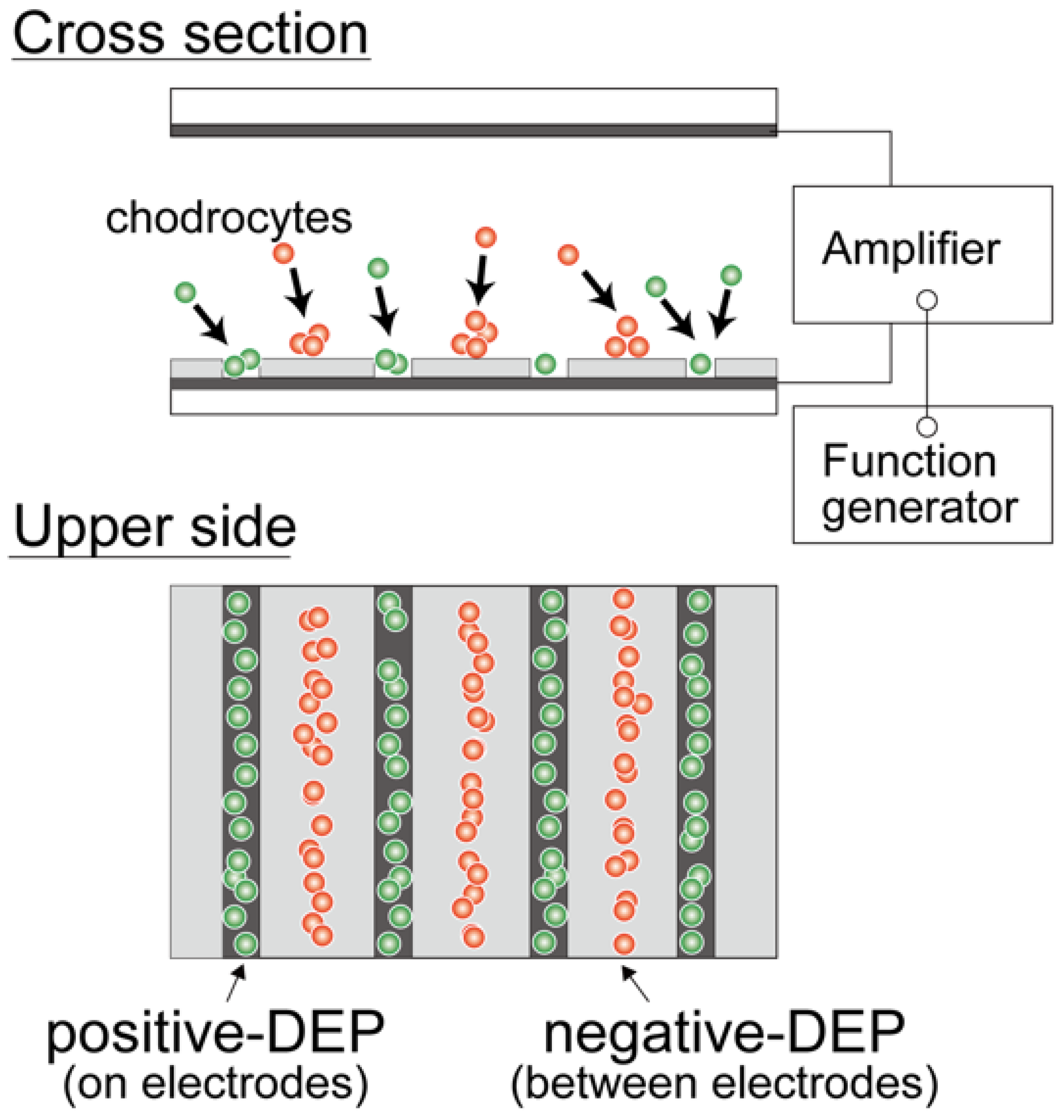
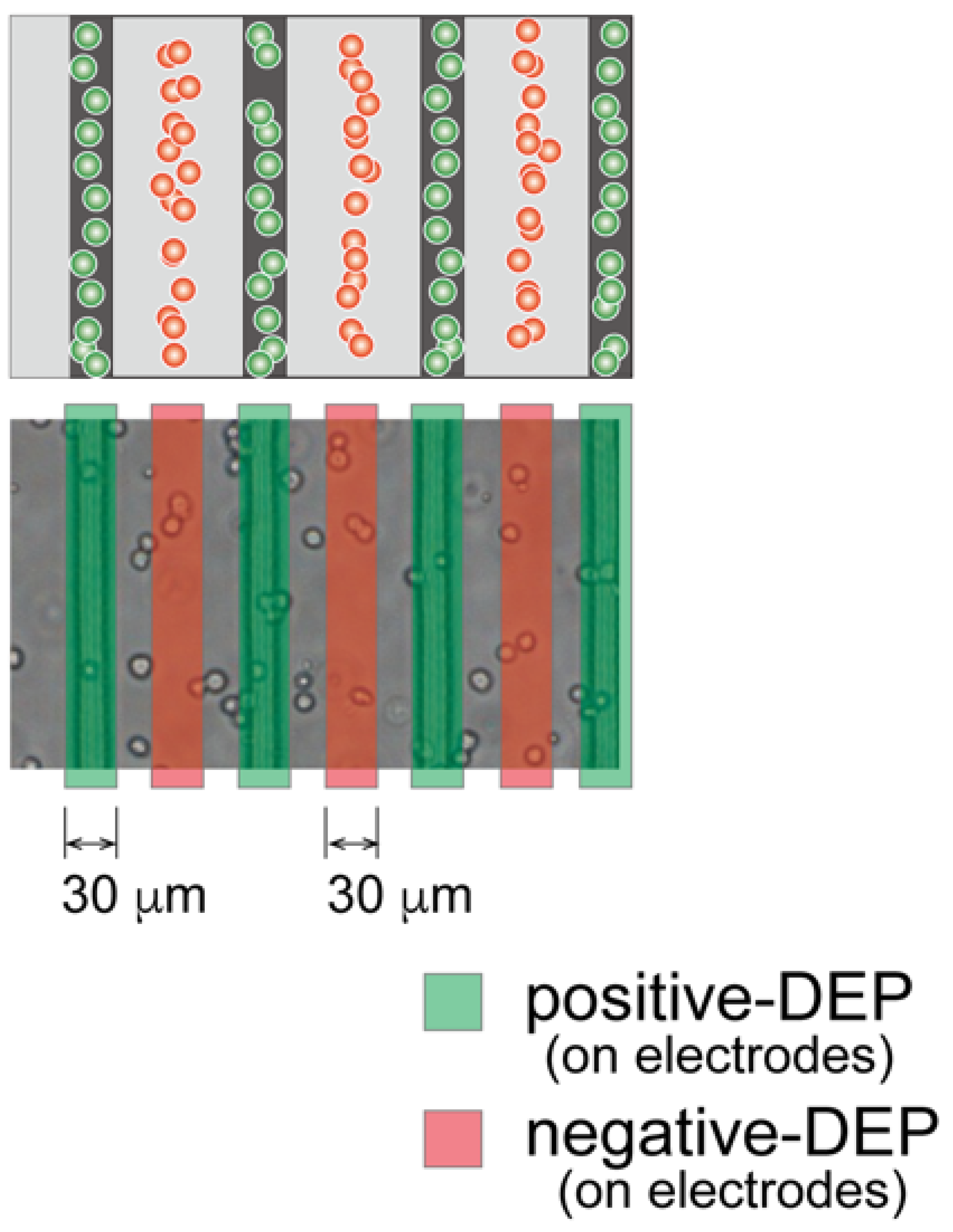
2.3. Dielectrophoretic Characterization of Chondrocytes
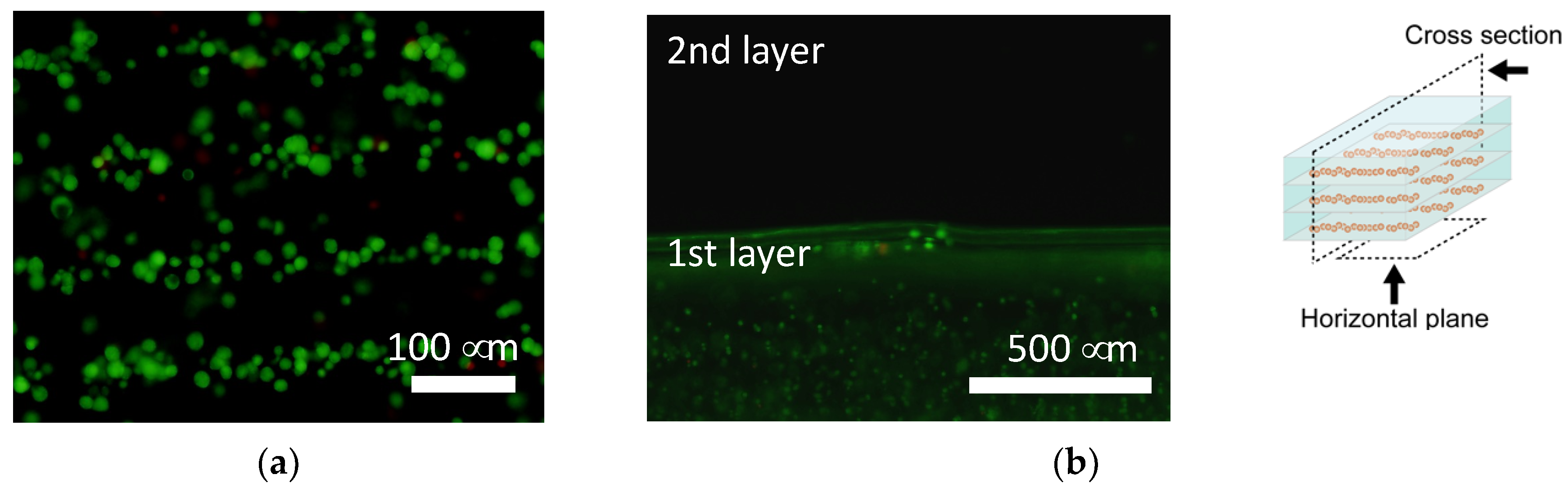
2.4. Dielectrophoretic Cell Accumulation in Agarose Gel to Fabricate Cell-Aligned Three-Dimensional Cultures
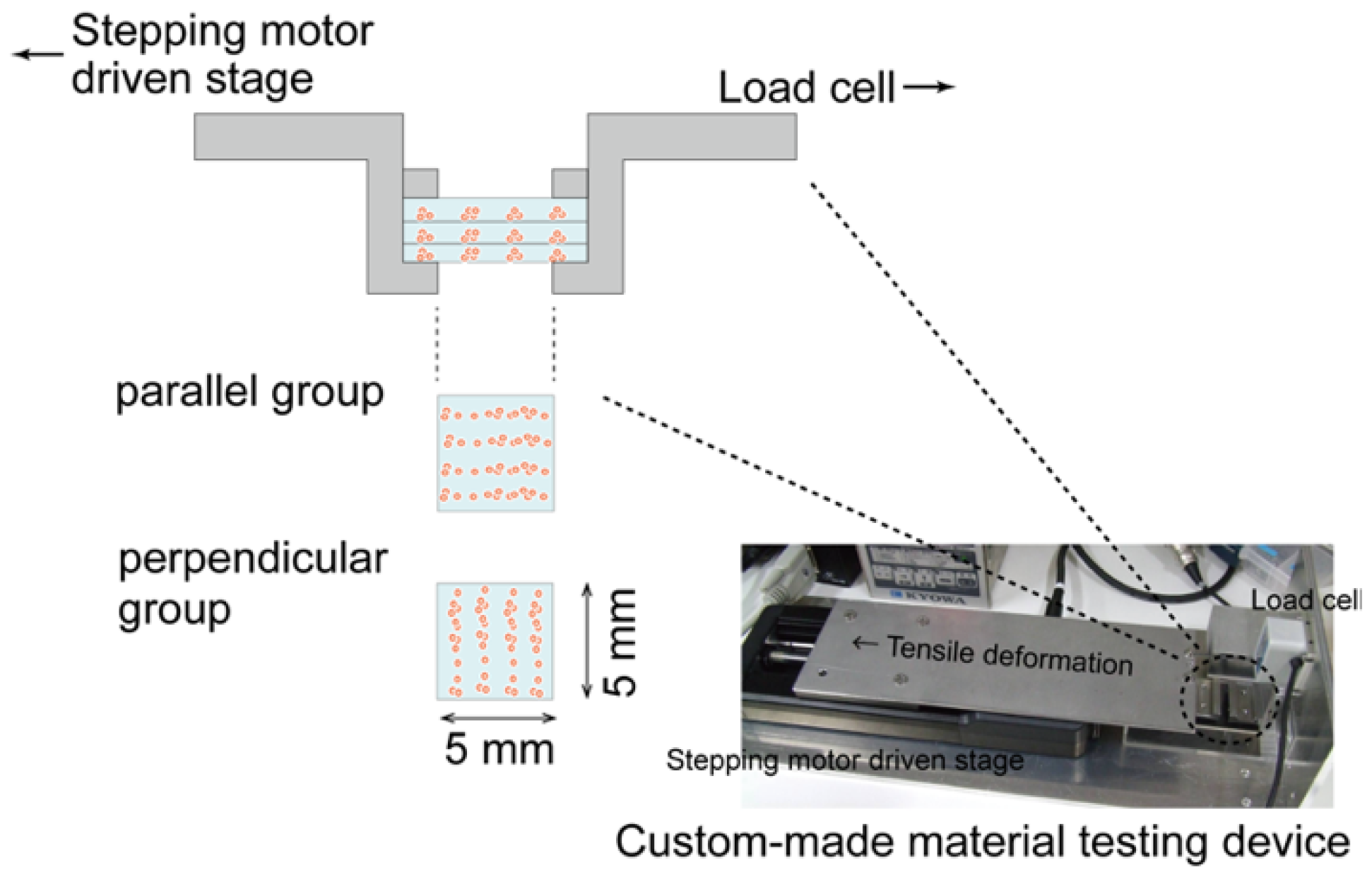
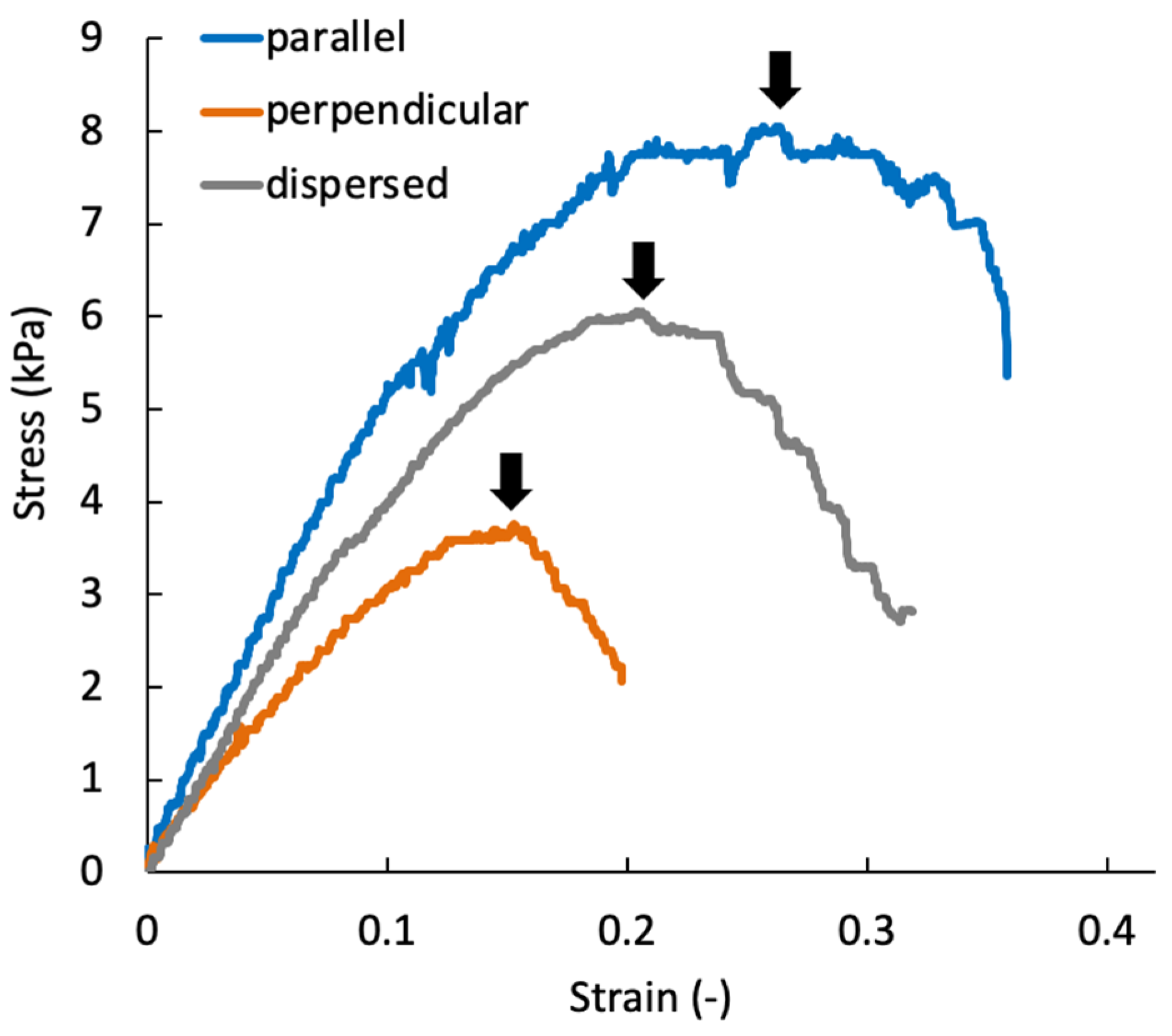
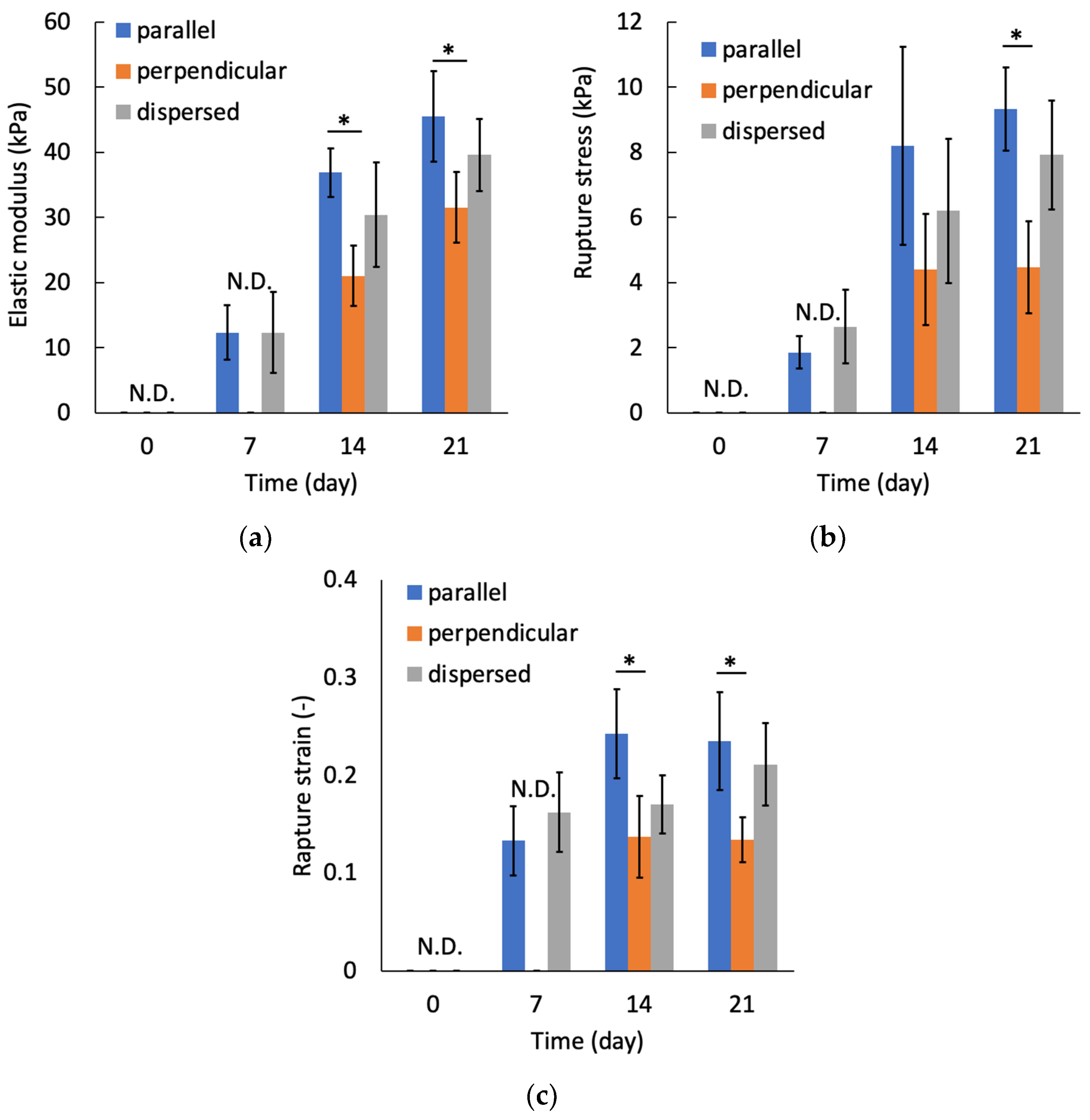
2.5. Biomechanical Characterization of the Regenerated Tissue
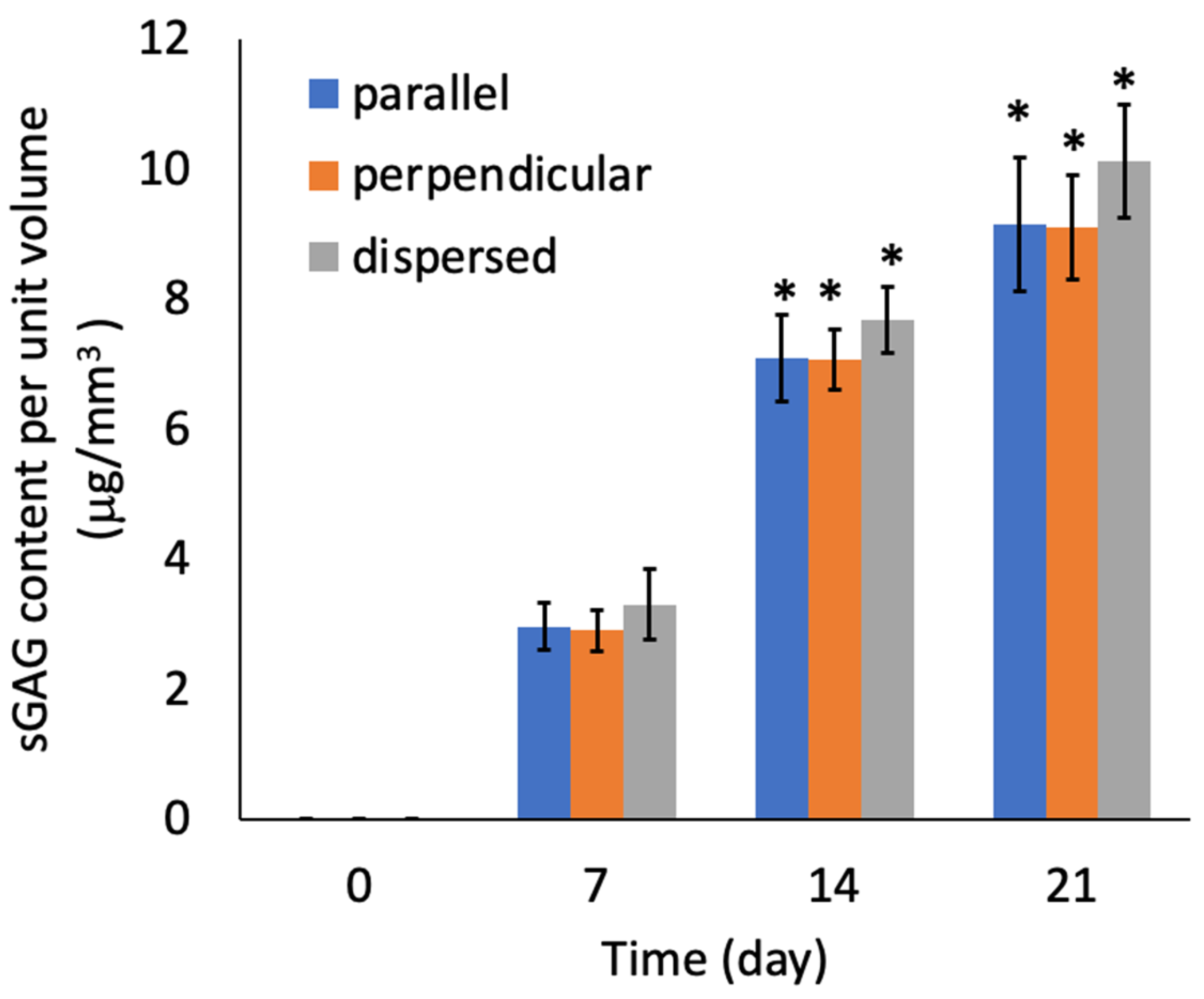
2.6. Cell Proliferation, Viability, and Biochemical Composition of the Regenerated Tissue
2.7. Statistical Analysis
3. Results
3.1. Dielectrophoretic Property of Primary Chondrocytes
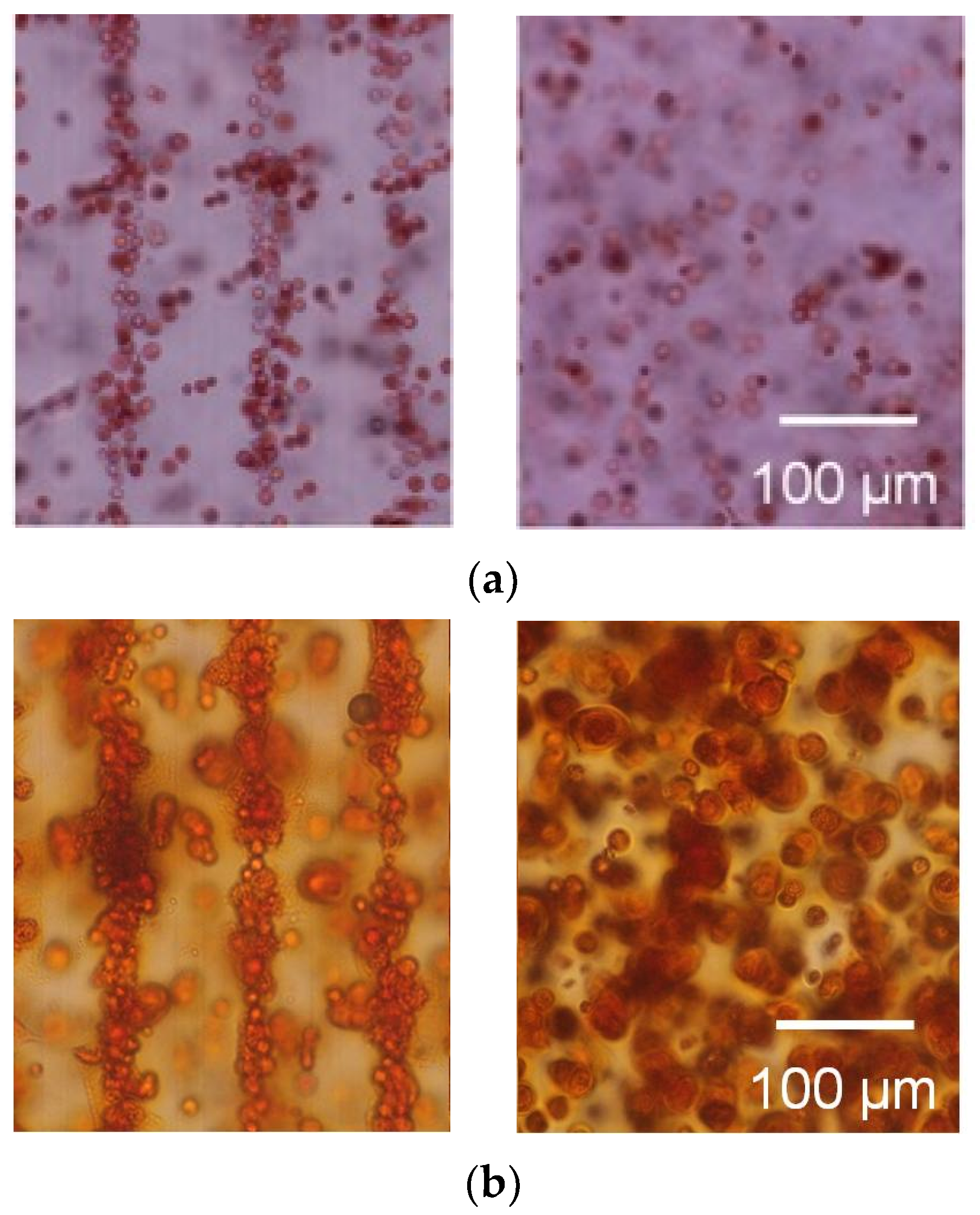
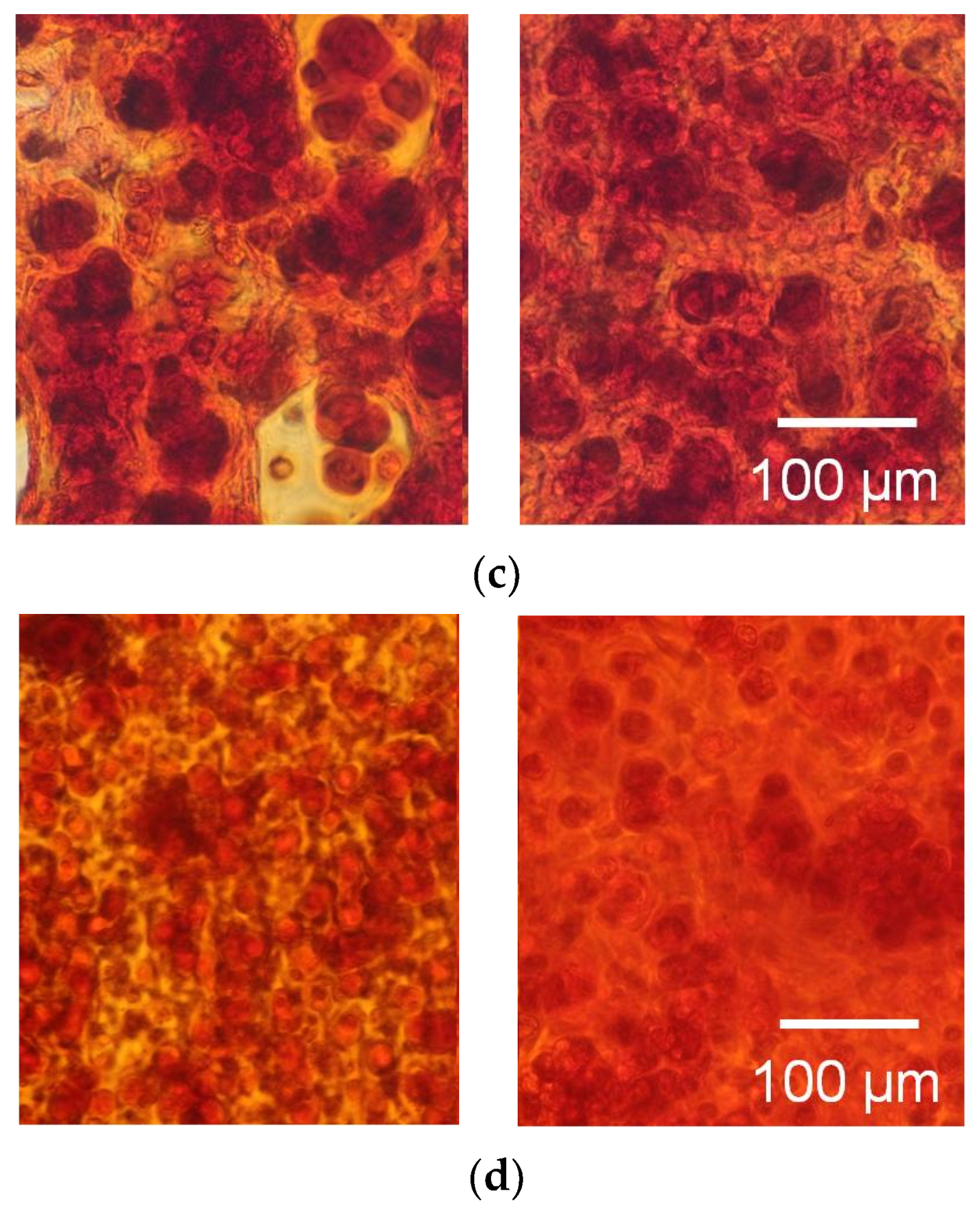
3.2. Tissue Reconstruction in Chondrocyte-Organized Agarose Gel Constructs
3.3. Mechanical Anisotropy of the Regenerated Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Holmes, M.H.; Michael Lai, W. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Partsalis, T.; Zheng, M.H. Articular cartilage repair: Procedures versus products. Expert Rev. Med. Devices 2007, 4, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Ochi, M.; Matsusaki, M.; Kurioka, H.; Katsube, K. Human chondrocyte proliferation and matrix synthesis cultured in atelocollagen gel. J. Biomed. Mater. Res. 2000, 50, 138–143. [Google Scholar] [CrossRef]
- Shimizu, R.; Kamei, N.; Adachi, N.; Hamanishi, M.; Kamei, G.; Mahmoud, E.E.; Nakano, T.; Iwata, T.; Yamato, M.; Okano, T.; et al. Repair mechanism of osteochondral defect promoted by bioengineered chondrocyte sheet. Tissue Eng. Part A 2015, 21, 1131–1141. [Google Scholar] [CrossRef]
- Hayashi, S.; Kamei, N.; Ikuta, Y.; Shimizu, R.; Ishikawa, M.; Adachi, N.; Ochi, M. Chondrocyte cell-sheet transplantation for treating monoiodoacetate-induced arthritis in rats. Tissue Eng. Part C Methods 2017, 23, 346–356. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Verteramo, A.; Seedhom, B.B. Zonal and directional variations in tensile properties of bovine articular cartilage with special reference to strain rate variation. Biorheology 2004, 41, 203–213. [Google Scholar]
- Steele, J.A.M.; McCullen, S.D.; Callanan, A.; Autefage, H.; Accardi, M.A.; Dini, D.; Stevens, M.M. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2014, 10, 2065–2075. [Google Scholar] [CrossRef]
- McCullen, S.D.; Autefage, H.; Callanan, A.; Gentleman, E.; Stevens, M.M. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng. Part A 2012, 18, 2073–2083. [Google Scholar] [CrossRef]
- Lee, J.K.; Huwe, L.W.; Paschos, N.; Aryaei, A.; Gegg, C.A.; Hu, J.C.; Athanasiou, K.A. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater. 2017, 16, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Holmes, J.W.; Costa, K.D. Remodeling of engineered tissue anisotropy in response to altered loading conditions. Ann. Biomed. Eng. 2008, 36, 1322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okuda, Y.; Konishi, R.; Miyata, S. Effect of cyclic compressive stimuli on mechanical anisotropy of chondrocyte-seeded agarose gel culture. Trans. Jpn. Soc. Mech. Eng. C 2013, 79, 1736–1743. [Google Scholar] [CrossRef][Green Version]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.B.; Wong, D.D.; Chao, P.H.G.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef]
- Mauck, R.L.; Nicoll, S.B.; Seyhan, S.L.; Ateshian, G.A.; Hung, C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003, 9, 597–611. [Google Scholar] [CrossRef]
- Kelly, T.A.N.; Ng, K.W.; Wang, C.C.B.; Ateshian, G.A.; Hung, C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J. Biomech. 2006, 39, 1489–1497. [Google Scholar] [CrossRef]
- Bougault, C.; Aubert-Foucher, E.; Paumier, A.; Perrier-Groult, E.; Huot, L.; Hot, D.; Duterque-Coquillaud, M.; Mallein-Gerin, F. Dynamic compression of chondrocyte-agarose constructs reveals new candidate mechanosensitive genes. PLoS ONE 2012, 7, e36964. [Google Scholar]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Rosenthal, A.; Voldman, J. Dielectrophoretic traps for single-particle patterning. Biophys. J. 2005, 88, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Tsang, V.L.; Sah, R.L.; Bhatia, S.N. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Underhill, G.H.; Wassermann, T.B.; Sah, R.L.; Bhatia, S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat. Methods 2006, 35, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Underhill, G.H.; Mendelson, A.; Bhatia, S.N. Multiphase electropatterning of cells and biomaterials. Lab Chip 2007, 7, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Yang, Y.-W.; Chen, Y.-D.; Wang, S.-S.; Chang, Y.-H.; Wu, M.-H. The application of an optically switched dielectrophoretic (ODEP) force for the manipulation and assembly of cell-encapsulating alginate microbeads in a microfluidic perfusion cell culture system for bottom-up tissue engineering. Lab Chip 2012, 12, 1164–1173. [Google Scholar] [CrossRef]
- Menad, S.; Franqueville, L.; Haddour, N.; Buret, F.; Frenea-Robin, M. nDEP-driven cell patterning and bottom-up construction of cell aggregates using a new bioelectronic chip. Acta Biomater. 2015, 17, 107–114. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyata, S. Continuous ES/feeder cell-sorting device using dielectrophoresis and controlled fluid flow. Micromachines 2020, 11, 734. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Miyata, S. Multi-layered cell assembling technology using dielectrophoresis and construction of skin tissue microelement. Trans. Jpn. Soc. Mech. Eng. C 2017, 83, 16-00387. [Google Scholar]
- Ojima, Y.; Miyata, S. Discrimination methodology of living-cells and microbeads using dielectrophoresis and fluid-induced shear force. J. Biorheol. 2015, 29, 42–50. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, Y.; Miyata, S. Micro cell patterning technology by dielectrophoresis and application to regenerated cartilage. Trans. Jpn. Soc. Mech. Eng. C 2010, 76, 3015–3020. [Google Scholar] [CrossRef][Green Version]
- Miyata, S.; Furukawa, K.S.; Ushida, T.; Nitta, Y.; Tateishi, T. Static and dynamic mechanical properties of extracellular matrix synthesized by cultured chondrocytes. Mater. Sci. Eng. C 2004, 24, 425–429. [Google Scholar] [CrossRef]
- Miyata, S.; Numano, T.; Homma, K.; Tateishi, T.; Ushida, T. Feasibility of noninvasive evaluation of biophysical properties of tissue-engineered cartilage by using quantitative MRI. J. Biomech. 2007, 40, 2990–2998. [Google Scholar] [CrossRef]
- Miyata, S.; Tateishi, T.; Ushida, T. Influence of cartilaginous matrix accumulation on viscoelastic response of chondrocyte/agarose constructs under dynamic compressive and shear loading. J. Biomech. Eng. 2008, 130, 051016. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Homma, K.; Numano, T.; Tateishi, T.; Ushida, T. Evaluation of negative fixed-charge density in tissue-engineered cartilage by quantitative MRI and relationship with biomechanical properties. J. Biomech. Eng. 2010, 132, 071014. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. BBA Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sah, R.L.Y.; Doong, J.Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Tanaka, N.; Ota, H.; Fukumori, K.; Miyake, J.; Yamato, M.; Okano, T. Micro-Patterned Cell-Sheets Fabricated with Stamping-Force-Controlled Micro-Contact Printing. Biomaterials 2014, 35, 9802–9810. [Google Scholar] [CrossRef]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]



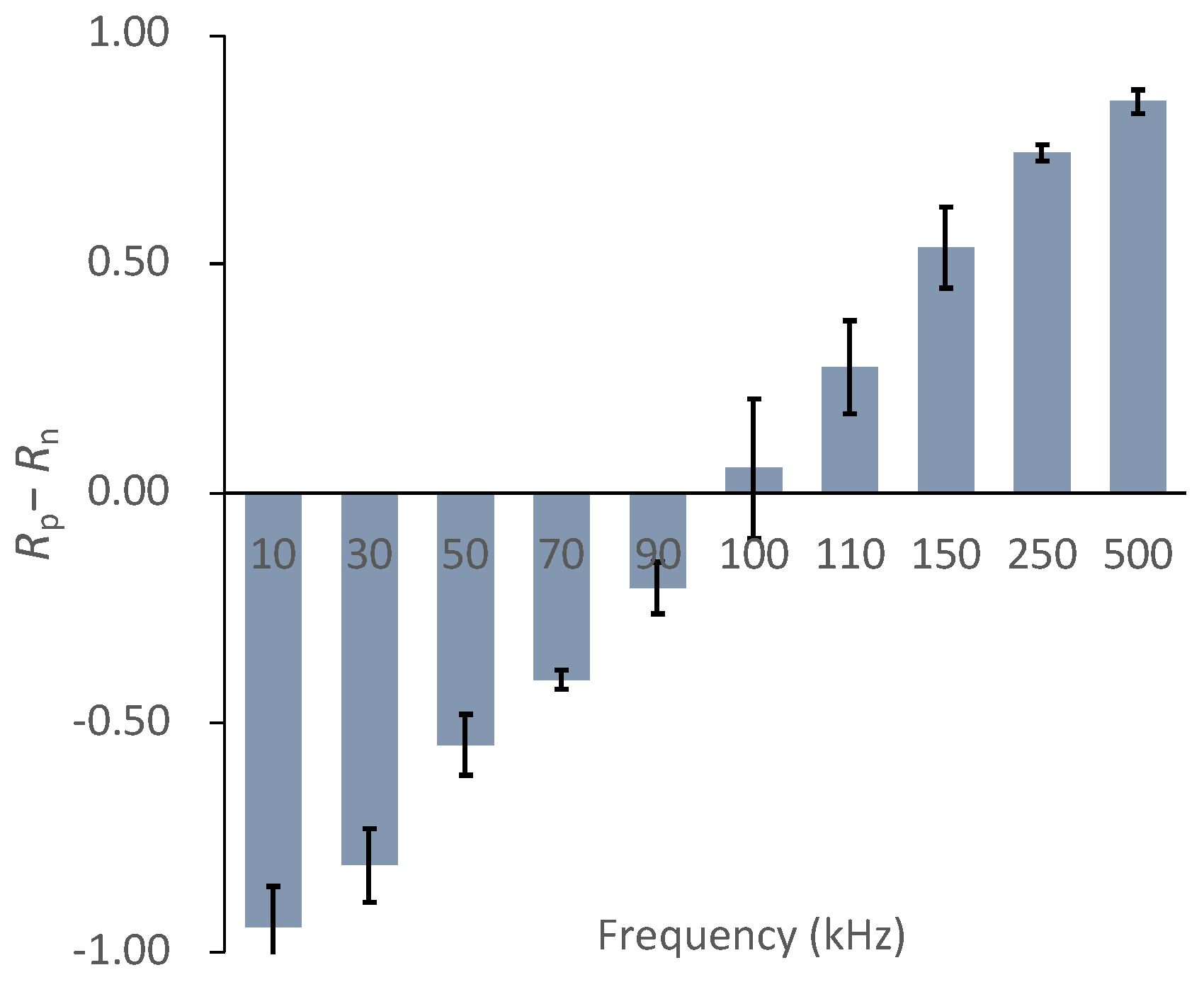

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, Y.; Miyata, S. Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue. Micromachines 2021, 12, 1098. https://doi.org/10.3390/mi12091098
Takeuchi Y, Miyata S. Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue. Micromachines. 2021; 12(9):1098. https://doi.org/10.3390/mi12091098
Chicago/Turabian StyleTakeuchi, Yoshitaka, and Shogo Miyata. 2021. "Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue" Micromachines 12, no. 9: 1098. https://doi.org/10.3390/mi12091098
APA StyleTakeuchi, Y., & Miyata, S. (2021). Dielectrophoretic Micro-Organization of Chondrocytes to Regenerate Mechanically Anisotropic Cartilaginous Tissue. Micromachines, 12(9), 1098. https://doi.org/10.3390/mi12091098





