Shear Thickening Polishing of Quartz Glass
Abstract
:1. Introduction
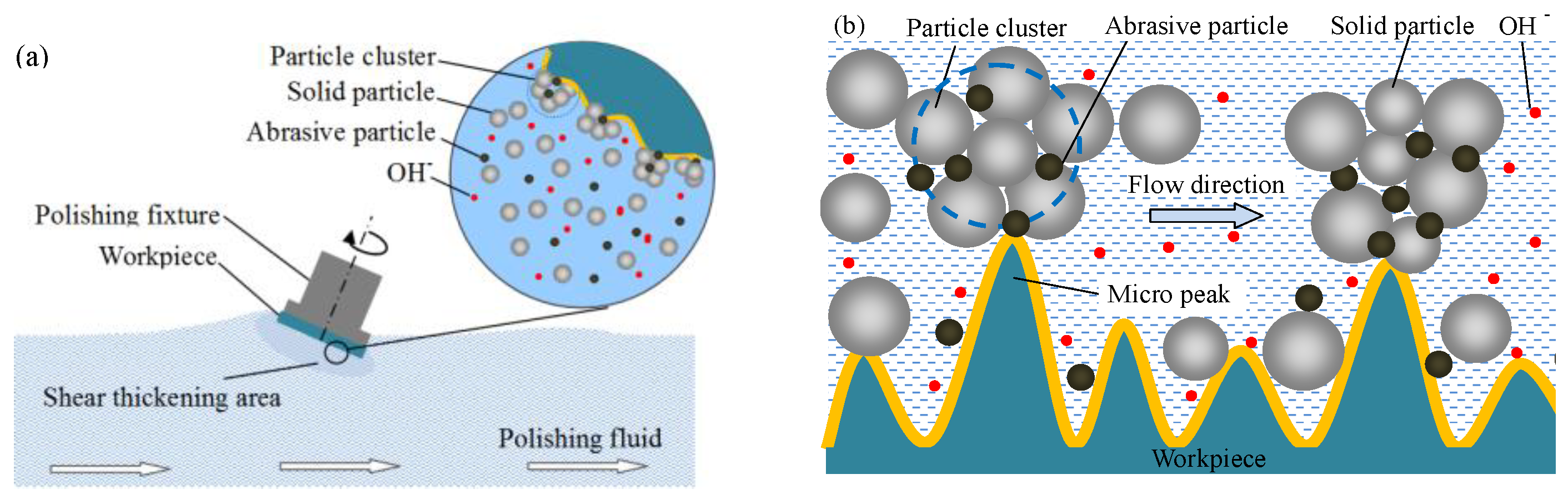
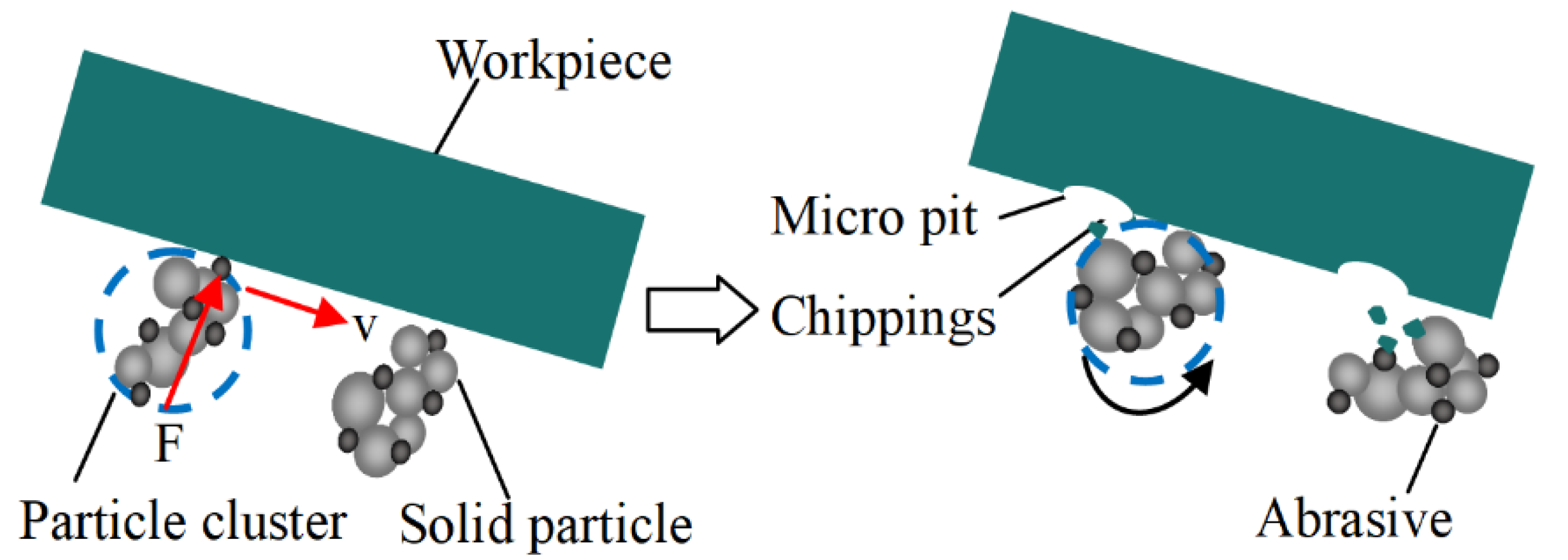
2. Principle of Shear Thickening Polishing
3. Experimental Method and Condition
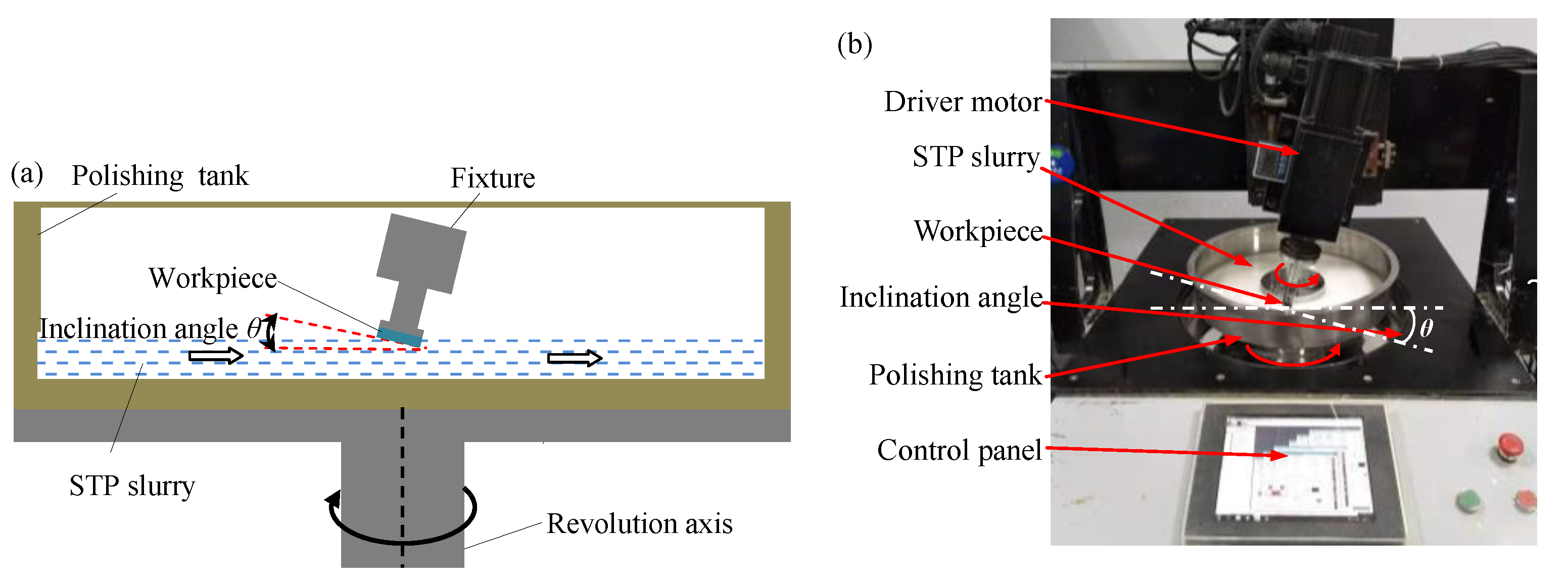
3.1. Experimental Process and Conditions
3.2. Preparation of STP Slurry
4. Results and Discussion
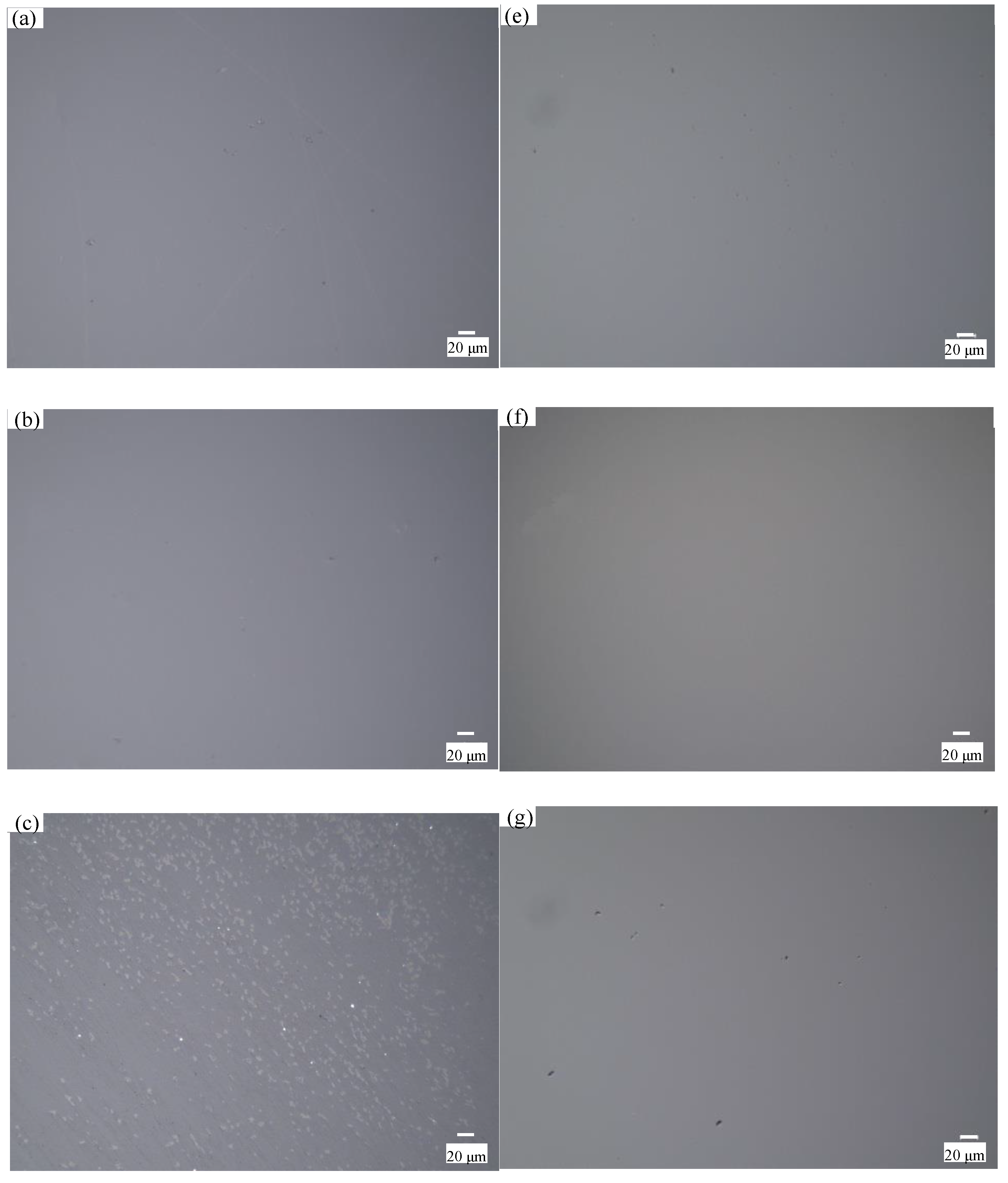
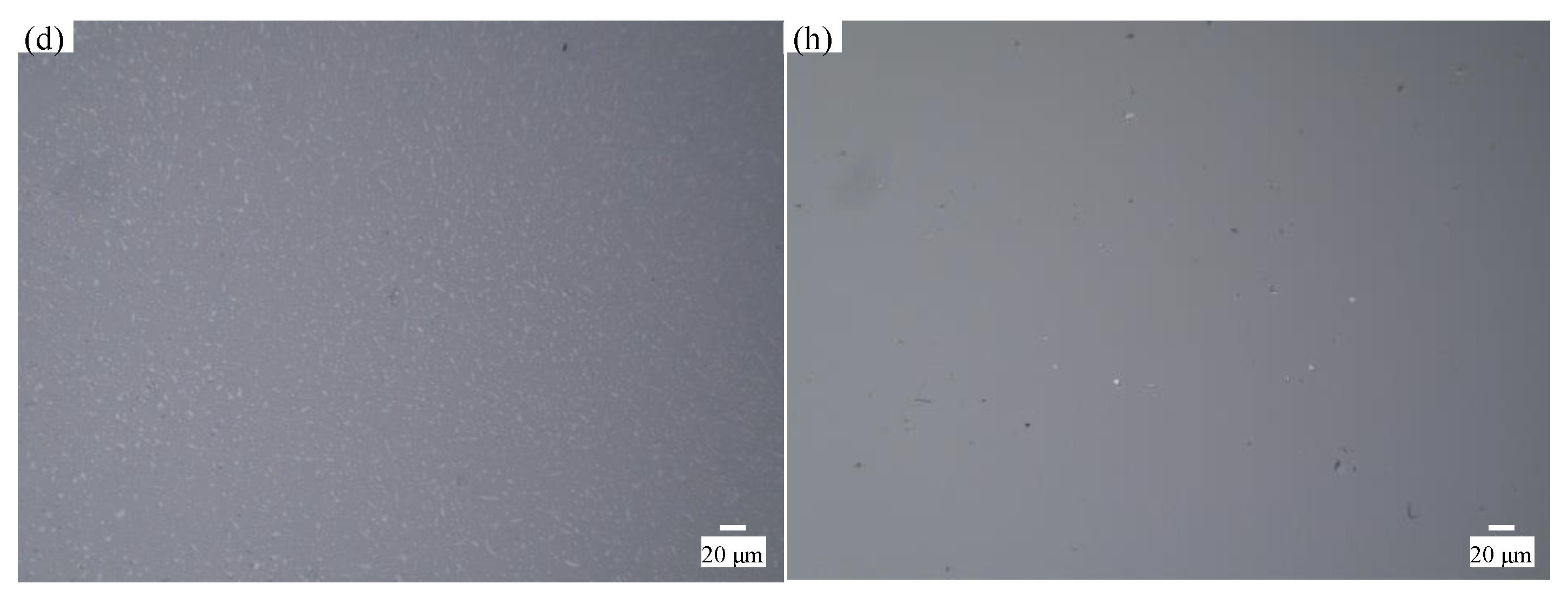
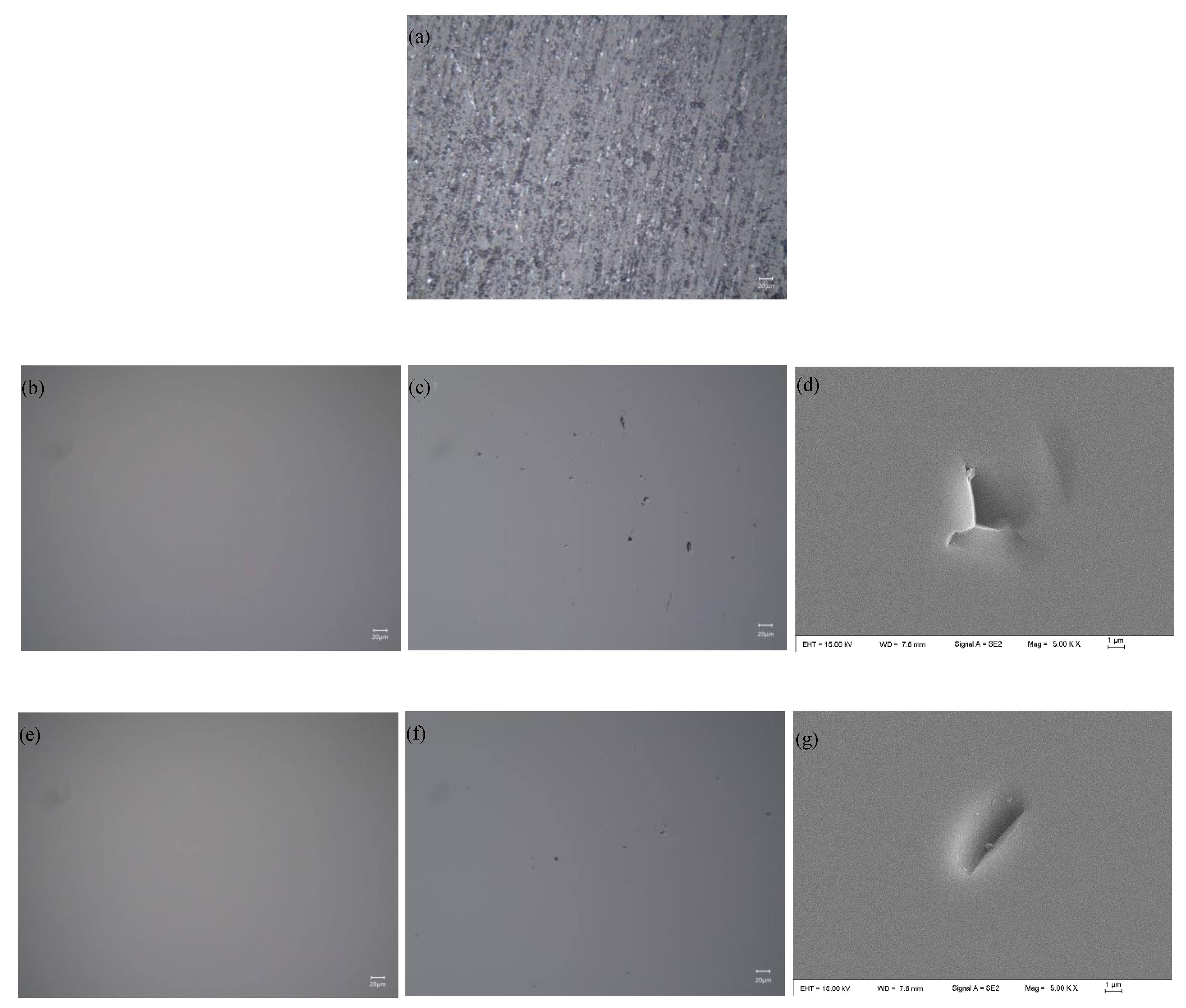
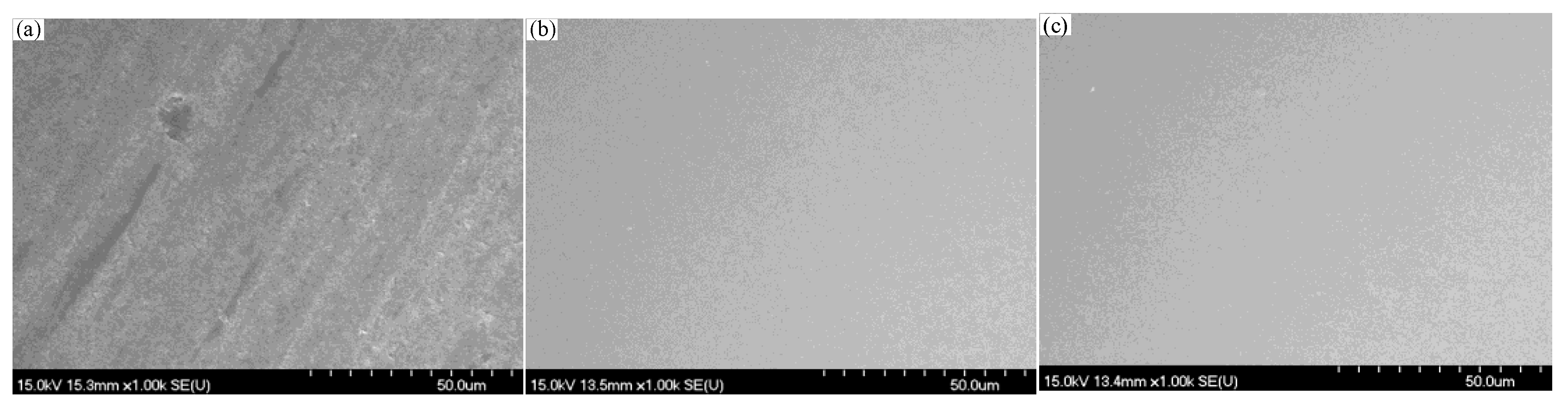
4.1. Material Removal Mechanism of Quartz Glass with Different Slurry
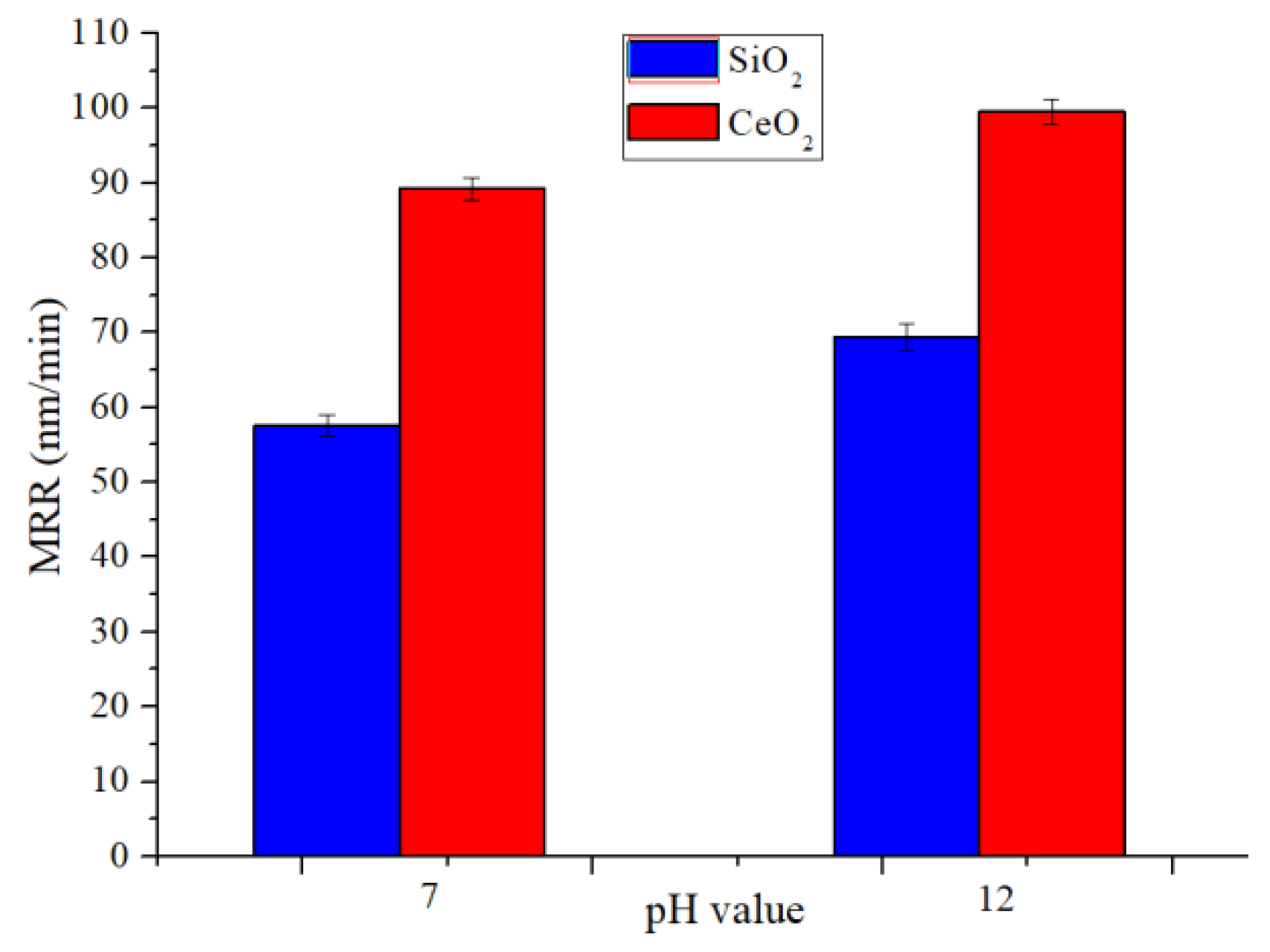
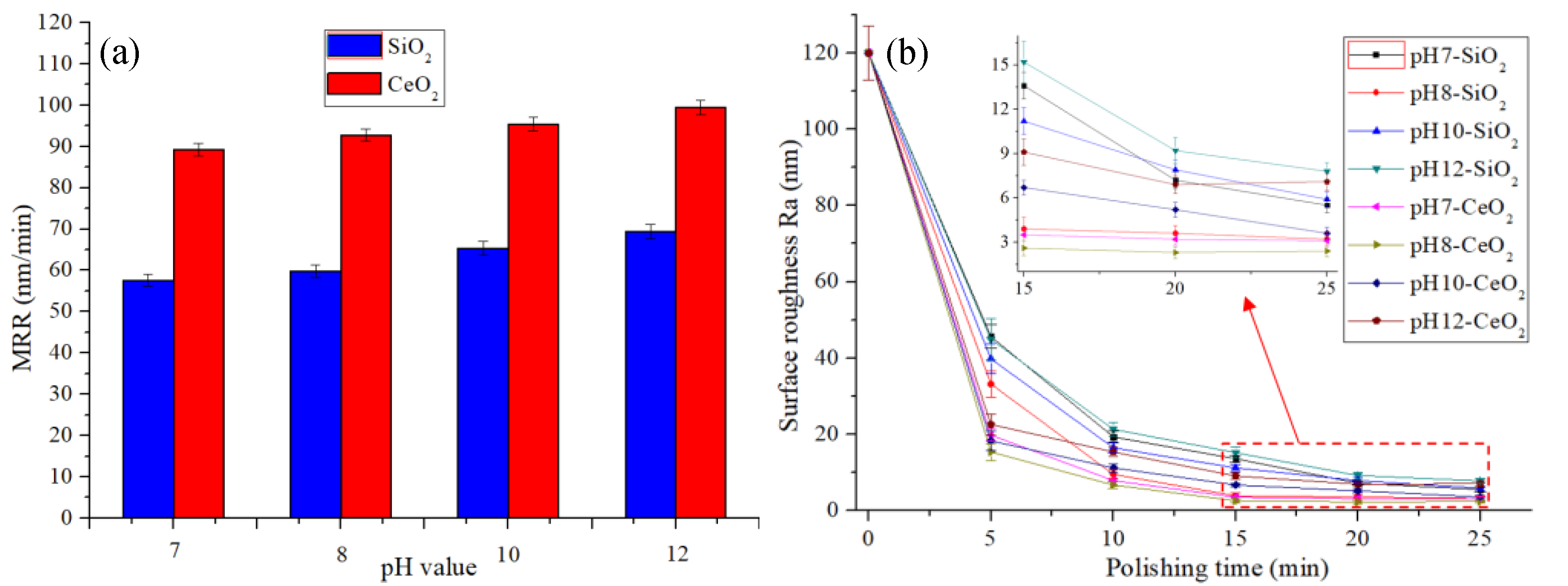
4.2. Polishing at Different pH Values
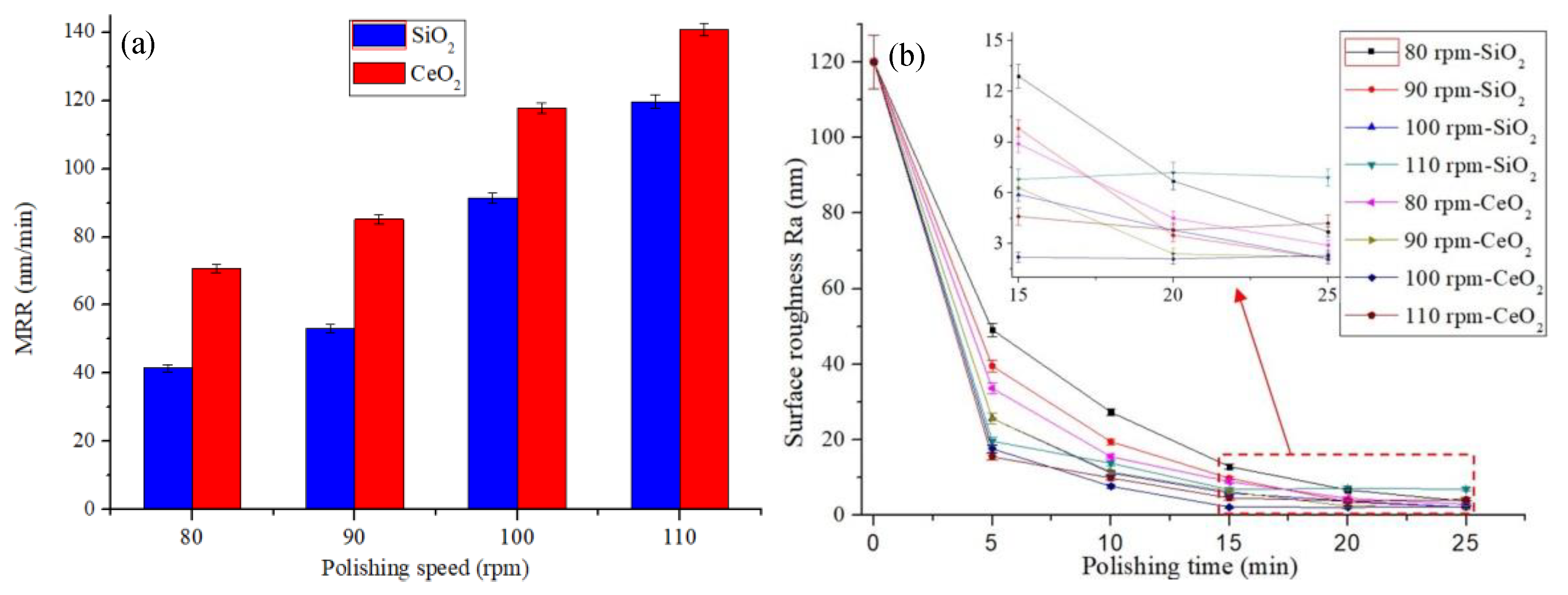
4.3. Polishing at Different Speeds
4.4. Polishing at Different Concentrations
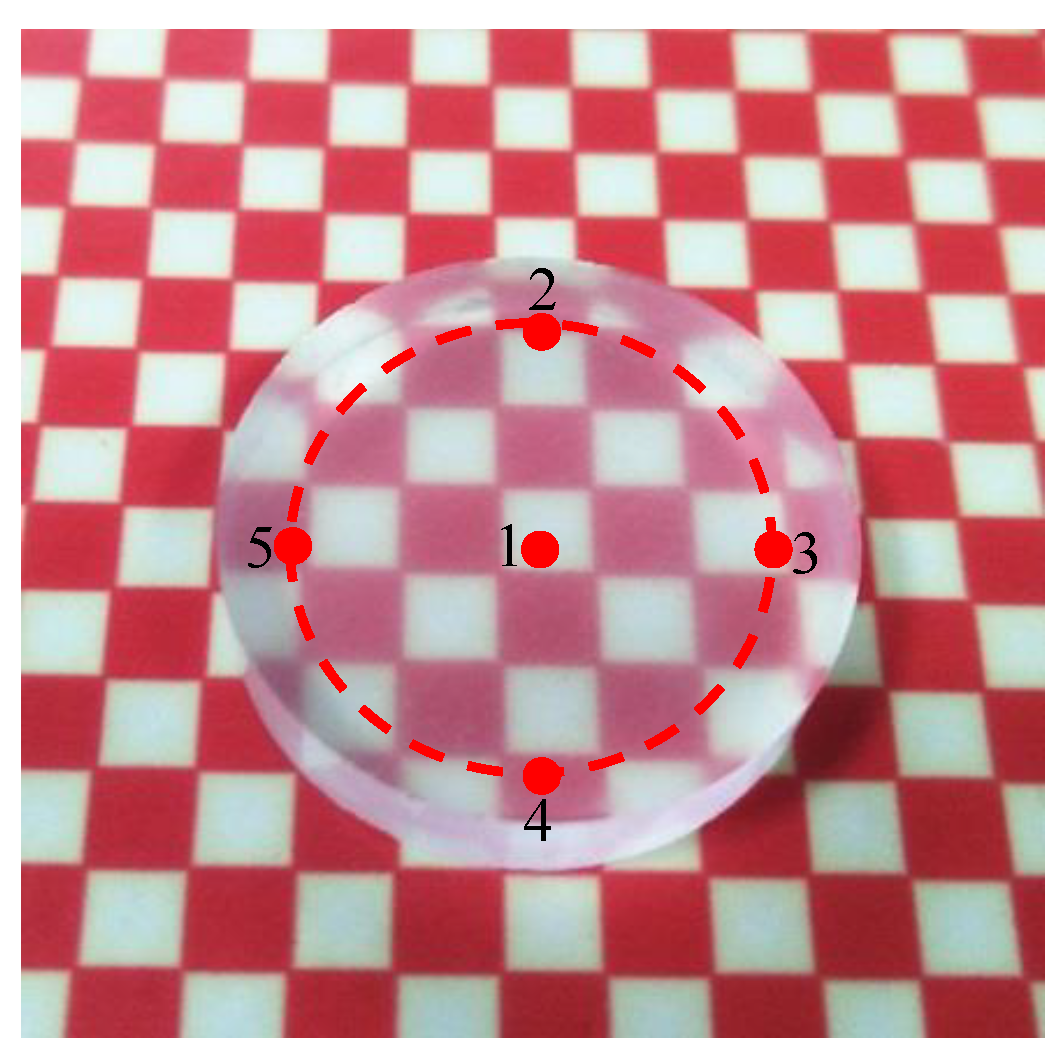
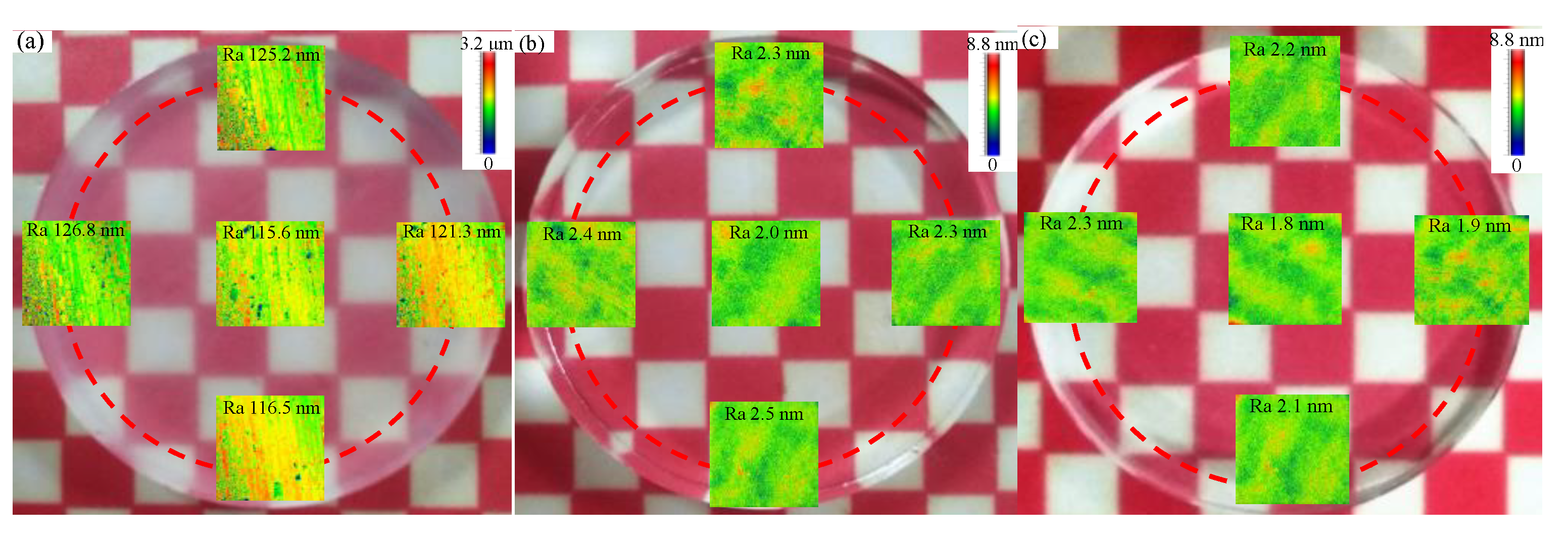
4.5. Polishing Experiment with Selected Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solinov, V.F. Study of defects in quartz glass with the action of high-velocity microparticles on port-holes. Glass Ceram. 2010, 67, 234–237. [Google Scholar] [CrossRef]
- Chen, S.T.; Jiang, Z.H. A force controlled grinding-milling technique for quartz-glass micromachining. J. Mater. Process. Technol. 2015, 216, 206–215. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Kurokawa, S.; Toyama, T.; Hayashi, T. CMP characteristics of quartz glass substrate by aggregated colloidal ceria slurry. Precis. Eng. 2019, 60, 458–464. [Google Scholar] [CrossRef]
- Jian, S.; Liu, S.; Yi, K.; He, H.; Shao, J.; Fan, Z. Subsurface damage in optical substrates. Optik 2005, 116, 288–294. [Google Scholar]
- Zhao, F.X.; Zhou, L.; Fan, Z.B.; Dai, Z.C. Research on Surface Processing of Quartz Wafer Based on Magnetorheological Finishing and Ion Beam Figuring. Procedia CIRP 2018, 71, 496–499. [Google Scholar] [CrossRef]
- Zhao, T.; Deng, Q.; Zhang, C.; Feng, K.; Yuan, J. Orthogonal experimental research on dielectrophoresis polishing (DEPP) of silicon wafer. Micromachines 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Song, Z.; Feng, S.L. Ceria concentration effect on chemical mechanical polishing of optical glas. Appl. Surf. Sci. 2007, 253, 4951–4954. [Google Scholar] [CrossRef]
- Shao, H.Y.; Ke, J.Z.; Yu, F.F.; Zhu, Y.J.; Yu, W. Influences of polishing tool’s shape on surface roughness in magneto-rheological finishing. Adv. Mater. Res. 2010, 97–101, 4092–4095. [Google Scholar]
- Mosavat, M.; Rahimi, A. Numerical-experimental study on the polishing of silicon wafers using coupled finite element-smoothed particle hydrodynamics. Appl. Opt. 2019, 58, 1569–1576. [Google Scholar] [CrossRef]
- Mosavat, M.; Rahimi, A.; Eshraghi, M.J.; Karami, S. Nano-finishing of the monocrystalline silicon wafer using magnetic abrasive finishing process. Appl. Opt. 2019, 58, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Fujitani, M.; Ishikawa, K.; Numazawa, M.; Ishi, D.; Otsubo, R.; Nagatoshi, H.; Suzuki, H.; Yuasa, T.; Ohashi, T.; et al. Grinding and chemical mechanical polishing process for micropore x-ray optics fabricated with deep reactive ion etching. Appl. Opt. 2019, 58, 5240–5247. [Google Scholar] [CrossRef]
- Li, M.; Lyu, B.H.; Yuan, J.L.; Dong, C.C.; Dai, W.T. Shear-thickening polishing method. Int. J. Mach. Tools. Manuf. 2015, 94, 88–99. [Google Scholar] [CrossRef]
- Lyu, B.H.; He, Q.K.; Chen, S.H.; Shao, Q.; Chen, Y.; Geng, Z.Y. Experimental study on shear thickening polishing of cemented carbide insert with complex shape. Int. J. Mach. Tools Manuf. 2019, 103, 585–595. [Google Scholar] [CrossRef]
- Lyu, B.H.; Shao, Q.; Hang, W.; Chen, S.H.; He, Q.K.; Yuan, J.L. Shear Thickening Polishing of Black Lithium Tantalite Substrate. Int. J. Precis. Eng. Manuf. 2020, 21, 1663–1675. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Dao, T.P.; Prakash, C.; Singh, S.; Pramanik, A.; Krolczyk, G.; Pruncu, C.I. Machining parameter optimization in shear thickening polishing of gear surfaces. J. Mater. Res. Technol. 2020, 9, 5112–5126. [Google Scholar] [CrossRef]
- Li, M.; Karpuschewski, B.; Ohmori, H.; Riemer, O.; Wang, Y.; Dong, T. Adaptive shearing-gradient thickening polishing (AS-GTP) and subsurface damage inhibition. Int. J. Mach. Tools Manuf. 2021, 160, 103651. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Riemer, O.; Song, F.Z.; Lyu, B.H. Anhydrous based shear-thickening polishing of KDP crystal. Chin. J. Aeronaut. 2020, 34, 90–99. [Google Scholar] [CrossRef]
- Shao, Q.; Lyu, B.H.; Yuan, J.L.; Wang, X.; Zhao, P. Shear thickening polishing of the concave surface of high-temperature nickel-based alloy turbine blade. J. Mater. Res. Technol. 2021, 11, 72–84. [Google Scholar] [CrossRef]
- Martin, C.; Legrand, C.; Mouret, M. Study of the shear thickening effect of superplasticizers on the rheological behaviour of cement pastes containing or not mineral additives. Cem. Concr. Res. 2000, 30, 1477–1483. [Google Scholar]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Rubio-Hernández, F.J.; Sevilla, A. An apparent viscosity function for shear thickening fluids. J. Non-Newton. Fluid Mech. 2011, 166, 321–325. [Google Scholar] [CrossRef]
- Osseo-Asare, K. Surface Chemical Processes in Chemical Mechanical Polishing Relationship Between Silica Material Removal Rate and the Point of Zero Charge of the Abrasive Material. J. Electrochem. Soc. 2002, 149, G651–G655. [Google Scholar] [CrossRef]
- Kakinuma, Y.; Konuma, Y.; Fukuta, M.; Tanaka, K. Ultra-precision grinding of optical glass lenses with La-doped CeO2 slurry. CIRP Ann. Manuf. Technol. 2019, 68, 345–348. [Google Scholar] [CrossRef]



| Parameters | Values |
|---|---|
| Abrasive particles | SiO2 (80 nm on average), CeO2 (100 nm on average) |
| The diameter of the polishing tank (mm) | 400 |
| Inclination angle (°) | 13 |
| Polishing speed (rpm) | 80, 90, 100, 110 |
| Concentration (wt%) | 2, 4, 6, 8 |
| The slurry pH value | 7, 8, 10, 12 |
| Parameters | Values |
|---|---|
| Content of SiO2 (%) | 99.95 |
| Density (g/cm3) | 2.2 |
| Melting point (°C) | 1150 |
| Mohs hardness | 7 |
| Poisson ratio ν | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Duan, S.; Fu, L.; Lyu, B.; Zhao, P.; Yuan, J. Shear Thickening Polishing of Quartz Glass. Micromachines 2021, 12, 956. https://doi.org/10.3390/mi12080956
Shao Q, Duan S, Fu L, Lyu B, Zhao P, Yuan J. Shear Thickening Polishing of Quartz Glass. Micromachines. 2021; 12(8):956. https://doi.org/10.3390/mi12080956
Chicago/Turabian StyleShao, Qi, Shixiang Duan, Lin Fu, Binghai Lyu, Ping Zhao, and Julong Yuan. 2021. "Shear Thickening Polishing of Quartz Glass" Micromachines 12, no. 8: 956. https://doi.org/10.3390/mi12080956
APA StyleShao, Q., Duan, S., Fu, L., Lyu, B., Zhao, P., & Yuan, J. (2021). Shear Thickening Polishing of Quartz Glass. Micromachines, 12(8), 956. https://doi.org/10.3390/mi12080956






