Simultaneous Detection of Viability and Concentration of Microalgae Cells Based on Chlorophyll Fluorescence and Bright Field Dual Imaging
Abstract
:1. Introduction
2. Materials and Methods
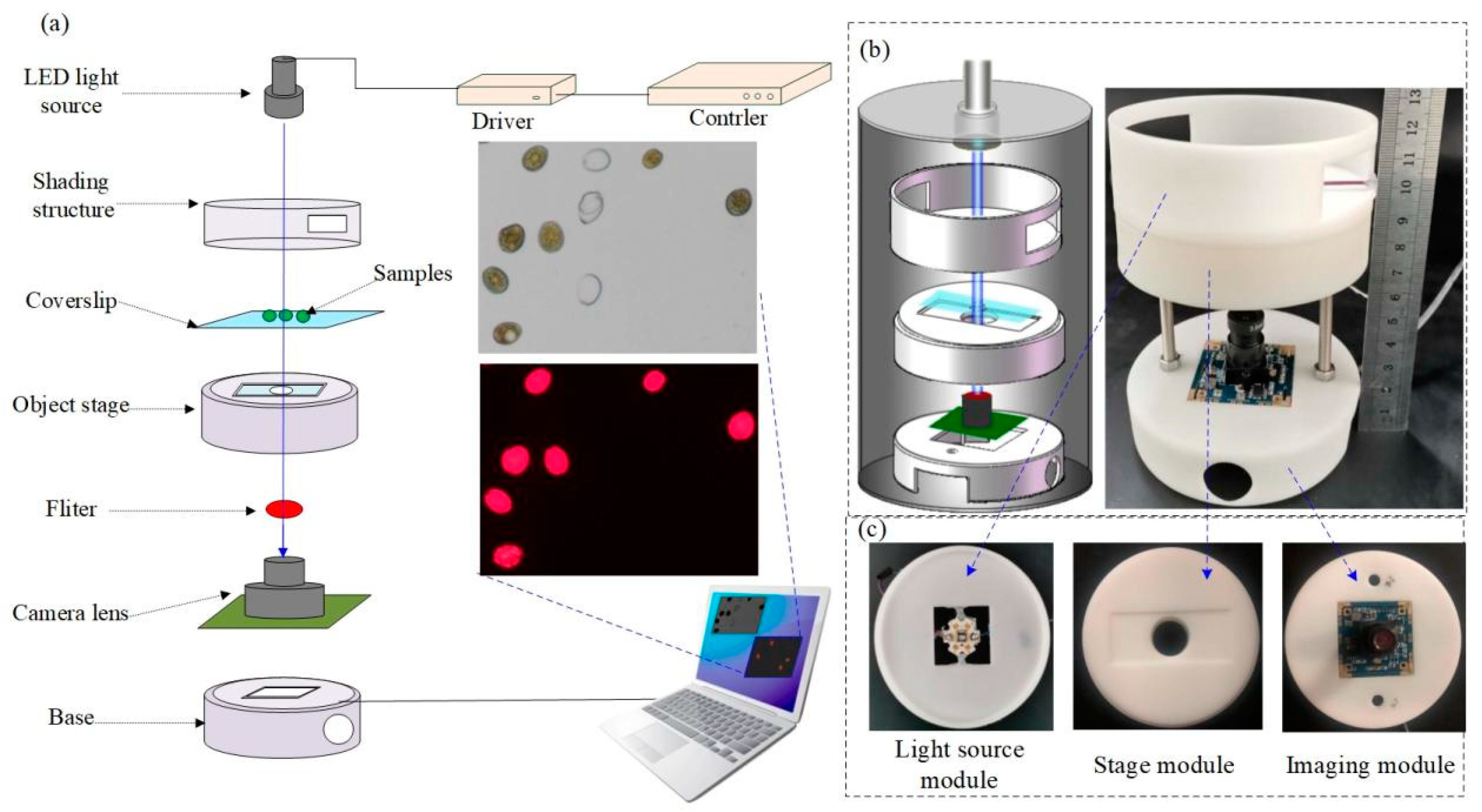
2.1. Principle and System Design
2.2. Viability Detection of Microalgae Cells
2.3. Concentration Detection
2.4. Sample Preparation
2.5. Experimental Setup
2.6. Cell Image Acquisition and Analysis
2.7. Image Registration and Fusion
3. Results and Discussion
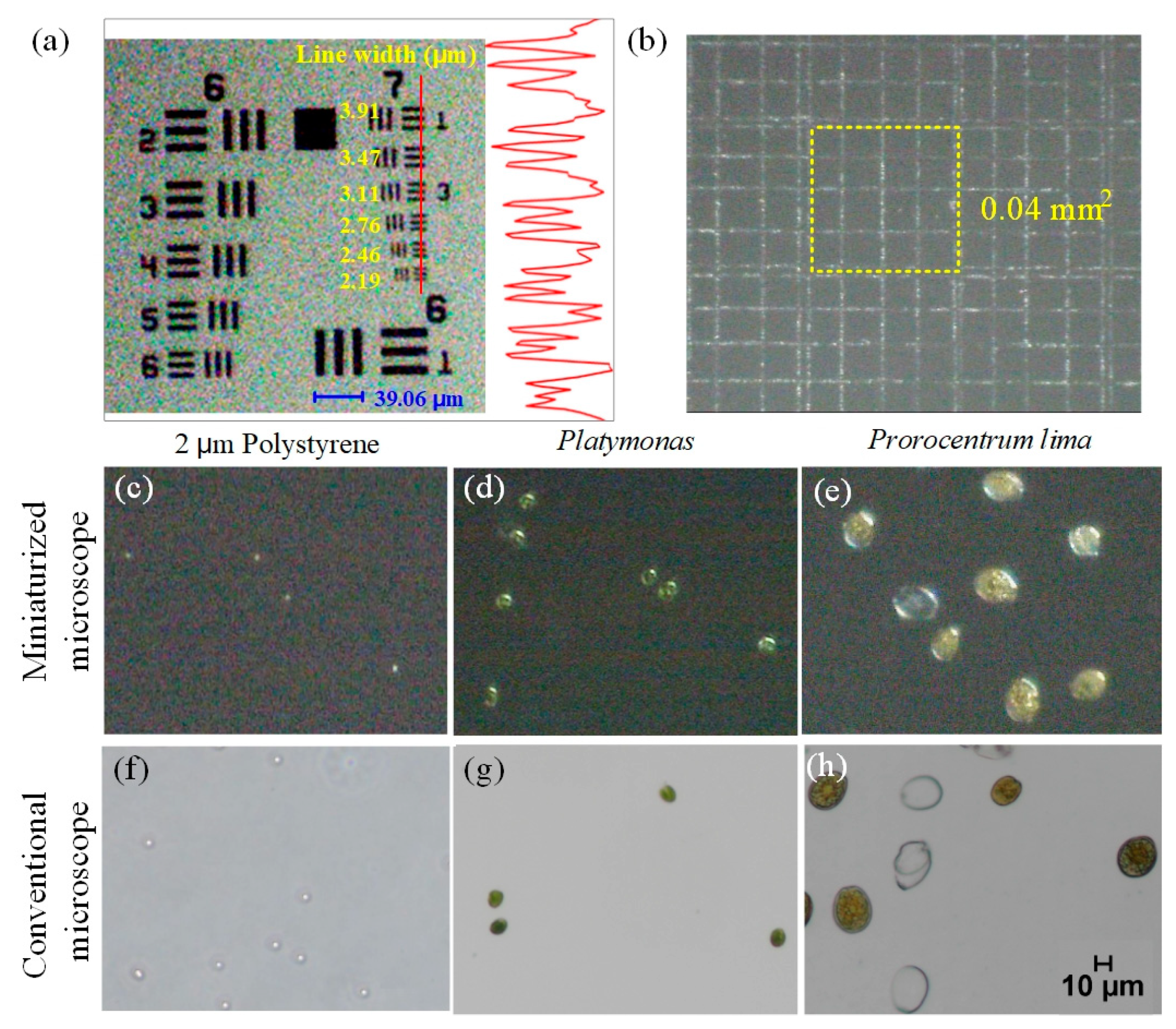
3.1. Resolution Analysis of Miniaturized Dual Imaging Microscopy System
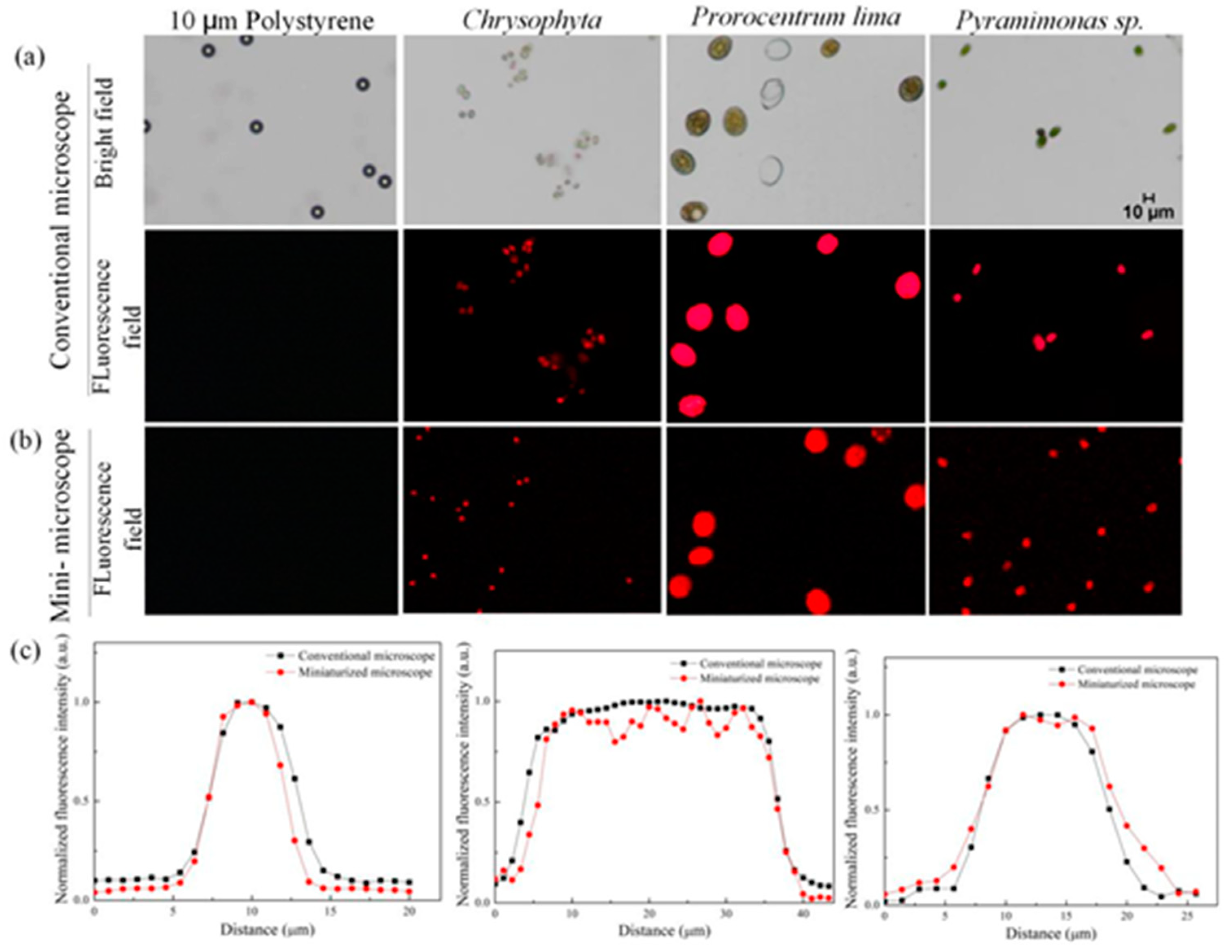
3.2. Fluorescence Analysis of Miniaturized Dual Imaging Microscopy System
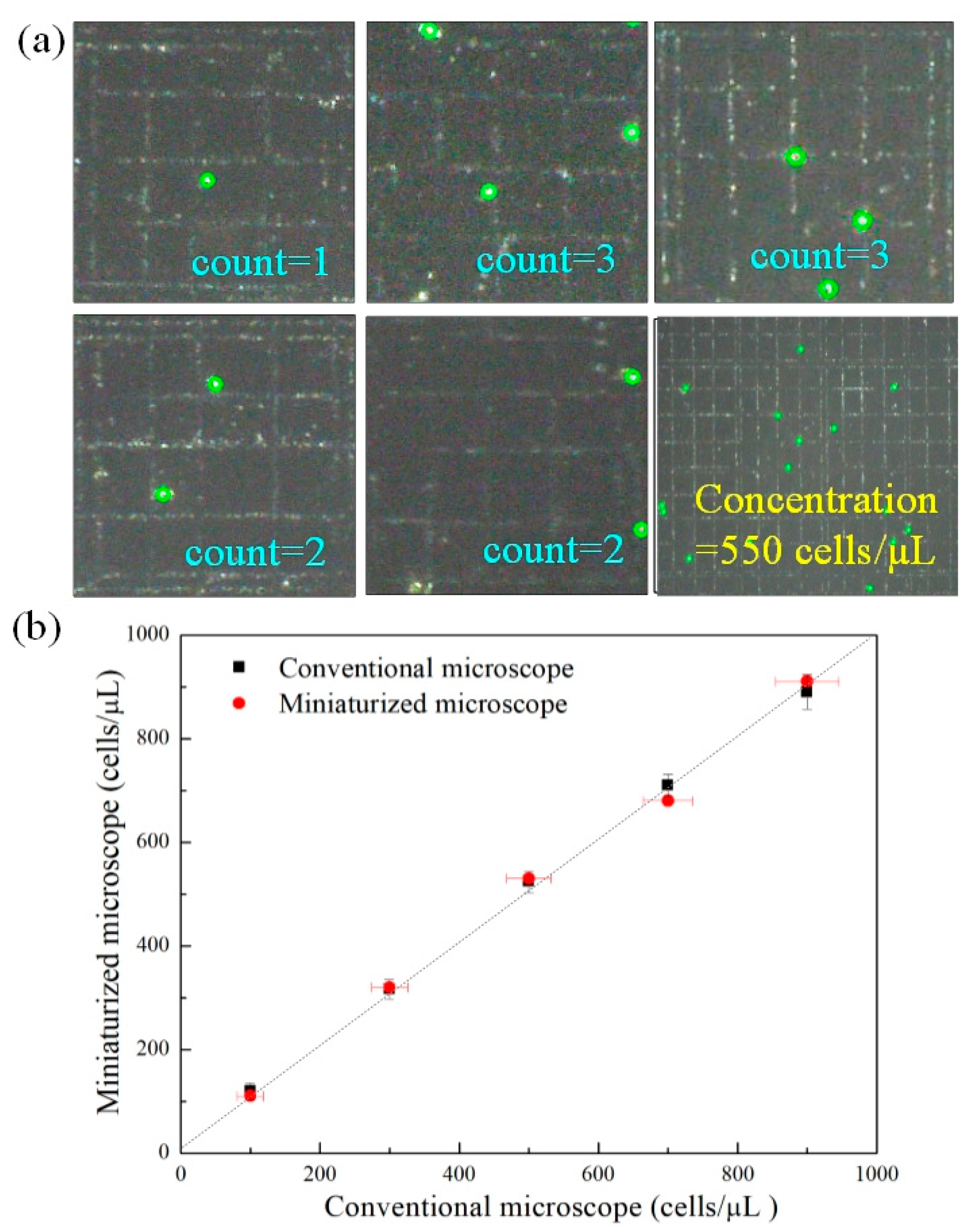
3.3. Concentration Detection of Microalgae Cell in Ballast Water
3.4. Viability Estimation of Microalgae Cells
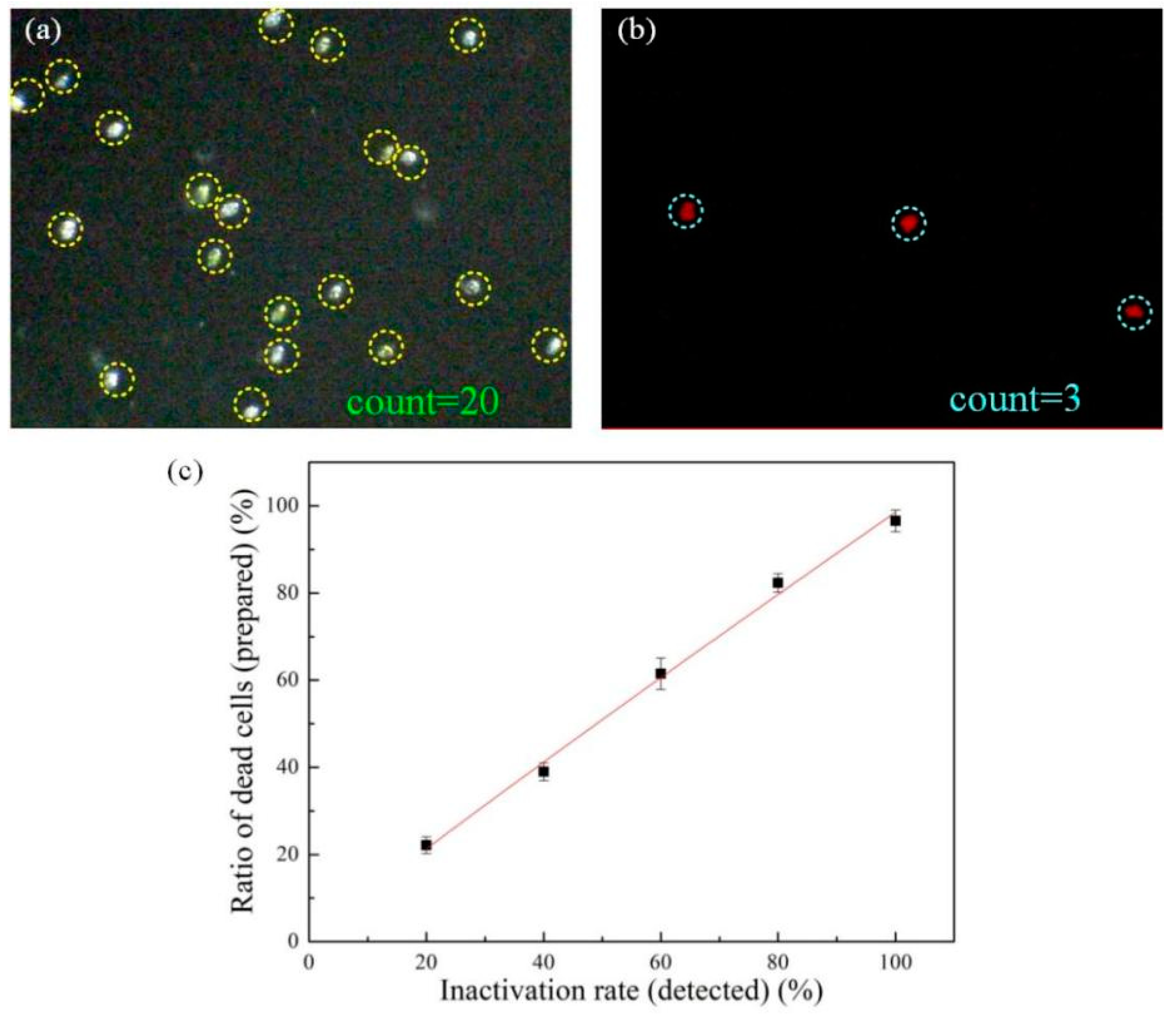
3.5. Evaluation of Ship Ballast Water Inactivation Effect
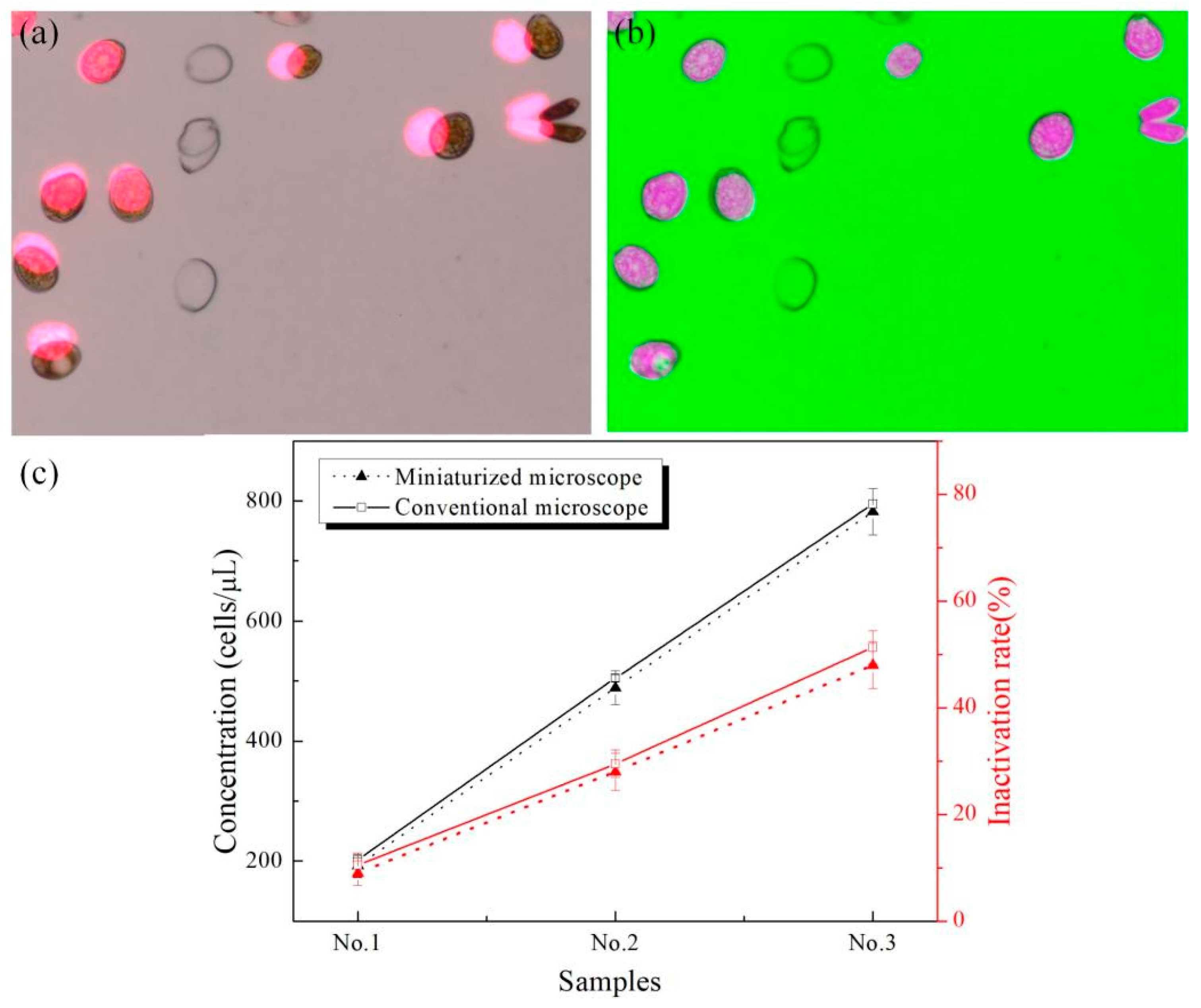
3.6. Simultaneous Detection of Viability and Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Zhao, J.; Wang, Y.; Wang, W. A new microfluidic device for classification of microalgae cells based on simultaneous analysis of chlorophyll fluorescence, side light scattering, resistance pulse sensing. Micromachines 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, I.C.; Scianni, C.; Minton, M.S.; Ruiz, G.M. A history of ship specialization and consequences for marine invasions, management, and policy. J. Appl. Ecol. 2018, 55, 1799–1811. [Google Scholar] [CrossRef]
- Olenin, S.; Gollasch, S.; Jonusas, S.; Rimkut, I. En-route investigations of plankton in ballast water on a ship’s voyage from the Baltic to the open Atlantic coast of Europe. Int. Rev. Hydrobiol. 2000, 85, 577–596. [Google Scholar] [CrossRef]
- Pagenkopp Lohan, K.M.; Fleischer, R.C.; Carney, K.J.; Holzer, K.K.; Ruiz, G.M. Amplicon-Based Pyrosequencing Reveals High Diversity of Protistan Parasites in Ships’ Ballast Water: Implications for Biogeography and Infectious Diseases. Microb. Ecol. 2016, 71, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulou, D.S.; Dobbs, F.C. Bacterial diversity in ships’ ballast water, ballast-water exchange, and implications for ship-mediated dispersal of microorganisms. Environ. Sci. Technol. 2017, 51, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.; Tamburri, M.N.; Fisher, D.J. Long-term algal toxicity of oxidant treated ballast water. Mar. Pollut. Bull. 2018, 133, 18–29. [Google Scholar] [CrossRef]
- Brinkmeyer, R. Diversity of bacteria in ships ballast water as revealed by next generation DNA sequencing. Mar. Pollut. Bull. 2016, 107, 277–285. [Google Scholar] [CrossRef]
- Joo, J.; Jung, G.; Oh, I.; Rhee, T. Validation Test on Real Scale UV Reactor for Ballast Water Treatment. Environ. Eng. Res. 2020, 26, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.-T.; Hong, J.H.; Tayyab, M.; Kim, D.-W. Continuous bubble reactor using carbon dioxide and its mixtures for ballast water treatment. Water Res. 2019, 154, 316–326. [Google Scholar] [CrossRef]
- Moreno-Andres, J.; Ambauen, N.; Vadstein, O.; Cynthia, H.; Acevedo, A.; Nebot, E.; Meyn, T. Inactivation of marine heterotrophic bacteria in ballast water by an Electrochemical Advanced Oxidation Process. Water Res. 2018, 140, 377–386. [Google Scholar] [CrossRef]
- Organization, International Maritime. International Convention for the Control and Management of Ship’s Ballast Water and Sediments. Available online: http://www.imo.org/Conventions/mainframe.asp?topic_id=867 (accessed on 16 February 2004).
- Tsimplis, M. Alien Species Stay Home: The International Convention for the Control and Management of Ships’ Ballast Water and Sediments 2004. Int. J. Mar. Coast. Law 2004, 19, 411–482. [Google Scholar] [CrossRef]
- Gollasch, S.; David, M.; Voigt, M.; Dragsun, E.; Hewitt, C.; Fukuyo, Y. Critical review of the IMO international convention on the management of ships’ ballast water and sediments. Harmful Algae 2007, 6, 585–600. [Google Scholar] [CrossRef]
- Valković, V.; Obhođaš, J. Sediments in the ship’s ballast water tank: A problem to be solved. J. Soils Sediments 2020, 20, 2717–2723. [Google Scholar] [CrossRef]
- Yu, H.F.; Liu, G.M.; Huang, H. Activity Assay of Microalgae Heterosigma akashiwo in Ballast Water by Neutral Red Staining Using the UV and UV/O3 as Inactivation Methods. Adv. Mater. Res. 2012, 573, 1079–1085. [Google Scholar] [CrossRef]
- Da Luz, D.S.; Da Silva, D.G.; Souza, M.M.; Giroldo, D.; Martins, C.D. Efficiency of Neutral Red, Evans Blue and MTT to assess viability of the freshwater microalgae D esmodesmus communisand P ediastrum boryanum. Phycol. Res. 2016, 64, 56–60. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, R.; Brans, T.; Lippens, S.; Parthoens, E.; Zanacchi, F.C.; Magrassi, R.; Singh, S.K.; Kurungot, S.; Szunerits, S.; et al. Repeated photoporation with graphene quantum dots enables homogeneous labeling of live cells with extrinsic markers for fluorescence microscopy. Light Sci. Appl. 2018, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, T.; Ramanna, L.; Rawat, I.; Bux, F. BODIPY staining, an alternative to the Nile Red fluorescence method for the evaluation of intracellular lipids in microalgae. Bioresour. Technol. 2012, 114, 507–511. [Google Scholar] [CrossRef]
- Sato, M.; Murata, Y.; Mizusawa, M.; Iwahashi, H.; Oka, S. A Simple and Rapid Dual-fluorescence Viability Assay for Microalgae. Microbiol. Cult. Coll. Dec. 2004, 20, 53–59. [Google Scholar]
- Laffin, R.J.; Chan, D.W.; Tanasijevic, M.J.; Fischer, G.A.; Markus, W.; Miller, J.; Matarrese, P.; Sokoll, L.J.; Bruzek, D.J.; Eneman, J.; et al. Hybritech Total and Free Prostate-specific Antigen Assays Developed for the Beckman Coulter Access Automated Chemiluminescent Immunoassay System: A Multicenter Evaluation of Analytical Performance. Clin. Chem. 2001, 47, 129–132. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Maw, M.M.; Song, Y.; Pan, X.; Sun, Y.; Li, D. Detection of size spectrum of microalgae cells in an integrated underwater microfluidic device. J. Exp. Mar. Biol. Ecol. 2015, 473, 129–137. [Google Scholar] [CrossRef]
- Nicolletti, I.; Magliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Yang, R.-J.; Fu, L.M.; Hou, H.H. Review and perspectives on microfluidic flow cytometers. Sens. Actuators B Chem. 2018, 266, 26–45. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wu, X.; Zong, J.; Wei, W. Dielectrophoretic separation of microalgae cells in ballast water in a microfluidic chip. Electrophoresis 2019, 40, 969–978. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Ribas, J.; Nadhman, A.; Aleman, J.; Selimović, S.; Lesherperez, S.C.; Wang, T.; Manoharan, V.; Shin, S.R.; Damilano, A. A Cost-Effective Fluorescence Mini-Microscope with Adjustable Magnifications for Biomedical Applications. Lab Chip 2015, 15, 3661. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.J.; Park, J.G.; Yoo, D.; Hahn, Y.K.; Choi, S. A portable somatic cell counter based on a multi-functional counting chamber and a miniaturized fluorescence microscope. Talanta 2017, 170, 238–243. [Google Scholar] [CrossRef]
- Dai, B.; Jiao, Z.; Zheng, L.; Bachman, H.; Zhang, D. Colour compound lenses for a portable fluorescence microscope. Light Sci. Appl. 2019, 8, 75. [Google Scholar] [CrossRef]
- Liu, T.; Rajadhyaksha, M.; Dickensheets, D.L. MEMS-in-the-lens architecture for a miniature high-NA laser scanning microscope. Light Sci. Appl. 2019, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaran, J.; Balasubramanian, H.; Tang, W.H.; Xue, W.N.; Wohland, T. Simultaneous spatiotemporal super-resolution and multi-parametric fluorescence microscopy. Nat. Commun. 2021, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Takagi, J.; Ito, E.; Ito, Y.; Nakano, A. Cargo sorting zones in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. Nat. Commun. 2021, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jin, Y.K.; Jeon, S.; Jin, W.B.; Cho, S.H.; Kim, C. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Yanny, K.; Antipa, N.; Liberti, W.; Dehaeck, S.; Waller, L. Miniscope3D: Optimized single-shot miniature 3D fluorescence microscopy. Light Sci. Appl. 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Z.; Zhang, J.; Jia, S.; Jia, H. MATRIEX imaging: Multiarea two-photon real-time in vivo explorer. Light Sci. Appl. 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Capretti, A.; Ringsmuth, A.K.; Velzen, J.; Rosnik, A.; Croce, R.; Gregorkiewicz, T. Nanophotonics of higher-plant photosynthetic membranes. Light Sci. Appl. 2019, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Song, Y.; Xu, Y.; Pan, X.; Sun, Y.; Li, D. A Label-Free Microfluidic Biosensor for Activity Detection of Single Microalgae Cells Based on Chlorophyll Fluorescence. Sensors 2013, 13, 16075–16089. [Google Scholar] [CrossRef]
- Jin, D.; Wong, D.; Li, J.; Luo, Z.; Guo, Y.; Liu, B.; Wu, Q.; Chih-Ming, H.; Fei, P. Compact Wireless Microscope for In-Situ Time Course Study of Large Scale Cell Dynamics within an Incubator. Sci. Rep. 2015, 5, 18483. [Google Scholar] [CrossRef]
- Wang, Z.; Boddeda, A.; Parker, B.; Samanipour, R.; Ghosh, S.; Menard, F.; Kim, K. A high-resolution mini-microscope system for wireless real-time monitoring. IEEE Trans. Biomed. Eng. 2017, 65, 1524–1531. [Google Scholar] [CrossRef]
- Sun, Y.L.; Dong, W.F.; Yang, R.Z.; Meng, X.; Zhang, L.; Chen, Q.D.; Sun, H.B. Dynamically Tunable Protein Microlenses. Angew. Chem. Int. Ed. 2012, 51, 1558–1562. [Google Scholar] [CrossRef]
- Sima, F.; Kawano, H.; Miyawaki, A.; Kelemen, L.; Ormos, P.; Wu, D.; Xu, J.; Midorikawa, K.; Sugioka, K. 3D Biomimetic Chips for Cancer Cell Migration in Nanometer-Sized Spaces Using “Ship-in-a-Bottle” Femtosecond Laser Processing. ACS Appl. Bio Mater. 2018, 1, 1667–1676. [Google Scholar] [CrossRef]
- Chen, Y.C.; Li, X.; Zhu, H.; Weng, W.H.; Fan, X. Monitoring Neuron Activities and Interactions with Laser Emissions. ACS Photonics 2020, 7, 2182–2189. [Google Scholar] [CrossRef]
- Khater, I.M.; Nabi, I.R.; Hamarneh, G. A Review of Super-Resolution Single-Molecule Localization Microscopy Cluster Analysis and Quantification Methods. Patterns 2020, 1, 100038. [Google Scholar] [CrossRef]
- Miao, X.; Yan, L.; Wu, Y.; Liu, P.Q. High-sensitivity nanophotonic sensors with passive trapping of analyte molecules in hot spots. Light: Sci. Appl. 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Qian, N.; Miao, Y.; Zhao, Z.; Min, W. Super-resolution vibrational microscopy by stimulated Raman excited fluorescence. Light Sci. Appl. 2021, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Chen, M.; Wang, Y.; Pan, X. An integrated microfluidic chip for treatment and detection of microalgae cells. Algal Res. 2019, 42, 101593. [Google Scholar] [CrossRef]


| Microalgae | Conventional Microscope | Miniaturized Microscope |
|---|---|---|
| Chrysophyta | 5.04 | 4.82 |
| Prorocentrum lima | 31.33 | 30.22 |
| Pyramimonas sp. | 12.86 | 13.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Wang, T.; Wang, C. Simultaneous Detection of Viability and Concentration of Microalgae Cells Based on Chlorophyll Fluorescence and Bright Field Dual Imaging. Micromachines 2021, 12, 896. https://doi.org/10.3390/mi12080896
Wang Y, Wang J, Wang T, Wang C. Simultaneous Detection of Viability and Concentration of Microalgae Cells Based on Chlorophyll Fluorescence and Bright Field Dual Imaging. Micromachines. 2021; 12(8):896. https://doi.org/10.3390/mi12080896
Chicago/Turabian StyleWang, Yanjuan, Junsheng Wang, Tianqi Wang, and Chengxiao Wang. 2021. "Simultaneous Detection of Viability and Concentration of Microalgae Cells Based on Chlorophyll Fluorescence and Bright Field Dual Imaging" Micromachines 12, no. 8: 896. https://doi.org/10.3390/mi12080896
APA StyleWang, Y., Wang, J., Wang, T., & Wang, C. (2021). Simultaneous Detection of Viability and Concentration of Microalgae Cells Based on Chlorophyll Fluorescence and Bright Field Dual Imaging. Micromachines, 12(8), 896. https://doi.org/10.3390/mi12080896








