Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis
Abstract
1. Introduction
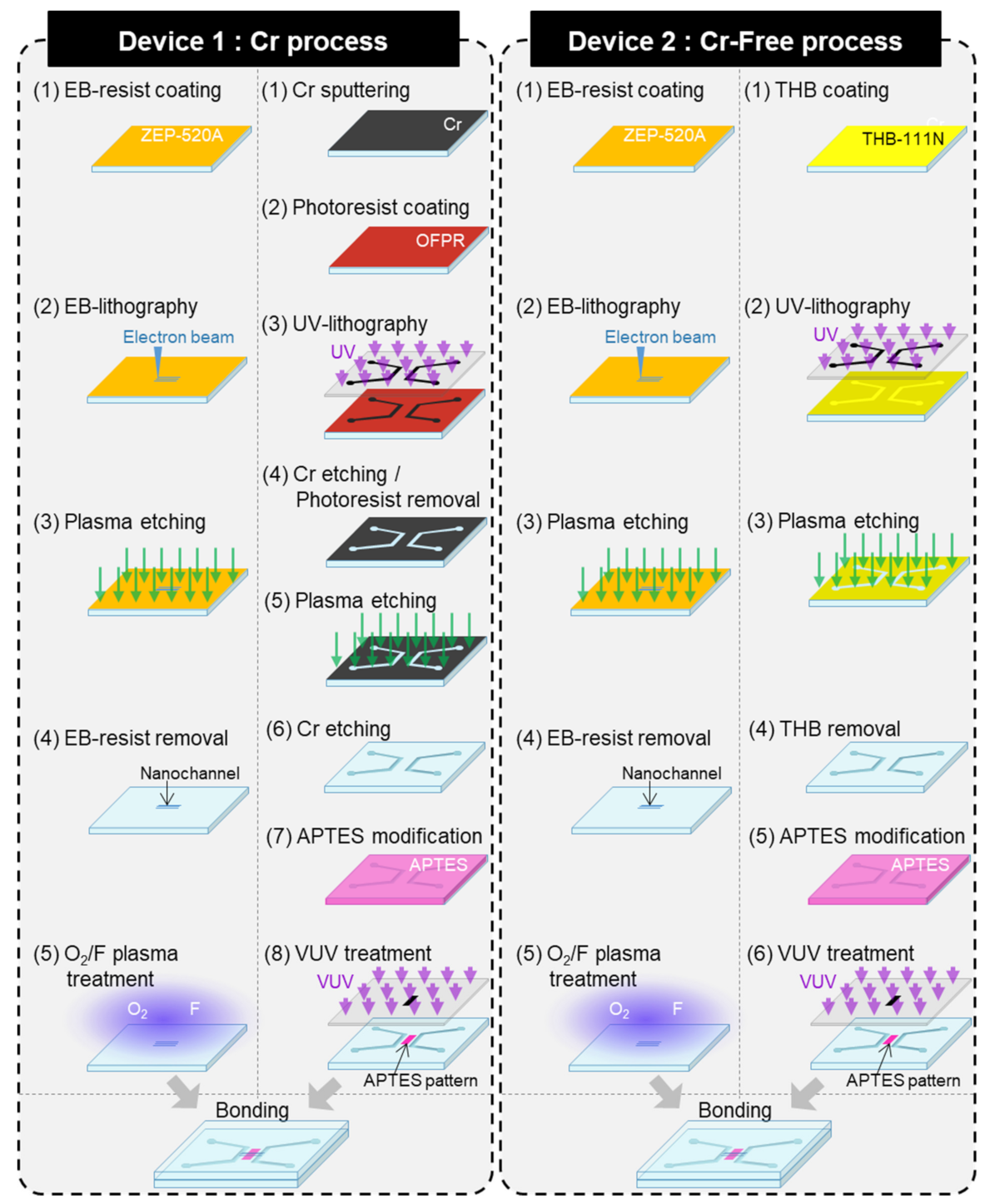
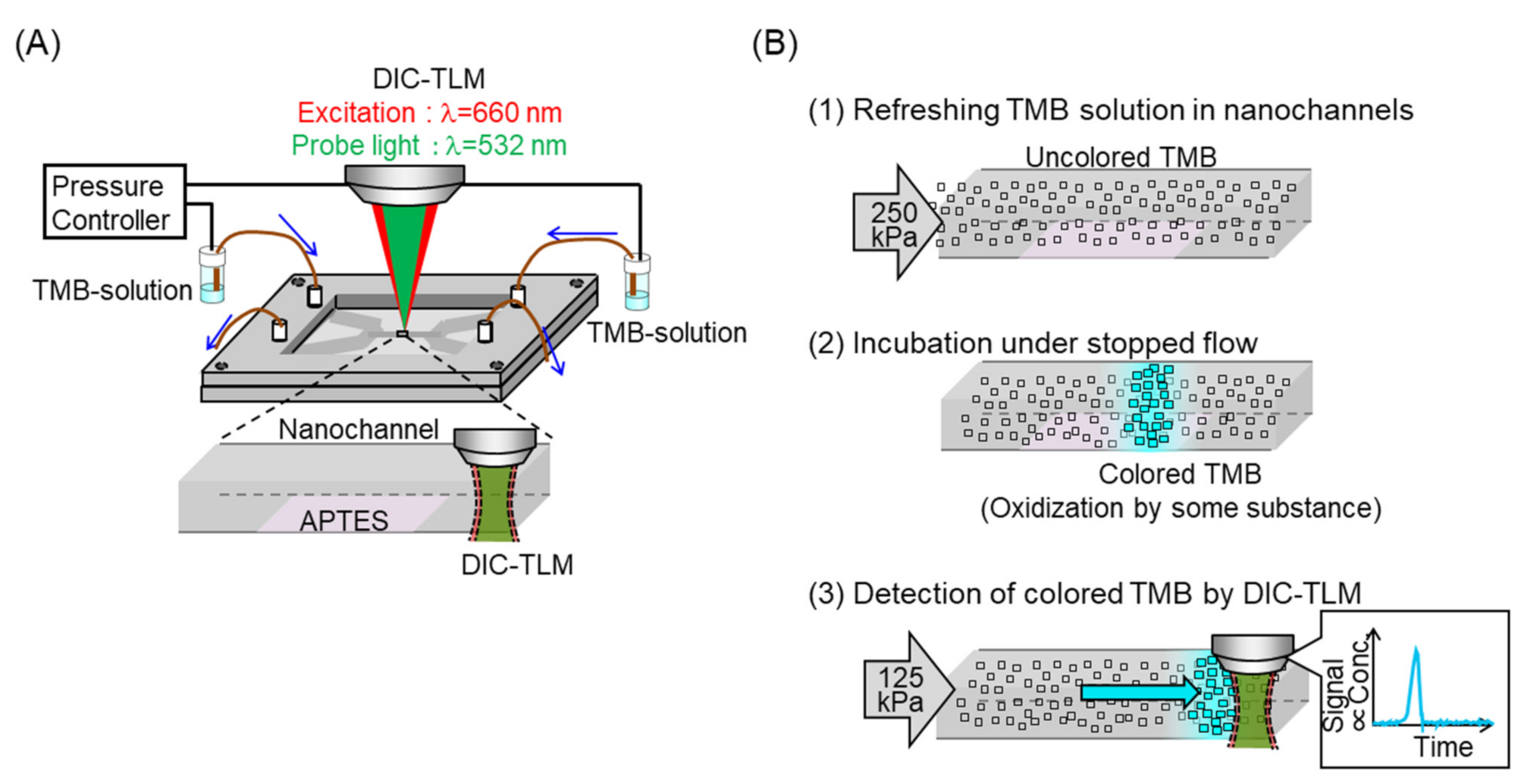
2. Materials and Methods
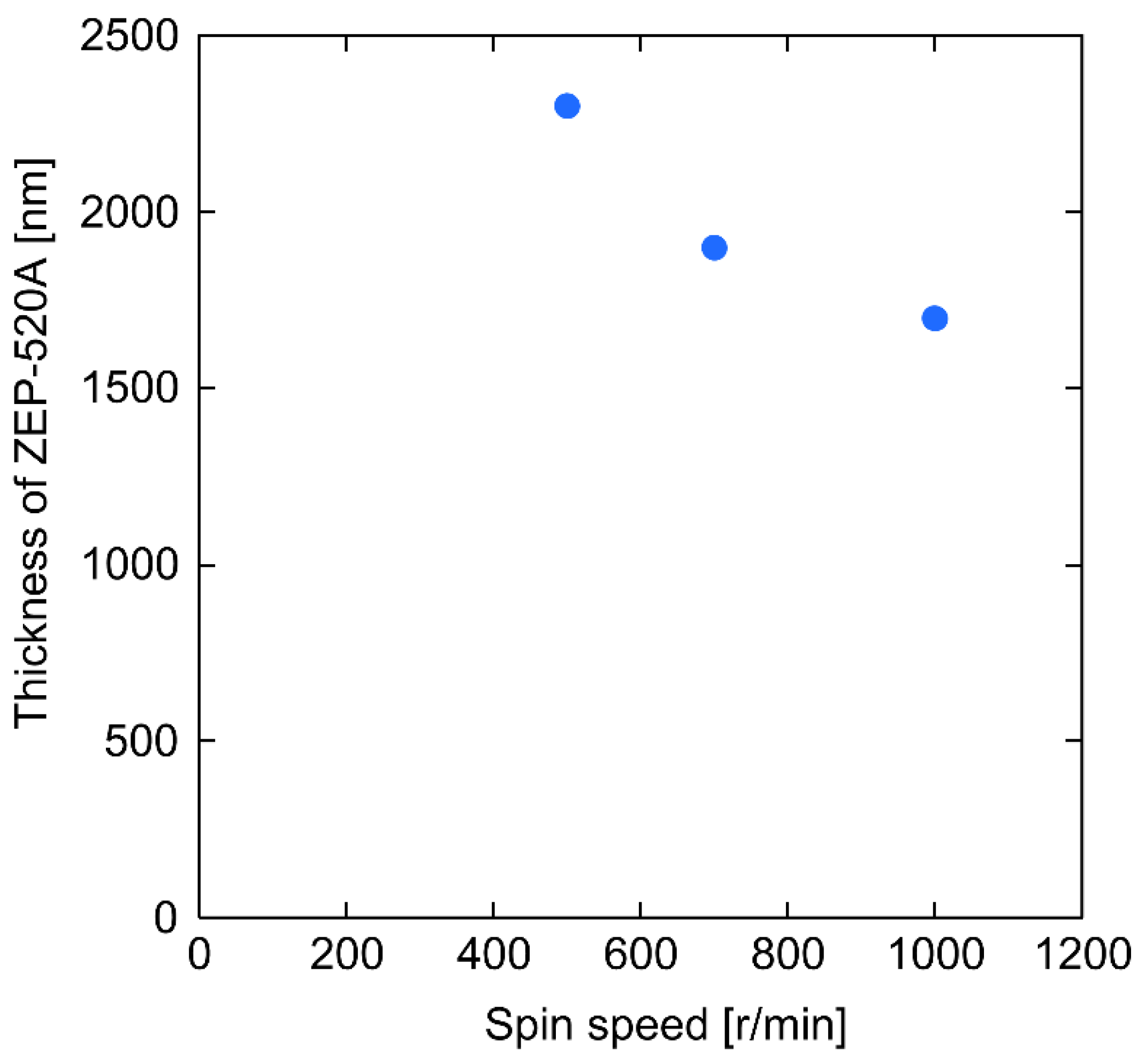
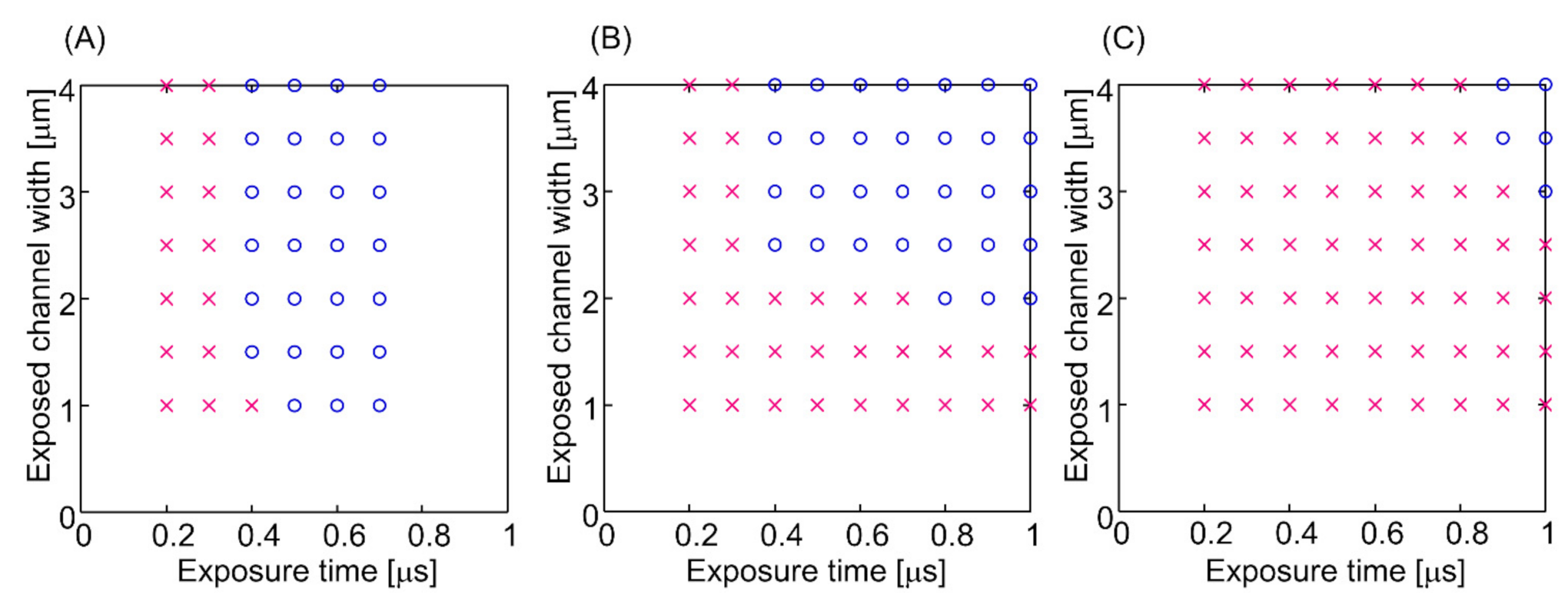
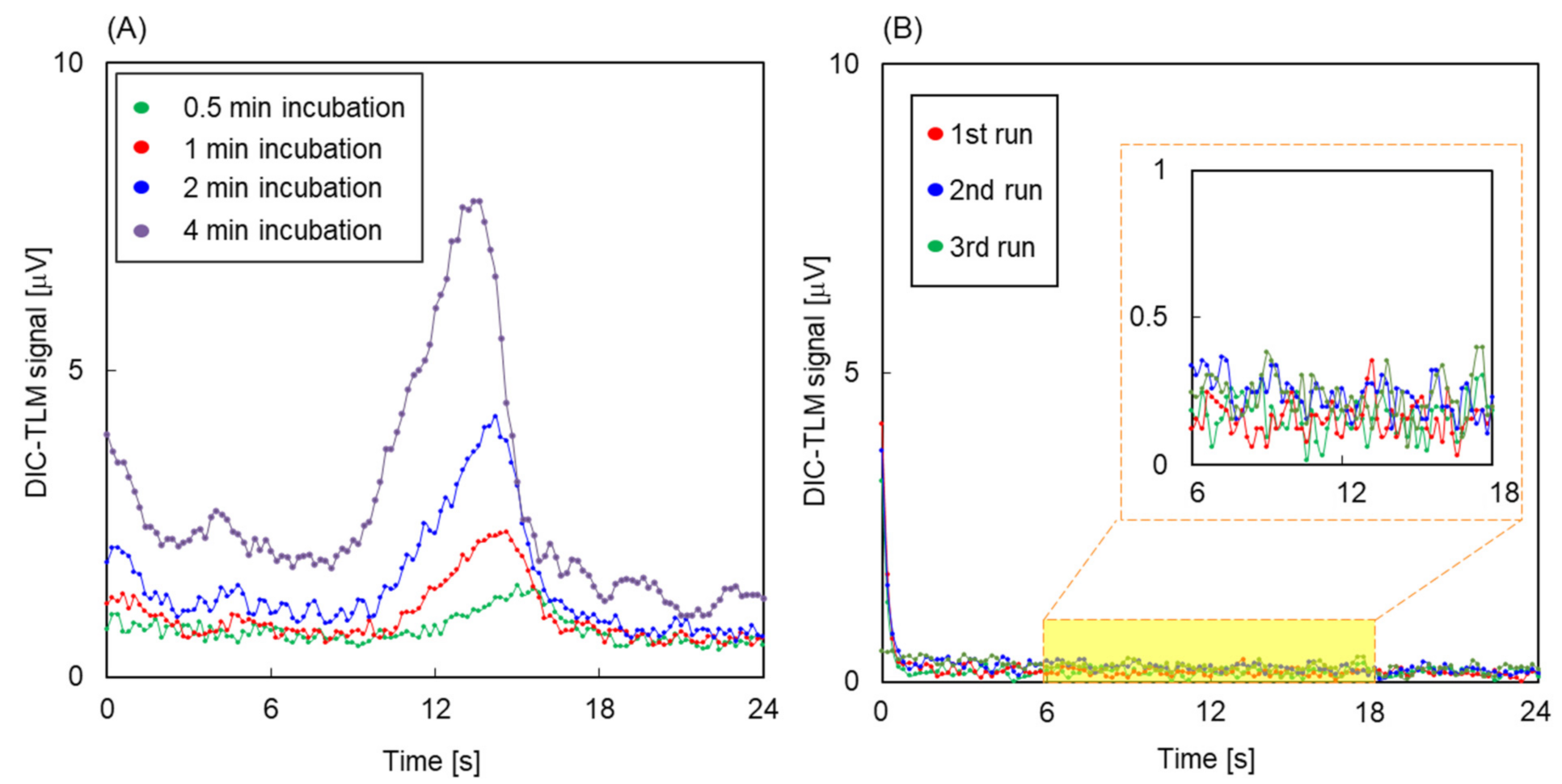
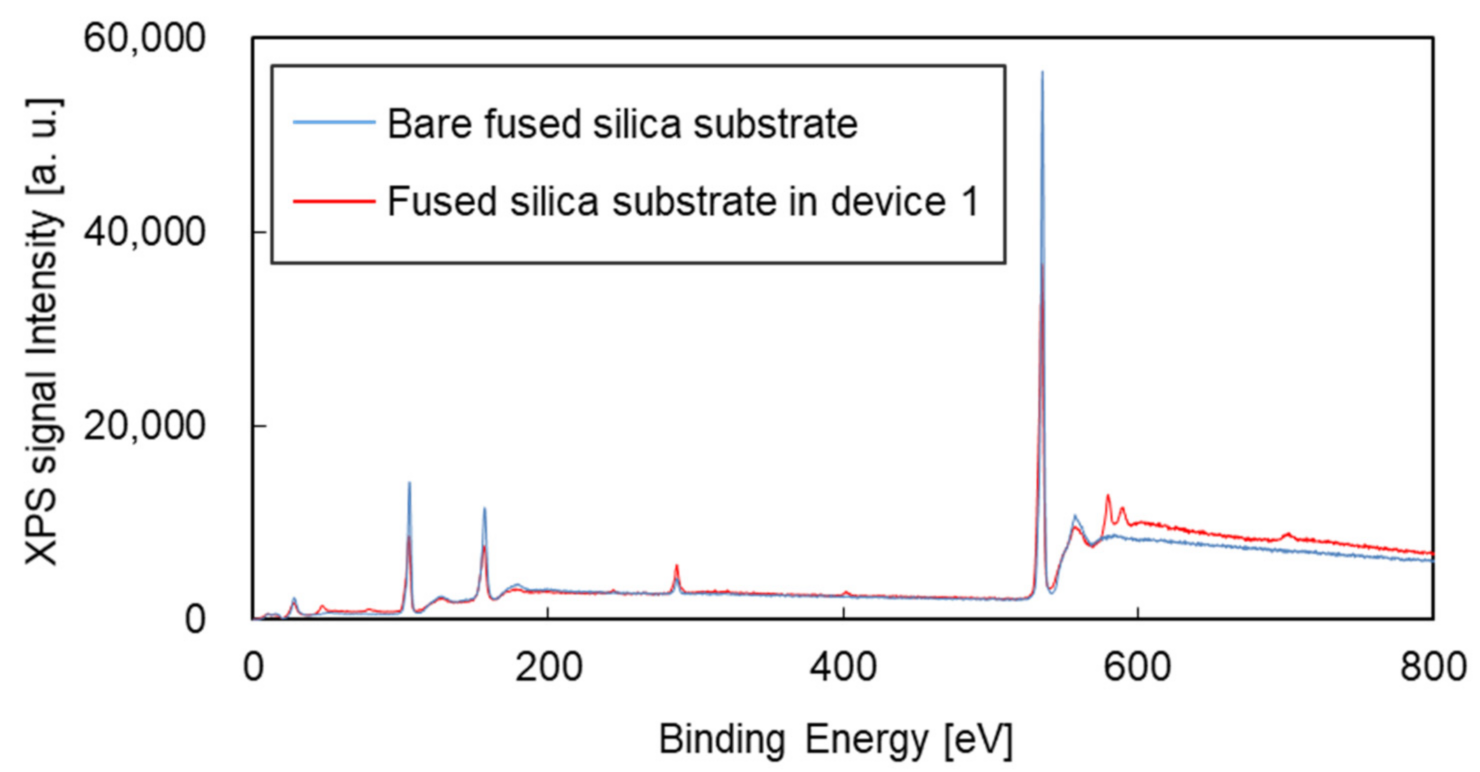
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, A. Micro total analysis systems. 1. introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Auroux, P.A.; Iossifidis, D.; Reyes, D.R.; Manz, A. Micro total analysis systems. 2. analytical standard operations and applications. Anal. Chem. 2002, 74, 2637–2652. [Google Scholar] [CrossRef]
- Song, H.; Li, H.W.; Munson, M.S.; Ha, T.G.V.; Ismagilov, R.F. On-chip titration of an anticoagulant argatroban and determination of the clotting time within whole blood or plasma using a plug-based microfluidic system. Anal. Chem. 2006, 78, 4839–4849. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab. Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Recent advances in the production of controllable multiple emulsions using microfabricated devices. Particuology 2016, 24, 1–17. [Google Scholar] [CrossRef]
- Bojang, A.A.; Wu, H.S. Design, fundamental principles of fabrication and applications of microreactors. Processes 2020, 8, 891. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab. Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.Q.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the lateral flow assay: A review of paper-based microfluidics. Microelectron. Eng. 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Leung, A.; Soleymani, L.; Didar, T.F. Biofunctionalization of glass- and paper-based microfluidic devices: A review. Adv. Mater. Interfaces 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Tokeshi, M.; Kikutani, Y.; Hibara, A.; Sato, K.; Hisamoto, H.; Kitamori, T. Chemical processing on microchips for analysis, synthesis, and bioassay. Electrophoresis 2003, 24, 3583–3594. [Google Scholar] [CrossRef]
- Ohira, S.I.; Toda, K. Micro gas analyzers for environmental and medical applications. Anal. Chim. Acta 2008, 619, 143–156. [Google Scholar] [CrossRef]
- Mijatovic, D.; Eijkel, J.C.T.; Van Den Berg, A. Technologies for nanofluidic systems: Top-down vs. bottom-up—A review. Lab. Chip 2005, 5, 492–500. [Google Scholar] [CrossRef]
- Bocquet, L.; Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Eijkel, J.C.T.; Pennathur, S. Nanofluidic technology for biomolecule applications: A critical review. Lab. Chip 2010, 10, 957–985. [Google Scholar] [CrossRef]
- Mawatari, K.; Kazoe, Y.; Shimizu, H.; Pihosh, Y.; Kitamori, T. Extended-nanofluidics: Fundamental technologies, unique liquid properties, and application in chemical and bio analysis methods and devices. Anal. Chem. 2014, 86, 4068–4077. [Google Scholar] [CrossRef]
- Le, T.H.H.; Shimizu, H.; Morikawa, K. Advances in label-free detections for nanofluidic analytical devices. Micromachines 2020, 11, 885. [Google Scholar] [CrossRef]
- Karnik, R.; Castelino, K.; Fan, R.; Yang, P.; Majumdar, A. Effects of biological reactions and modifications on conductance of nanofluidic channels. Nano Lett. 2005, 5, 1638–1642. [Google Scholar] [CrossRef]
- Schoch, R.B.; Cheow, L.F.; Han, J. Electrical detection of fast reaction kinetics in nanochannels with an induced flow. Nano Lett. 2007, 7, 3895–3900. [Google Scholar] [CrossRef]
- Liao, T.; Li, X.; Tong, Q.; Zou, K.; Zhang, H.; Tang, L.; Sun, Z.; Zhang, G.J. Ultrasensitive Detection of MicroRNAs with Morpholino-functionalized nanochannel biosensor. Anal. Chem. 2017, 89, 5511–5518. [Google Scholar] [CrossRef]
- Duan, C.; Alibakhshi, M.A.; Kim, D.K.; Brown, C.M.; Craik, C.S.; Majumdar, A. Label-Free electrical detection of enzymatic reactions in Nanochannels. ACS Nano 2016, 10, 7476–7484. [Google Scholar] [CrossRef]
- Zevenbergen, M.A.G.; Singh, P.S.; Goluch, E.D.; Wolfrum, B.L.; Lemay, S.G. Stochastic sensing of single molecules in a nanofluidic electrochemical device. Nano Lett. 2011, 11, 2881–2886. [Google Scholar] [CrossRef]
- Rassaei, L.; Mathwig, K.; Kang, S.; Heering, H.A.; Lemay, S.G. Integrated biodetection in a nanofluidic device. ACS Nano 2014, 8, 8278–8284. [Google Scholar] [CrossRef]
- Yamamoto, K.; Morikawa, K.; Imanaka, H.; Imamura, K.; Kitamori, T. Picoliter enzyme reactor on a nanofluidic device exceeding the bulk reaction rate. Analyst 2020, 145, 5801–5807. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Morikawa, K.; Liu, Y.; Smirnova, A.; Mawatari, K.; Kitamori, T. Femtoliter high-performance liquid chromatography using extended-nano channels. Analyst 2016, 141, 6068–6072. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, Y.; Morikawa, K.; Mawatari, K. Integration of sequential analytical processes into sub-100 nm channels: Volumetric sampling, chromatographic separation, and label-free molecule detection. Nanoscale 2021, 13, 8855–8863. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Mawatari, K.; Ohta, R.; Shimizu, H.; Kitamori, T. A single-molecule ELISA device utilizing nanofluidics. Analyst 2018, 143, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Tokeshi, M.; Minagawa, T.; Uchiyama, K.; Hibara, A.; Sato, K.; Hisamoto, H.; Kitamori, T. Continuous-flow chemical processing on a microchip by Combining microunit operations and a multiphase flow network tion of complicated chemical processing on a microchip. Anal. Chem. 2002, 74, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Kazoe, Y.; Mori, E.; Morikawa, K.; Fukasawa, T.; Yoshizaki, A.; Kitamori, T. Cytokine analysis on a countable number of molecules from living single cells on nanofluidic devices. Analyst 2019, 144, 7200–7208. [Google Scholar] [CrossRef]
- Morikawa, K.; Kazoe, Y.; Takagi, Y.; Tsuyama, Y.; Pihosh, Y.; Tsukahara, T.; Kitamori, T. Advanced Top-Down fabrication for a fused silica nanofluidic device. Micromachines 2020, 11, 995. [Google Scholar] [CrossRef]
- Morikawa, K.; Matsushita, K.; Tsukahara, T. Rapid plasma etching for fabricating fused silica microchannels. Anal. Sci. 2017, 33, 1453–1456. [Google Scholar] [CrossRef][Green Version]
- ZEON Corporation Technical Report ZEP520A. Available online: https://www.nanophys.kth.se/nanolab/resists/zep520a-7-2.pdf (accessed on 18 June 2021).
- Roy, S.; Gao, Z. Nanostructure-based electrical biosensors. Nano Today 2009, 4, 318–334. [Google Scholar] [CrossRef]
- Fine, D.; Grattoni, A.; Goodall, R.; Bansal, S.S.; Chiappini, C.; Hosali, S.; Van De Ven, A.L.; Srinivasan, S.; Liu, X.; Godin, B.; et al. Silicon micro- and nanofabrication for medicine. Adv. Healthc. Mater. 2013, 2, 632–666. [Google Scholar] [CrossRef]
- JSR Negative Tone THB Photoresists. Available online: https://www.jsrmicro.com/electronic-materials/packaging-materials/jsr-negative-tone-thb-photoresists (accessed on 20 July 2021).
- Ohta, R.; Mawatari, K.; Takeuchi, T.; Morikawa, K.; Kitamori, T. Detachable glass micro/nanofluidic device. Biomicrofluidics 2019, 13, 024104. [Google Scholar] [CrossRef]
- Holland, V.R.; Saunders, B.C.; Rose, F.L.; Walpole, A.L. A safer substitute for benzidine in the detection of blood. Tetrahedron 1974, 30, 3299–3302. [Google Scholar] [CrossRef]
- Shimizu, H.; Mawatari, K.; Kitamori, T. Sensitive determination of concentration of nonfluorescent species in an extended-nano channel by differential interference contrast thermal lens microscope. Anal. Chem. 2010, 82, 7479–7484. [Google Scholar] [CrossRef]
- Tang, T.; Yuan, Y.; Yalikun, Y.; Hosokawa, Y.; Li, M.; Tanaka, Y. Glass based micro total analysis systems: Materials, fabrication methods, and applications. Sens. Actuators B Chem. 2021, 339, 129859. [Google Scholar] [CrossRef]
- Ohashi, T.; Mawatari, K.; Sato, K.; Tokeshi, M.; Kitamori, T. A micro-ELISA system for the rapid and sensitive measurement of total and specific immunoglobulin e and clinical application to allergy diagnosis. Lab. Chip 2009, 9, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Dutta, D. Enhancement in the sensitivity of microfluidic enzyme-linked immunosorbent assays through analyte preconcentration. Anal. Chem. 2012, 84, 7029–7036. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.; McAllister, S.J.; Dutta, D. A glass microchip device for conducting serological survey of West Nile viral antibodies. Biomed. Microdevices 2014, 16, 737–743. [Google Scholar] [CrossRef] [PubMed]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 24 June 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, K.; Ohta, R.; Mawatari, K.; Kitamori, T. Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis. Micromachines 2021, 12, 917. https://doi.org/10.3390/mi12080917
Morikawa K, Ohta R, Mawatari K, Kitamori T. Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis. Micromachines. 2021; 12(8):917. https://doi.org/10.3390/mi12080917
Chicago/Turabian StyleMorikawa, Kyojiro, Ryoichi Ohta, Kazuma Mawatari, and Takehiko Kitamori. 2021. "Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis" Micromachines 12, no. 8: 917. https://doi.org/10.3390/mi12080917
APA StyleMorikawa, K., Ohta, R., Mawatari, K., & Kitamori, T. (2021). Metal-Free Fabrication of Fused Silica Extended Nanofluidic Channel to Remove Artifacts in Chemical Analysis. Micromachines, 12(8), 917. https://doi.org/10.3390/mi12080917





